Translate this page into:
Asian type atopic dermatitis

*Corresponding author: Chia-Yu Chu, Department of Dermatology, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan. chiayu@ntu.edu.tw
-
Received: ,
Accepted: ,
How to cite this article: Wu W, Chan TC, Chu C. Asian type atopic dermatitis. CosmoDerma 2022;2:48.
Abstract
Atopic dermatitis (AD) is a common chronic inflammatory skin disorder. Recent clinical and basic research has demonstrated that AD is an immune-mediated disease involving multiple inflammatory pathways and is considered a T helper (TH)2-centered disease involving a common TH22 component. Recently, some reports demonstrated that Asian patients with AD are more likely to present with clearly demarcated lesions with prominent scaling and lichenification and may exhibit distinct immune and barrier features compared with European American patients with AD. Besides TH2 activation, patients of Asian descent (Japanese, Korean, and Chinese) with AD had strong TH17 activation, overlapping clinically and molecularly with some hallmarks of psoriasis.
Keywords
Atopic dermatitis
Han Chinese
Lichenification
Psoriasis
INTRODUCTION
Atopic dermatitis (AD) is a common chronic inflammatory skin disorder, with a lifetime prevalence of 10-20% in children and 1-3% in adults.[1] Recent clinical and basic research have demonstrated that AD is an immune-mediated disease involving multiple inflammatory pathways and is considered a T helper (TH)2-centered disease involving a common TH22 component.[2] AD can be classified into different subtypes according to serum IgE levels (extrinsic or intrinsic), ethnicity (European American [EA], Asian, or African American), and age (pediatric or adult).[3-5]
Extrinsic and intrinsic AD
Extrinsic AD is associated with high total serum IgE levels and specific IgEs that target environmental and food allergens, whereas intrinsic AD presents with normal total IgE values and the absence of specific IgEs.[6] Increased IgE levels are detected in approximately 80% of AD cases (categorized as extrinsic), whereas normal IgE levels (categorized as intrinsic) are detected in 20% of cases.[6] Extrinsic AD is frequently accompanied by allergic bronchial asthma or allergic rhinoconjunctivitis, whereas intrinsic AD presents with normal serum IgE levels despite the development of skin lesions and distribution patterns similar to those observed in extrinsic AD. Patients with intrinsic AD are negative for in vitro tests that detect the presence of environmental or food allergens and are not associated with other atopic diseases.[7] However, metal allergy, which is common in AD, is more frequently associated with intrinsic AD than with extrinsic AD.[6] Intrinsic AD is also associated with a female predominance, the absence of other atopic diseases, Dennie-Morgan fold, later disease onset, and milder disease severity.[8] By contrast, palmar hyperlinearity, keratosis pilaris, pityriasis alba, and hand and/or food eczema are significantly less common in cases of intrinsic AD.[8] AD is associated with a decreased skin barrier function. Loss-of-function mutations in Filaggrin (FLG), which encodes the epidermal barrier protein filaggrin, have been identified as the cause of ichthyosis vulgaris and serve as major predisposing factors for AD with allergic sensitization.[9-11] Filaggrin facilitates the terminal differentiation of the epidermis and the formation of the skin barrier, including the regulation of skin pH and epidermal hydration. The loss of filaggrin expression leads to the enhanced percutaneous transfer of allergens and epicutaneous sensitization to protein antigens.[12,13] AD patients who harbor FLG mutations have more persistent disease, a higher incidence of eczema herpeticum, and a greater risk of developing multiple allergies and asthma.[14]
Extrinsic AD is considered the prototypical form of AD, and an association between FLG mutations and extrinsic AD has been observed.[9,15] In a Japanese study, the incidence of FLG mutations was significantly lower (10.5%) in the IgE-low group (IgE < 200kU/L) than in the AD patients with IgE > 500kU/L (44.4%).[16] Palmar hyperlinearity and keratosis pilaris, which present more frequently in patients with extrinsic AD than in those with intrinsic AD, are phenotypic characteristics of ichthyosis vulgaris and are both highly associated with FLG mutations.[17,18] Patients with extrinsic AD show increased transepidermal water loss and reduced skin surface hydration, whereas no significant differences in these measures were observed in patients with intrinsic AD.[7]
