Translate this page into:
Clinico-etiological profile of atopic dermatitis in Northeast India

*Corresponding author: Shikha Verma, Department of Dermatology and STD, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India. shikha.b.thakur@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Lamba R, Verma S, Thappa DM, Marak A, Paul D, Chhangte MZ. Clinico-etiological profile of atopic dermatitis in Northeast India. CosmoDerma. 2023;3:191. doi: 10.25259/CSDM_252_2023
Abstract
Objectives:
Atopic dermatitis (AD) is a chronic, inflammatory skin disorder with relapsing and remitting course affecting all age groups and has multifactorial pathogenesis. As multiple genetic and environmental factors contribute to the pathogenesis of AD, its prevalence and presentation vary among different ethnic groups residing in different geographical areas. This study was conducted to study the clinical profile and aggravating factors in cases of AD attending a tertiary hospital in Meghalaya, India, and to find a correlation of disease with serum immunoglobulin E (IgE) levels.
Material and Methods:
Patients suspected of AD were diagnosed on the basis of Hanifin and Rajka criteria and included in this study. All relevant demographic, anthropometric, clinical, and biochemical data were collected as per a preset pro forma. Serum IgE levels were tested for every patient using electrochemiluminescence immunoassay.
Results:
A total of 50 diagnosed cases of AD were included in our study with ages ranging from 5 months to 61 years, and the median age for the study population was 7.9 years. Male-to-female ratio was 1:1.17. Urban patients (66%) outnumbered rural patients and the majority of these patients (90%) belonged to the upper middle class (class II) according to the modified Kuppuswamy scale. The disease was aggravated in winters in 70%, in summer in 10%, due to wool in 48%, and due to food items in 22% of patients. Allergic rhinitis was found to be coexisting in 8% of patients, whereas asthma was found in 2% of patients. The predominant site of involvement was the face (91.9%) in children and the flexor surface (92.3%) among adults. The most common clinical presentation included pruritus (100%) and xerosis (98%). Serum IgE was raised in 58% of patients.
Conclusion:
Atopic dermatitis of chronic type with predominant facial involvement in children and predominant flexural involvement in adults was common in our study population. Seasonal changes, food items, and woolen clothes were common causative and exacerbating factors. Some atypical presentations included posterior thigh eczema, infra-auricular fissures, retroauricular fissures, eyelid eczema, genital dermatitis, juvenile plantar dermatoses, infranasal fissure, and follicular variant of the disease.
Keywords
Atopic dermatitis
Atopic eczema
Serum IgE
Northeast India
Hanifin and Rajka criteria
INTRODUCTION
Atopic dermatitis (AD) is an itchy, chronically relapsing inflammatory skin condition that often starts in early childhood presenting with erythema, itchy papules or vesicles, which may become excoriated and lichenified and typically has a flexural distribution.[1] Although it is considered a predominantly pediatric disease, it affects a significant number of adults. Its clinical presentation can vary based on age, ethnicity, and underlying pathological mechanism.[2-5] Various studies conducted in India in different age groups from 2002 to 2020 have reported prevalence ranging from 0.55% to 29.9%.[6] A two- to three-fold increase in disease prevalence has been observed in the last few decades, which has been attributed to changing environmental conditions and urbanization.[7]
Pathogenesis of AD is an extensively explored area, and multiple factors have been postulated such as dysfunctional skin barrier, genetic, immunological, environmental, and microbiological factors. Increased serum IgE is a common finding in AD with some studies suggesting a correlation between the levels of IgE and severity of the disease.[8] The tribal ethnicity and peculiar environmental conditions of the area including higher altitude, prolonged winters, and low temperature make the population of Meghalaya a unique study group. Moreover, there is no data available on AD in this population. With the above considerations, the aim of our study was to assess the unexplored clinical spectrum and exacerbating and precipitating factors associated with AD in Meghalaya, India.
MATERIAL AND METHODS
The present study was undertaken in the Department of Dermatology, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences (NEIGRIHMS), Shillong, India. Ethical approval was obtained from the Institution Ethics Committee (IEC), NEIGRIHMS on 14th May 2020 (NEIGR/IEC/M10/T6/2020).
All patients attending the Dermatology outpatient department were clinically screened for AD and diagnosed on the basis of Hanifin and Rajka criteria and included in the study.[1,8] Written consent was obtained from patients/guardians after explaining about the nature and purpose of the study. A detailed history was obtained from patients including present age, age at onset, residence, personal and family history of atopy, history of pruritus, seasonal variation, and history of allergy and associated diseases. A thorough clinical examination was done, and types of lesions were noted along with the distribution of eczema and predominant sites of involvement (face, flexor, extensor limbs). All findings were recorded in the pro forma. Serum IgE levels were tested for every patient using electrochemiluminescence immunoassay (ECLIA).
Descriptive statistics were used for data analysis. The distribution of frequencies in the demographic data of the patients in the study has been presented. The continuous variables are presented as mean ± standard deviation or median (range) if the data is skewed. Categorical variables are presented as absolute numbers and percentages.
RESULTS
A total number of 50 patients with AD were included in the study, and the prevalence was found to be 2.85%.
Age and sex distribution
The median of the age distribution was found to be 94.5 months (7.9 years) with minimum and maximum being 5 months and 732 months (61 years), respectively. Among them, 12 (24%) patients were 0–2 years old (five males and seven females), eight (16%) patients were 2–6 years old (five males and three females), 12 (24%) patients were 6–12 years old (five males and seven females), five (10%) patients were 12–18 years (three males and two females), and 13 (26%) patients were ≥18 years old (five males and eight females). The highest number of our patients were in the age group of ≥18 years.
Among 50 patients, 27 (54.0%) patients were female and 23 (46.0%) patients were male. The male-to-female ratio was found to be 1:1.17.
Age of onset
The median age of onset was found to be 21.5 months with minimum and maximum being 2 months and 728 months, respectively. The mean age of onset in infantile, children, and adult age groups were 11.4 months, 45.5 months, and 305 months, respectively.
Duration of disease
The median duration of disease in our study population was found to be 5.5 months with minimum and maximum being 1 month and 432 months, respectively. The mean duration of disease in infantile, children, and adult age groups were 3.1 months, 48 months, and 93.4 months, respectively.
Residence and socioeconomic background
In our study, 17 (34.0%) patients were residents of rural areas and 33 (66.0%) patients were hailing from urban areas. According to the modified Kuppuswamy scale, the majority of our patients, 45 (90%), belonged to the upper middle class (class II) while 2% of patients belonged to the lower middle class (class III), and 6% belonged to the upper lower class (class IV).