Pathophysiology of extrinsic and intrinsic AD
Atopic dermatitis (AD) is well known as a TH2-polarized disease, and both extrinsic and intrinsic AD show marked TH2 activation (high expression levels of interleukin [IL]-4/IL-13). Extrinsic AD patients have elevated TH2 cells and decreased TH1 cells in peripheral blood, associated with elevated levels of IL-4, IL-5, and IL-13 expression and increased eosinophil counts. By contrast, intrinsic AD is associated with much lower IL-4 and IL-13 levels.[9,20] In mouse models, skin barrier damage drives the production of TH2 cells and eosinophil-produced chemokines (C-C motif chemokine ligand [CCL]17, CCL22, and CCL5) and augments the expression of IL-4 and C-C motif chemokine receptor (CCR)4, leading to the dermal infiltration of eosinophils.[21] Keratinocytes that are differentiated in the presence of IL-4 and IL-13 exhibit significantly reduced FLG expression, even in patients without FLG mutations.[22] Atopic immune responses contribute to acquired skin barrier defects in AD, resulting in a vicious cycle. Another recent study showed similar increases in TH2 cells in lesional skin from patients with both intrinsic and extrinsic AD, although the higher activation of TH1, TH17, and TH22 cytokines was detected in patients with intrinsic AD.[16,23] Although IL-22 messenger ribonucleic acid (mRNA) expression increases significantly in lesional skin compared with non-lesional skin in both intrinsic and extrinsic AD, this increase is significantly higher in intrinsic AD than in extrinsic AD.[23] Intrinsic AD was also associated with Increased TH1 signaling factors (interferon-gamma [IFN-γ], C-X-C chemokine motif ligand [CXCL]9, CXCL10, and MX dynamin-like GTPase 1 [MX-1]) and more pronounced TH17/ TH22 activation (IL-17A, IL-12/IL-23p40, CCL20, Elafin, and IL-22).[23] Patients with extrinsic AD showed strong correlations between disease activity and TH2 cytokine levels (IL-4 and IL-5) and negative correlations with barrier products (loricrin, periplakin, and filaggrin). By contrast, disease activity in patients with intrinsic AD was correlated with the expression of TH1/IFN-related genes (IL-1β and IFN-α) and the IL-17-related CCL20 chemokine.[23] In another study, the expression of IL22, IL36A, IL36G, CCL19, and CCL22 was upregulated in intrinsic AD and psoriasis but not in extrinsic AD. These findings suggest that the inflammation signature of intrinsic AD is more similar to that of psoriasis, than that of extrinsic AD.[24]
Epidemiology and clinical characteristics of Asian AD
Recently the heterogeneity of AD has become apparent, supported by the identification of clinical, molecular, and genetic differences associated with different racial backgrounds.[25] A high prevalence of AD has been reported in the Asian population. In the Asia-Pacific region, overall AD prevalence was 10.1% in children between the ages of 6 and 7 years, a higher level than reported for Western Europe (8.1%) or the total global prevalence of 7.9% reported by the International Study of Asthma and Allergies in Childhood Phase Three.[6,27] In the United States, Asians and Pacific Islanders are 7 times more likely to visit physicians for AD compared with whites.[28] There is a rising prevalence of both pediatric and adult AD in Asian populations worldwide, particularly in Asians living in urban areas. Potentially contributing to the evolving prevalence of AD is the rapid urbanization and increased pollution in many metropolitan areas in Asia.[29] A prospective cohort study in the United States found the adjusted odds ratio (OR) for risk of AD among infants born to Asian mothers in the first 6 months of life was 2.58, compared with infants born to white mothers.[30] Studies suggest that the children of Asian immigrants may be at higher risk for developing AD, potentially resulting from epigenetic phenomena unique to immigrant populations.[29]
Clinically, Asian patients with AD are more likely to present with clearly demarcated lesions with prominent scaling and lichenification compared with EA patients with AD, which is consistent with some molecular features of psoriasis in this patient population [Figure 1].[25,31]

- (a-c) Representative clinical images of Asian patients with AD, which characterized by clearly demarcated lesions with prominent scaling and lichenification. (d) Representative hematoxylin and eosin staining for lesional skin of an Asian patient with AD, characterized by epidermal hyperplasia, parakeratosis and hypogranulosis.
Differences in skin barrier impairments in Asian AD
Several studies have found that up to 27% of Asian patients with AD harbor relevant FLG null mutations, whereas similar research in Europe reported one or more FLG mutations in 50% of all AD cases.[31-33] The most frequent loss-of-function FLG mutations (R501X, 2282del4, S3247X, and R2447X) have been identified in 7-10% of the white European population; however, these mutations are usually absent in Asian individuals, who exhibit FLG null mutations that are unique to their respective ethnic groups.[34] In the white European population, two prevalent FLG mutations (R501X and 2282del4) account for over 80% of all FLG null alleles, whereas in the Singaporean Chinese population, seven different FLG null mutations have been identified (3321delA, 6950_6957del8, S1515X, S2706X, Q2417X, E2422X, and G323X), which account for 80% of the FLG mutation spectrum.[34,35] In another study, the FLG mutations K4671X and 3321delA were two of the most commonly identified FLG mutations of patients with AD in northern China (K4671X:11.2%, 3321delA:9.7%).[36] The OR for eczema was found to be higher for the R501X mutation than for the 2282del4 mutation in a meta-analysis, suggesting a possible differential risk effect associated with the FLG mutation site; however, an insufficient number of large studies have examined other uncommon recurrent null alleles, limiting the ability to explore this possibility further.[37] Serine protease inhibitor Kazal-type 5 (SPINK5 ) encodes lymphoepithelial Kazal-type related inhibitor (LEKTI), which is also involved in skin barrier function and AD development. LEKTI is a serine protease inhibitor that degrades corneodesmosome, leading to defects in barrier permeability. LEKTI polymorphisms are associated with common atopy and AD, especially in Eastern Asian populations.[38-42]
Different immunological status in Asian AD
Atopic dermatitis (AD) is thought to be associated with skin barrier dysfunction and adaptive immune responses to common environmental allergens mediated by TH2 cells. Polymorphic differences among ethnic groups have been described in genes involved in innate and adaptive immunity, especially TH2 signaling pathways. IL-4 polymorphisms are significantly associated with atopic AD in Egyptian, Japanese, and Chinese populations.[43-46] Several IL4R polymorphisms have been associated with AD in Japanese populations.[47] Linkage and association studies found that polymorphisms in the gene encoding signal transducer and activator of transcription 6 were significantly associated with allergic diseases, including AD, in Egyptian and Japanese populations.[44,48] IL-13 polymorphisms were significantly associated with an increased risk of AD in Japanese populations.[49,50]
Recent findings suggest that AD is more heterogeneous across more aspects than differences in TH2-centric inflammation. In addition to TH2/TH22 pathways, TH1 and TH17 immune pathways have been implicated in AD pathogenesis. The epidermal hyperplasia response in chronic AD is likely driven directly by IL-22 produced by TH22 cells.[51] Some AD subtypes are associated with TH17, resulting in increased production of IL-17.[52] Intrinsic AD showed the stronger activation of TH17 and TH22 responses than extrinsic AD.[23] Early pediatric AD is associated with a significant increase in the induction of TH17-related cytokines and antimicrobials (IL-17A, IL-19, CCL20, LL37, and peptidase inhibitor 3/elafin) compared with adult AD.[53] Although TH2 activation has been described across all races and ethnicities investigated, studies in the Japanese population have demonstrated the expansion of IL-17+ T cells in both skin and blood, suggesting that the Asian AD phenotype may exhibit distinct immune and barrier features compared with the EA AD phenotype. In additional to TH2 activation, patients of Asian descent (Japanese or Korean) with AD had strong TH17 activation, overlapping clinically and molecularly with some hallmarks of psoriasis.