Associated and exacerbating features
Winter exacerbation was seen in 35 (70%) patients, summer exacerbation was seen in 5 (10%), and a family history of atopy was present in 35 (70%) patients. Allergic rhinitis was found to be coexisting in 4 (8%) patients whereas asthma was found in only 1 (2%) patient.
Clinical findings
Clinical findings including features in Hanifin and Rajka criteria and other atypical features are presented in Tables 1-4 and Figures 1-8.
| Major criteria | Present | Absent | Total |
|---|---|---|---|
| Pruritus | 50 | 0 | 50 |
| Typical morphology and distribution | 44 | 6 | 50 |
| Chronic or relapsing dermatitis | 42 | 8 | 50 |
| Personal or family history of atopy | 39 | 11 | 50 |
| Minor criteria | Present | Absent | Total |
|---|---|---|---|
| Xerosis | 49 | 1 | 50 |
| Ichthyosis, palmar hyperlinearity, or keratosis pilaris |
39 | 11 | 50 |
| Immediate (type 1) skin-test reactivity | - | - | - |
| Raised serum IgE | 29 | 21 | 50 |
| Early age of onset | 35 | 15 | 50 |
| Tendency toward cutaneous infections | 21 | 29 | 50 |
| Tendency toward non-specific hand or foot dermatitis |
18 | 32 | 50 |
| Nipple eczema | 1 | 49 | 50 |
| Cheilitis | 18 | 32 | 50 |
| Recurrent conjunctivitis | 9 | 41 | 50 |
| Dennie-Morgan infraorbital fold | 23 | 27 | 50 |
| Keratoconus | 1 | 49 | 50 |
| Anterior subcapsular cataracts | 1 | 49 | 50 |
| Orbital darkening | 10 | 40 | 50 |
| Facial pallor or facial erythema | 38 | 12 | 50 |
| Pityriasis alba | 22 | 28 | 50 |
| Anterior neck folds | 31 | 19 | 50 |
| Itch when sweating | 31 | 19 | 50 |
| Intolerance to wool and lipid solvents |
24 | 26 | 50 |
| Perifollicular accentuation | 6 | 44 | 50 |
| Food intolerance | 11 | 39 | 50 |
| Course influenced by environmental or emotional factors | 35 | 15 | 50 |
| White dermographism or delayed blanch |
15 | 35 | 50 |
IgE: Immunoglobulin E
| Atypical Features | Present | Absent | Total |
|---|---|---|---|
| Infra-auricular fissure | 14 | 36 | 50 |
| Retroauricular fissure | 11 | 39 | 50 |
| Infranasal fissure | 3 | 47 | 50 |
| Eyelid eczema | 10 | 40 | 50 |
| Genital dermatitis | 4 | 46 | 50 |
| Posterior thigh eczema | 18 | 32 | 50 |
| Juvenile plantar dermatoses | 4 | 46 | 50 |
| Follicular variant | 2 | 48 | 50 |
| Others | 10 | 40 | 50 |
| Sites of involvement | Total (n=50) | Pediatric (n=37) | Adult (n=13) | |||
|---|---|---|---|---|---|---|
| Present | Absent | Present | Absent | Present | Absent | |
| Flexures | 37 | 13 | 25 | 12 | 12 | 1 |
| Extensors | 34 | 16 | 26 | 11 | 8 | 5 |
| Face | 39 | 11 | 34 | 3 | 5 | 8 |

- A child with prominent facial eczema and pityriasis alba.

- Palmer hyperlinearity in child with atopic dermatitis.
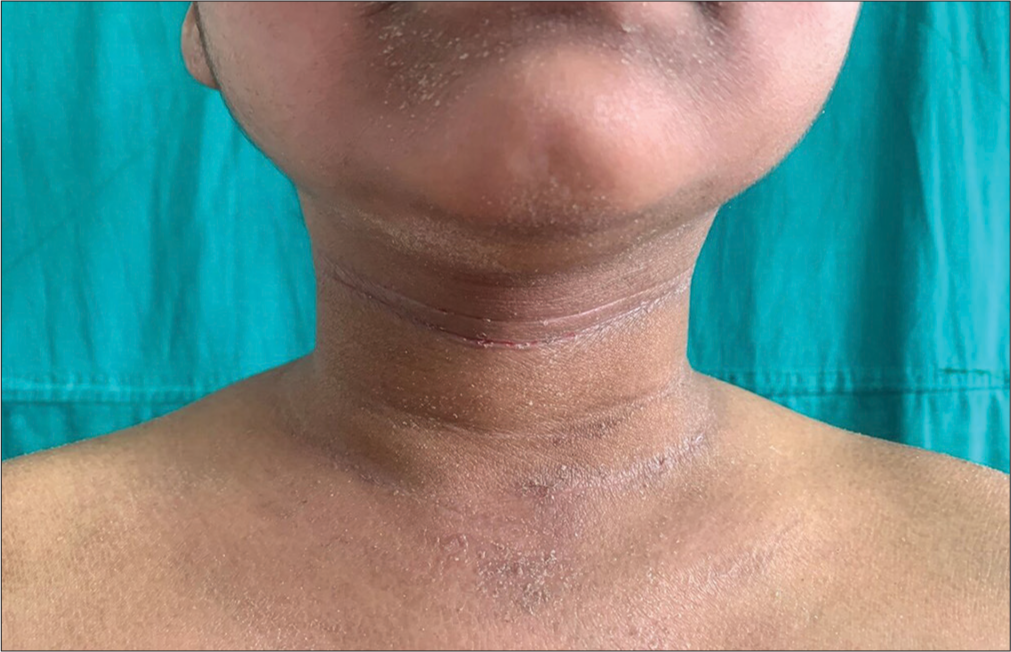
- Prominent anterior neck folds seen in a child with atopic dermatitis.

- Cheilitis and perioral dermatitis.
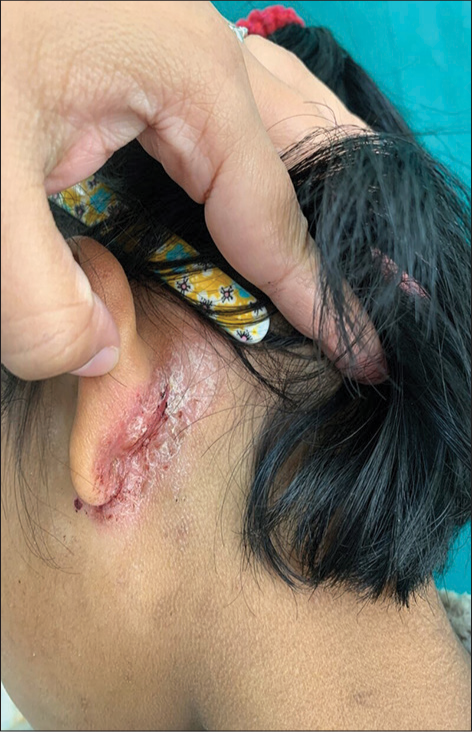
- Atopic child with posterior and infra-auricular fissures.
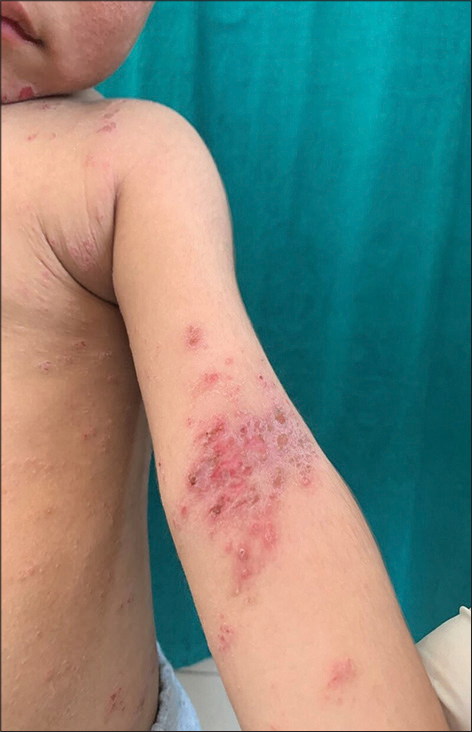
- Flexure involvement in atopic dermatitis.

- Extensor involvement in atopic dermatitis.

- Facial involvement in atopic dermatitis.
Serum IgE
Serum IgE was measured in all patients by ECLIA. It was found to be raised in 29 (58%) patients. In patients aged 0–2 years, 41.6% had raised IgE; in the age group of 2–6 years, 62.5% had raised IgE; in the age group 6–12 years, 41.6% had raised IgE; in the age group 12–18, 80% had raised IgE, and in age group ≥18 years, 76.9% had raised IgE.
DISCUSSION
Atopic dermatitis is a common, chronic inflammatory skin disease with a relapsing course that primarily affects young children and is often associated with raised serum IgE.[8] Some recent studies suggest a delayed onset of the disease, calling the same as “adult-onset AD.”[9,10]
The prevalence of AD in our study was found to be 2.85%, which was higher when compared to reports in previously conducted studies by Pandya and Agrawal,[7] Dhar et al.,[11] Swamy et al.,[12] and Sehgal et al.[13] who reported the prevalence of 1.9%, 0.55%, 0.58%, and 0.98%, respectively. This variation in findings can be possibly attributed to differences in geographical factors, environmental factors, and food habits. Meghalaya with a cooler climate experiences a prolonged winter and low humidity from October to March, especially in the Shillong plateau. Cold and dry weather conditions are known to increase the risk of flares in AD by decreasing skin barrier function and increasing susceptibility towards mechanical stress. Skin becomes more reactive towards irritants and allergens as pro-inflammatory cytokines, and cortisol are released by keratinocytes, and the number of dermal mast cells increases.[14]
The median age of onset in our study population was found to be 21.5 months (1.8 years) with minimum and maximum being 2 months and 728 months (61 years), respectively. Another study conducted in the Indian subcontinent reported a mean age-of-onset of 3.63± 1.42, with a minimum of one year to a maximum of six years.[13]
In infants, a mean age of onset of 11.4 months and a mean duration of disease of 3.1 months were found in our study. Dhar and Kanwar[15]and Sarkar and Kanwar[16] reported a mean age of onset of 4.2 months and 4.5 months, respectively, and a mean duration of disease of 3.3 months and 3 months, respectively, in infants.