[31,54] Lesional epidermis derived from Japanese and Korean patients with AD showed greater acanthosis, higher Ki67 counts, frequent parakeratosis, focal hypogranulosis, and more elongated rete ridges but relatively preserved barrier proteins expression, including filaggrin and loricrin, compared with the EA AD population. Significantly higher TH17 and TH22 (IL17A, IL19, and S100 calcium-binding protein A12 [S100A12] in lesional and IL-22 in non-lesional skin) and lower TH1/interferon (CXCL9, CXCL10, MX1, and IFN-γ in non-lesional skin) gene induction was observed in AD skin from Japanese and Korean patients.[31] IL-19 is induced by both IL-17 and IL-4/IL-13, which induces epidermal hyperplasia and amplifies many IL-17A effects on keratinocytes, and showed robust and significant increases in the Asian AD phenotype compared with the EA AD phenotype.[31] The psoriasis-like histologic features in the skin of the Asian AD population might be attributed to increased TH17/TH22 polarization and IL-19 induction.[31] Peripheral blood from these Asian patients with AD show significant increases in the TH2/IL-13/CCL26 and TH22/IL-22 axes, with decreases in TH1/IFN-γ (IFN-γ, CCL2, CCL3, and CCL4) axis, which is highly correlated with the pattern found in the skin.[55] Decreased expression of TH1 pathway genes in Asian patients with AD might be attributed to a negative regulatory effect of IL-17 on the IFN/TH1 pathway.[31] Elevated IL-22 levels in Asian patients with AD are concordant with TH17/TH22 upregulation and the occurrence of more prominent epidermal hyperplasia and parakeratosis in the Asian AD population.[31,55] Similar to the results observed in Japanese and Korean populations with AD, skin lesions in Chinese individuals with AD show prominent hyperplasia, parakeratosis, and increased TH17-skewing with decreased TH1 activation compared with EA AD.[56] A positive correlation exists between IL-17A expression and severity (as measured using the scoring atopic dermatitis [SCORAD] scale) in Chinese patients with AD.[56] The TH17-produced cytokine IL-17 is a key inducer of antimicrobial peptides and neutrophil chemoattractants.[57] Neutrophil infiltration has been described more consistently in AD patients of East Asian descent (Japanese, Korean, and Chinese) compared with EA patients with AD.[56,58] Interestingly, several TH2 markers were significantly upregulated in Chinese psoriasis lesions from Chinese patients compared with those from EA patients. These data revealed overlapping tissue patterns between AD and psoriasis in the Chinese population, with variable degrees of TH2 and TH17 upregulation in both diseases. CCL26 was significantly upregulated in Chinese patients with AD and downregulated in Chinese patients with psoriasis and can be used to discriminate AD from psoriasis in the Chinese population.[56] The significant increase in CCL26 levels observed in Asian patients with AD is in line with reports describing increased circulating eosinophil counts in Asian patients compared with EA patients.[55] Thus, in the Asian population, AD is a disease with features observed in both EA AD and psoriasis (parakeratosis and increased neutrophils) and is characterized by a unique combination of immune dysregulation, including TH17/TH22 upregulation accompanied by comparable or even greater TH2 activation (IL-13 and CCL26) and decreased TH1/IFN-γ levels.[31,55,56]
Therapeutic implications for Asian patients with AD
Treatment of AD consists of initial assessment should include detailed history and the extent and severity of AD, followed by delivering basic care with emollients, therapeutic patient education and avoidance of irritants/allergens. Induction pharmacologic therapy is to provide immediate control of pruritus and inflammation by antihistamines and topical corticosteroids (TCSs). If the signs and symptoms of the disease is inadequately controlled, treatment options such as burst use of systemic corticosteroids, phototherapy, or control of infections might be considered. Systemic immunomodulatory agents, potent TCSs, alternative medicine or psychotherapeutic approach might be helpful for severe recalcitrant disease. All patients may be switched to maintenance therapy once the disease is under control, and they may return to continuing basic care if the lesions achieve complete remission [Figure 2].[4]
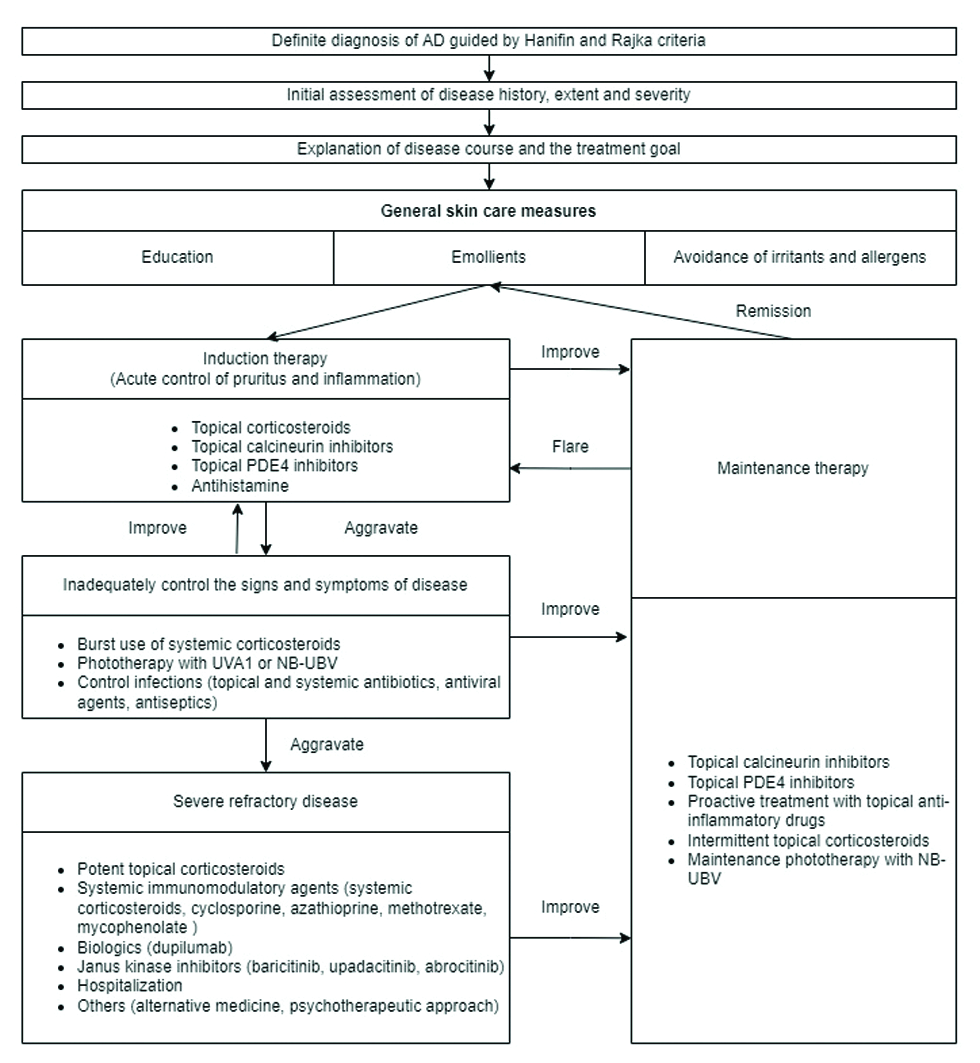
- Atopic dermatitis treatment algorithm.
Although Western medicine and ideas about AD have become popular in many Asian countries, local beliefs about the causes of AD and the types of treatment often prevail. Complementary and alternative medicine, traditional Chinese medicine, and integrative medicine usage are prevalent among the Asian population.[57] Despite its popular use in Asian societies, studies examining the safety and efficacy of traditional Chinese herbal mixtures in children with AD have reported inconsistent results.[58] Western medicine practitioners are often ignorant about complementary and alternative medicine. In addition, many of the cultural practices are preserved among the southeast Asian minorities residing in the United Kingdom and North America.[57] Parents are concerned about the potential side effects of Western medicine and are more likely to cause nonadherence to medication. Nonadherence to TCSs is associated with concern/fear of their side effects (TCS phobia or corticophobia). In a large international study evaluating levels of corticophobia by the validated Topical Corticosteroid Phobia score, Taiwan is among the top three counties with the highest levels of corticophobia.[59] Recognizing this phenomenon and providing accurate information are necessary for improving medication adherence and treatment outcome.[4]
Given its key role in the pathway of TH2 mediated immune response, IL-4 receptor subunit alpha (IL-4Rα) blockade was anticipated to represent a therapeutic approach to treating allergic diseases. Dupilumab is a fully IgG4 human monoclonal antibody that binds IL-4Rα and inhibits IL-4R signaling induced by both IL-4 and IL-13, and down-regulates TH2 inflammation.[60] Although TH2-skewing is common across all AD subtypes, as suggested by the efficacy of dupilumab across populations with AD, therapeutic targeting in AD is complicated by the contributions of multiple immune axes to different pathogenic disease features. Approximately 60% of patients attain a 75% reduction from baseline in the Eczema Area and Severity Index following dupilumab treatment, which suggests that AD is not a pure TH2 disease.[60-62] Additional targeted therapies are likely to contribute to improved AD resolution across all subtypes. AD might be considered a multi-axis immune disease requiring the combined blockade of the TH2, TH22, and potentially TH17 immune axes. Some AD subtypes with significant TH17/TH22 activation, such as intrinsic and pediatric AD and AD in Asian populations, may benefit from therapeutic agents that target the TH17/IL-23 or TH22/IL-22 axes. A randomized, double-blind, placebo-controlled trial examining monotherapy with intravenous fezakinumab (an IL-22 monoclonal antibody) showed improvements in SCORAD evaluations, body surface area involvement, and Investigator Global Assessment compared with placebo. IL-22 blockade only appears to elicit significant clinical effects in patients with severe AD.[63] Greater mean SCORAD improvements were observed following fezakinumab administration in the IL-22-high group than in the IL-22-low group.[64] Future studies may evaluate whether Asian patients with AD can benefit from IL-22 antagonism therapy.