In children, a mean age of onset of 3.75 years and a mean duration of disease of 4 years were found in our study, which is similar to previous studies.[15,16]
The male-to-female ratio found in our study was 1:1.17 suggesting slight female preponderance, though not statistically significant (P = 0.57), which was also seen in studies conducted by Upendra et al.[17] and Kumar et al.[18] who reported male-to-female ratio of 1:1.73 and 1:1.3, respectively. A male preponderance was reported by Dhar and Kanwar[15] with a ratio of 2.13:1, Sarkar and Kanwar[16] with a ratio of 2.25:1 and Pandya and Agrawal[7] with a ratio 2:1. This difference in findings signifies the difference in the sex ratio in parts of the country and better healthcare seeking behavior of women in Meghalaya.
In our study, 66% of patients were urban residents whereas 34% were rural residents, which were in concordance with the findings of a previous Indian study conducted by Sehgal et al.[13] (70% urban and 30% rural). Other Indian studies also reported urban predominance including Sarkar and Kanwar[16] (76.9% urban and 23.1% rural) and Swamy et al.[12] (76% urban and 24% rural). These findings are depicting that urbanization, modified lifestyle, changed food habits, and reduced exposure to antigens are playing a role in increasing disease prevalence.
The majority of our patients (90%) belonged to the upper middle class (socioeconomic class II) while 2% of patients belonged to the lower middle class (class III) and 6% to the upper lower class (class IV) according to modified Kuppuswamy scale. Swamy et al.[12] also reported middle socio economic class as most commonly affected (52%). This is possibly due to awareness and better healthcare seeking behavior in the upper middle class as compared to people from low socioeconomic strata.
We found that 70% of patients had winter exacerbation of symptoms while summer exacerbation was seen only in 10% of patients. Similar findings were noted by Dhar and Kanwar[15] where 67.14% of patients had aggravation during winters, and 23.36% had a summer exacerbation. Exacerbation in winter is attributed to dry climate and low humidity whereas increased sweating leading to itching and secondary infections cause exacerbation of symptoms in summer.
Family history of respiratory or cutaneous atopy was noted in 70% of patients, which was the same as reported by Hanifin and Rajka.[1] A family history of atopy was reported by Dhar et al.[11] in 65%, Kumar et al.[18] in 68.9%, and Singh and Yadav[19] in 32.9% of the patients.
Allergic rhinitis was found to be coexisting with AD in 8% of patients whereas a prior diagnosis of asthma was found in only 2% of patient in our study. Ellis et al.[20] have reported parallel findings with allergic rhinitis in 7% of patients with AD and asthma in 5% of patients with AD. Rystedt[21] reported that 32% of patients of AD with bronchial asthma and 60% with allergic rhinitis.
Pruritus was the most consistent symptom and was present in all patients in our study, which was consistent with the findings of studies by Swamy et al.[12] and Nadeem et al.,[22] where they noted pruritus in all patients. Pruritus has been reported as the most common symptoms in other studies conducted in India and internationally.[13,16,23,24]
Among our study population, 74% of patients had flexures involved [Figure 6], 68% of patients had extensors involved [Figure 7], and facial involvement [Figure 8] was seen in 78% of patients. Among adult patients (≥18 years) in our study, 92.3% patients had flexures involved, 61.5% patients had extensors involved, and 38.4% had facial involvement whereas among pediatric patients, 67.6% had flexures involved, 70.3% had extensors involved, and 91.9% had facial involvement. In a study conducted by Dhar and Kanwar,[15] 42% of patients with flexural involvement, 52.3% with extensor involvement, and 79% with facial involvement were reported in the infantile group, and in the childhood group, corresponding figures were 35.5%, 56.32%, and 74.5%, respectively. Sarkar and Kanwar[16] have noted flexures, extensors, and face involvement in 15.4%, 26.9%, and 80.8% of infants and 45.4%, 37.4%, and 66.7% of children. In another Indian study, 39%, 38%, and 20% of patients had flexor, extensor, and face involvement, respectively.[11]
Among the minor criteria of Hanifin and Rajka, the most common feature present in our study population was xerosis, which was noted in 98% of patients that was similar to the findings in studies conducted by Nadeem et al.[22]and Swamy et al.,[12] who reported xerosis in 100% and 99% of patients, respectively. Xerosis was also the most common feature reported by Barbarot et al.[24]
A second most common feature among minor criteria noted in our study was presence of ichthyosis, palmar hyperlinearity [Figure 2] or keratosis pilaris, which were seen in 78% patients in contrast to 51% patients in study conducted by Swamy et al.,[12] 50% patients in study conducted by Sehgal et al.,[13] and 34.9% patients in study conducted by Kumar et al.[18] Facial pallor or facial erythema was noted in 76% of patients in our study whereas it was reported in only 6% of patients by Swamy et al.[12]
History of disease activity being influenced by environmental and emotional factors was present in 70% of patients in our study, which was in conformity to those reported by Sehgal et al.,[13] whereas Swamy et al.[12] reported a history of emotional and environmental influence in only 49% of patients. Anterior neck folds [Figure 3] and itching with sweating were seen in 62% of patients while it was seen in only 2% of patients in a study conducted by Swamy et al.[12]
We found the history of intolerance to wool and lipid solvents in 48% of patients whereas Sehgal et al.[13] has reported this feature in 68%, and Swamy et al.[12] has reported the same feature in only 9% of patients.