Recently, a phase 2 randomized, double-blinded study examined the use of the IL-17-targeting agent secukinumab on intrinsic and extrinsic AD. The entire cohort of patients receiving secukinumab versus placebo was analyzed, with further analysis of patients with intrinsic AD versus patients with extrinsic AD. However, compared the secukinumab-treated with placebo groups, no significant differences were observed in either group in the primary outcome of changes in epidermal hyperplasia, clinical outcome measures, or secondary translational endpoints, as assessed by evaluating the mRNA expression of TH17/IL-23-related products, even in in the secukinumab-treated patients with intrinsic AD.[65] Overall, 16 Asian patients were enrolled in this study, but no significant changes were found between secukinumab treatment and placebo in this subgroup. The outcomes of this study show that IL-17 may not represent a valid therapeutic target in patients with AD, including subsets of patients with higher TH17 activation, such as those with intrinsic AD and Asian patients, in contrast to the hypothesis that IL-17A is a sole pathogenic contributor to AD. Janus kinase inhibitors (JAK inhibitors or jakinibs) inhibit signaling pathways through a variety of cytokine and hematopoietic growth factor receptors, including in the TH1 (IL-2, IL-12, IFN-γ), TH2 (IL-4, IL-5, IL-13), and TH17 (IL-17, IL-22) axes, which may provide therapeutic relief for Asian patients with AD.[66]
CONCLUSION
The AD phenotype observed among patients of Asian descent might be associated with differences in epidemiology, clinical phenotype, histopathology, barrier defects, and immune characteristics than the better-studied phenotype in the EA population [Table 1]. Asian patients with AD present with a unique immune phenotype that combines the characteristics of AD and psoriasis and might require new clinical trials with specific cytokine antagonists, in addition to TH2-specific therapies. Combination therapy with TH2-targeting strategies and strategies that target the cytokines IL-17, IL-23, and IL-22 must be further evaluated and may lead to personalized or precision medicine approaches for the various AD subtypes.
| Asian AD | European American AD | |
|---|---|---|
| Epidemiology | High prevalence (~10%) | ~8% |
| Clinical phenotype | Clearer demarcation of lesions, with prominent scaling and lichenification. | Indistinct borders, wet and erythematous. In patients with chronic disease, lichenification, dry, and hyperpigmentation |
| Histopathology | Prominent hyperplasia, parakeratosis, hypogranulosis, and neutrophil infiltration. | Rare parakeratosis and rare neutrophils. |
| Epidermal barrier | Fewer FLG null mutations (27%), and FLG null mutations unique to ethnic groups. | Common FLG null mutations (~50%), especially R501X, 2282del4, S3247X, and R2447X. |
| Immune polarization | TH1↑ TH2↑↑↑ TH17↑↑ TH22↑↑↑ |
TH1↑↑ TH2↑↑↑ TH17↑ TH22↑↑↑ |
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflict of interest
Author Prof. (Dr.) Chia-Yu Chu is on the Editorial Board of the journal.
References
- Epidemiology of atopic dermatitis. Immunology and Allergy Clinics of North America. 2002;22:1-24.
- [CrossRef] [Google Scholar]
- Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344-54.
- [CrossRef] [PubMed] [Google Scholar]
- Subtypes of atopic dermatitis: From phenotype to endotype. Allergol Int. 2022;71:14-24.
- [CrossRef] [PubMed] [Google Scholar]
- Taiwanese Dermatological Association consensus for the management of atopic dermatitis: A 2020 update. J Formos Med Assoc. 2021;120:429-442.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of baseline comorbidities in patients with atopic dermatitis: A population-based cohort study in Taiwan. JAAD Int. 2020;1:50-58.
- [CrossRef] [PubMed] [Google Scholar]
- Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010;58:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of transepidermal water loss, capacitance and pH values in the skin between intrinsic and extrinsic atopic dermatitis patients. J Korean Med Sci. 2003;18:93-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical differences between atopic and atopiform dermatitis. J Am Acad Dermatol. 2008;58:407-14.
- [CrossRef] [PubMed] [Google Scholar]
- Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214-9.
- [CrossRef] [PubMed] [Google Scholar]
- Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalent and low-frequency null mutations in the filaggrin gene are associated with early-onset and persistent atopic eczema. J Invest Dermatol. 2008;128:1591-4.
- [CrossRef] [PubMed] [Google Scholar]
- Deciphering the complexities of atopic dermatitis: Shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134:769-79.
- [CrossRef] [PubMed] [Google Scholar]
- Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009;124:485-93. 493.e1
- [CrossRef] [PubMed] [Google Scholar]
- Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315-27.
- [CrossRef] [PubMed] [Google Scholar]
- Filaggrin mutations strongly predispose to early-onset and extrinsic atopic dermatitis. J Invest Dermatol. 2007;127:724-6.
- [CrossRef] [PubMed] [Google Scholar]
- A group of atopic dermatitis without IgE elevation or barrier impairment shows a high Th1 frequency: Possible immunological state of the intrinsic type. J Dermatol Sci. 2012;67:37-43.
- [CrossRef] [PubMed] [Google Scholar]
- The prevalence of accentuated palmoplantar markings and keratosisin pilaris in atopic dermatitis, autosomal dominant ichthyosis and control dermatological patients. Br J Dermatol. 1985;112:679-85.
- [CrossRef] [PubMed] [Google Scholar]
- Filaggrin null mutations and childhood atopic eczema: A population-based case-control study. J Allergy Clin Immunol. 2008;121:940-46.e3.
- [CrossRef] [PubMed] [Google Scholar]
- Immune dysregulation in atopic dermatitis. Allergy Asthma Proc. 2006;27:451-5.
- [CrossRef] [PubMed] [Google Scholar]
- Differential in vivo cytokine mRNA expression in lesional skin of intrinsic vs. extrinsic atopic dermatitis patients using semiquantitative RT-PCR. Clin Exp Allergy. 2003;33:1717-24.
- [CrossRef] [PubMed] [Google Scholar]
- Induction of eosinophil- and Th2-attracting epidermal chemokines and cutaneous late-phase reaction in tape-stripped skin. Exp Dermatol. 2009;18:1036-43.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150-5.
- [CrossRef] [PubMed] [Google Scholar]
- Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol. 2013;132:361-70.
- [CrossRef] [PubMed] [Google Scholar]
- Distinct molecular signatures of mild extrinsic and intrinsic atopic dermatitis. Exp Dermatol. 2016;25:453-9.
- [CrossRef] [PubMed] [Google Scholar]
- Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-55.
- [CrossRef] [PubMed] [Google Scholar]
- ISAAC phase three study group. The International Study of Asthma and Allergies in Childhood (ISAAC) phase three: A global synthesis. Allergol Immunopathol (Madr). 2013;41:73-85.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of atopic dermatitis in Asia. J Dermatol. 2019;46:825-34.
- [CrossRef] [PubMed] [Google Scholar]
- In the United States, blacks and Asian/Pacific Islanders are more likely than whites to seek medical care for atopic dermatitis. Arch Dermatol. 2002;138:634-7.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and characterization of atopic dermatitis in East Asian populations: A systematic review. Dermatol Ther (Heidelb). 2021;11:707-717.
- [CrossRef] [PubMed] [Google Scholar]
- Perinatal predictors of atopic dermatitis occurring in the first six months of life. Pediatrics. 2004;113:468-74.
- [CrossRef] [PubMed] [Google Scholar]
- The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254-64.
- [CrossRef] [PubMed] [Google Scholar]
- Eczema genetics: Current state of knowledge and future goals. J Invest Dermatol. 2009;129:543-52.
- [CrossRef] [PubMed] [Google Scholar]
- Novel FLG null mutations in Korean patients with atopic dermatitis and comparison of the mutationalin spectra in Asian populations. J Dermatol. 2015;42:867-73.
- [CrossRef] [PubMed] [Google Scholar]
- One remarkable molecule: Filaggrin. J Invest Dermatol. 2012;132:751-62.
- [CrossRef] [PubMed] [Google Scholar]
- Widespectrum of filaggrin-null mutations in atopic dermatitis highlights differences between Singaporean Chinese and European populations. Br J Dermatol. 2011;165:106-14.
- [CrossRef] [PubMed] [Google Scholar]
- Mutations analysis in filaggrin gene in northern China patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2013;27:169-74.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of filaggrin polymorphisms in eczema and asthma: Robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123:1361-70.