Pityriasis alba [Figure 1] was seen in 44% of patients in our study population in contrast to previous studies where a 27% frequency of the same feature was reported by Swamy et al.[12], and a frequency of 20% was reported by Sehgal et al.[13]
In contrast to the study conducted by Sehgal et al.,[13] where they found Dennie-Morgan infraorbital fold, hand and foot dermatitis, and white dermographism to be occurring very commonly; these were found in 46%, 36%, and 30% of patients, respectively, in our study.
In our study, an increased tendency towards cutaneous infections was noted in 42% of patients whereas Sehgal et al.[13] has reported it in 62% of patients, and Swamy et al.[12] has reported the same in 68% of patients in their study.
Cheilitis [Figure 4] was noted in 36% and orbital darkening in 20% of patients, in the current study, which was higher in comparison to findings of Swamy et al.[12], who found cheilitis in 8% of patients and orbital findings in only 2% of patients whereas the frequency of both was found to be 58% of patients in a study conducted by Sehgal et al.[13]
We found the history of food intolerance in 22% of patients in our study population. The most common items reported by patients included brinjal followed by egg and dry fish. Sehgal et al.,[13] Swamy et al.,[12] Sendrasoa et al.,[25] and Upendra et al.[17] have reported a history of food intolerance in 70%, 8%, 16.5%, and 2.2% patients, respectively.
Recurrent conjunctivitis was present in 18% of patients in our study, which was consistent with the findings of the previous studies.[12,13]
Perifollicular accentuation was seen in 12% of patients in our study, which was higher than as seen by Swamy et al. (2%).[12] Nipple eczema was seen in only 2% of patients in our study, which was the same as observed by Swamy et al.,[12] but lower than as observed by Sehgal et al. (20%).[13] Anterior subcapsular cataract and keratoconus were seen in 2% of patients each in our study while Swamy et al.[12] noted anterior subcapsular cataract in 2% of patients and did not find any patient with keratoconus.
Serum IgE was found to be raised in 58% of patients in our study, which was in consonance with the findings of the meta-analysis done by Yew et al.[23] Akdis et al.[26] have reported an elevated serum IgE levels in about 80% of AD patients. Somani[27] conducted a study on allergen specific IgE antibody in patients of AD and found IgE to be raised in 88% of patients.
Other than features mentioned in Hanifin and Rajka criteria for diagnosis of AD, we came across some other common features in patients as well including posterior thigh eczema, infra- and retroauricular fissures [Figure 5], eyelid eczema, genital dermatitis, juvenile plantar dermatoses, and infranasal fissures.
Limitations of study
The number of patients attending OPD during the COVID-19 pandemic reduced drastically. As a result, the sample size was smaller than the expected one
Lack of appropriate criteria for diagnosing adult-onset AD led to underdiagnozing the adult-onset AD resulting in lesser sample size.
CONCLUSION
Atopic dermatitis of chronic type with slight female preponderance having predominant facial involvement in children and predominant flexural involvement in adults was common in our study. Pruritus and xerosis were the most frequent presenting features. Seasonal changes, urbanization, food items (egg, fish, and milk), and woolen cloths were principal causative and exacerbating factors; and allergic rhinitis, asthma, and family history of atopy were associated features found. Some atypical presentations included posterior thigh eczema, infra-auricular fissures, retroauricular fissures, eyelid eczema, genital dermatitis, juvenile plantar dermatoses, infranasal fissure, and follicular variant of the disease. The majority of patients were found to have raised serum IgE during exacerbation of the disease. These findings help us in better understanding of disease dynamics and preventive aspects of the disease; however, confirmation of any such correlation needs further study.
Ethical approval
The research/study is approved by the North Eastern Indira Gandhi Regional Institute of Health & Medical Sciences, Shillong, number NEIGR/IEC/M10/T6/2020, dated 14th May 2020 .
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of Interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Atopic eczema In: Rook's textbook of dermatology (9th ed). Ch. 41. Chichester, West Sussex: John Wiley and Sons Inc; 2016. p. :1-34.
- [CrossRef] [Google Scholar]
- Phenotypes of atopic dermatitis depending on the timing of onset and progression in childhood. JAMA Pediatr. 2017;171:655-62.
- [CrossRef] [PubMed] [Google Scholar]
- 164 Changes in the geographic distribution of atopic dermatitis on the body with age. J Investig Dermatol. 2017;137(5 Suppl 1):S28.
- [CrossRef] [Google Scholar]
- Phenotypical differences of childhood-and adult-onset atopic dermatitis. J Allergy Clin Immunol Pract. 2018;6:1306-12.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical practice. Atopic dermatitis. N Engl J Med. 2005;352:2314-24.
- [CrossRef] [PubMed] [Google Scholar]
- Burden of disease, unmet needs in the diagnosis and management of atopic dermatitis: An Indian expert consensus. Clin Cosmet Investig Dermatol. 2021;14:1755-65.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of clinical profile of children aged 9-16 years with atopic dermatitis. Indian J Child Health. 2020;7:223-6.
- [CrossRef] [Google Scholar]
- Role of IgE in atopic dermatitis. Curr Opin Immunol. 1993;5:956-62.
- [CrossRef] [PubMed] [Google Scholar]
- Is there something called adult onset atopic dermatitis in India? Indian J Dermatol Venereol Leprol. 2013;79:145.
- [CrossRef] [PubMed] [Google Scholar]
- Adult-onset atopic dermatitis. Australas J Dermatol. 2000;41:225-8.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and clinical pattern of atopic dermatitis in 100 children seen in a city hospital. Indian J Dermatol. 2002;47:202-4.
- [Google Scholar]
- Epidemiological profile and clinical pattern of atopic dermatitis in South Indian teaching hospital. Indian J Clin Exp Dermatol. 2019;5:146-53.
- [CrossRef] [Google Scholar]
- Atopic dermatitis: A cross-sectional (descriptive) study of 100 cases. Indian J Dermatol. 2015;60:519.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of environmental humidity and temperature on skin barrier function and dermatitis. J Eur Acad Dermatol Venereol. 2016;30:223-49.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and clinical pattern of atopic dermatitis in a North Indian pediatric population. Pediatr Dermatol. 1998;15:347-51.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-epidemiological profile and factors affecting severity of atopic dermatitis in north Indian children. Indian J Dermatol. 2004;49:117.
- [Google Scholar]
- The clinicoepidemiological profile of atopic dermatitis in residential schoolchildren: A study from South Chhattisgarh, India. Indian J Paediatr Dermatol. 2017;18:281.
- [CrossRef] [Google Scholar]
- Clinico-immunological profile and their correlation with severity of atopic dermatitis in Eastern Indian children. J Nat Sci Biol Med. 2014;5:95-100.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Atopic dermatitis among children in Jaipur. Sch J App Med Sci. 2017;5:1875-8.
- [Google Scholar]
- Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361-70.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic factors in atopic dermatitis. Acta Derm Venereol. 1985;65:206-13.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis in children: A clinico epidemiological study and the role of dietary restrictions in disease severity. Int J Res Dermatol. 2017;3:168-74.
- [CrossRef] [Google Scholar]
- A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80:390-401.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy. 2018;73:1284-93.
- [CrossRef] [PubMed] [Google Scholar]
- Birth month and prevalence of atopic dermatitis in children under 3 years in Antananarivo, Madagascar. J Asthma Allergy. 2020;13:265-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL consensus report. Allergy. 2006;61:969-87.
- [CrossRef] [PubMed] [Google Scholar]
- A study of allergen-specific IgE antibodies in Indian patients of atopic dermatitis. Indian J Dermatol Venereol Leprol. 2008;74:100-4.
- [CrossRef] [PubMed] [Google Scholar]