- [CrossRef] [PubMed] [Google Scholar]
- The genetics and epigenetics of atopic dermatitis-filaggrin and other polymorphisms. Clin Rev Allergy Immunol. 2016;51:315-28.
- [CrossRef] [PubMed] [Google Scholar]
- Association between polymorphisms in the SPINK5 gene and atopic dermatitis in the Japanese. Genes Immun. 2003;4:515-7.
- [CrossRef] [PubMed] [Google Scholar]
- SPINK5 polymorphism is associated with disease severity and food allergy in children with atopic dermatitis. J Allergy Clin Immunol. 2005;115:636-8.
- [CrossRef] [PubMed] [Google Scholar]
- Association of SPINK5 gene polymorphisms with atopic dermatitis in the Japanese population. Br J Dermatol. 2003;148:665-9.
- [CrossRef] [PubMed] [Google Scholar]
- Association of SPINK5 gene polymorphisms with atopic dermatitis in Northeast China. J Eur Acad Dermatol Venereol. 2012;26:572-7.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular genetic of atopic dermatitis: An update. Int J Health Sci (Qassim). 2016;10:96-120.
- [PubMed] [Google Scholar]
- Association between genes encoding components of the IL-4/IL-4 receptor pathway and dermatitis in children. Gene. 2014;545:276-81.
- [CrossRef] [PubMed] [Google Scholar]
- Linkage and association of an interleukin 4 gene polymorphism with atopic dermatitis in Japanese families. J Med Genet. 1998;35:502-4.
- [CrossRef] [PubMed] [Google Scholar]
- IL-4 gene polymorphism may contribute to an increased risk of atopic dermatitis in children. Dis Markers. 2016;2016:1021942.
- [CrossRef] [PubMed] [Google Scholar]
- Polymorphisms in the promoter of the interleukin-4 receptor alpha chain gene are associated with atopic dermatitis in Japan. J Invest Dermatol. 2004;122:843-5.
- [CrossRef] [PubMed] [Google Scholar]
- Linkage and association studies of STAT6 gene polymorphisms and allergic diseases. Int Arch Allergy Immunol. 2003;131:33-8.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-13 gene polymorphism G4257A is associated with atopic dermatitis in Japanese patients. J Dermatol Sci. 2002;30:100-7.
- [CrossRef] [PubMed] [Google Scholar]
- Case-control study of eczema associated with IL13 genetic polymorphisms in Japanese children. Int Arch Allergy Immunol. 2011;154:328-35.
- [CrossRef] [PubMed] [Google Scholar]
- Th17 cytokines interleukin (IL)—17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092-102.
- [CrossRef] [PubMed] [Google Scholar]
- The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139:S65-76.
- [CrossRef] [PubMed] [Google Scholar]
- Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol. 2016;138:1639-51.
- [CrossRef] [PubMed] [Google Scholar]
- Possible pathogenic role of Th17cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625-30.
- [CrossRef] [PubMed] [Google Scholar]
- Serum from Asian patients with atopic dermatitis is characterized by TH2/TH22 activation, which is highly correlated with non-lesional skin measures. J Allergy Clin Immunol. 2018;142:324-328.e11.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis in Chinese patients shows TH2/TH17 skewing with psoriasiform features. J Allergy Clin Immunol. 2018;142:1013-17.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis: Conventional and integrative medicine. Curr Pediatr Rev. 2022;18:84-96.
- [CrossRef] [PubMed] [Google Scholar]
- Integrative medicine as adjunct therapy in the treatment of atopic dermatitis-the role of traditional Chinese medicine, dietary supplements, and other modalities. Clin Rev Allergy Immunol. 2013;44:242-53.
- [CrossRef] [PubMed] [Google Scholar]
- Topical corticosteroid phobia in atopic dermatitis: International feasibility study of the TOPICOP score. Allergy. 2017;72:1713-19.
- [CrossRef] [PubMed] [Google Scholar]
- Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-48.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis and psoriasis: Two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-303.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J Am Acad Dermatol. 2018;78:872-81.e6.
- [CrossRef] [PubMed] [Google Scholar]
- Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J Allergy Clin Immunol. 2019;143:142-54.
- [CrossRef] [PubMed] [Google Scholar]
- Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis. J Allergy Clin Immunol. 2021;147:394-7.
- [CrossRef] [PubMed] [Google Scholar]
- Basic mechanisms of JAK inhibition. Mediterr J Rheumatol. 2020;31:100-4.
- [CrossRef] [PubMed] [Google Scholar]






