Translate this page into:
Understanding skin aging: Exploring anti-aging modalities

-
Received: ,
Accepted: ,
How to cite this article: Srivastava G, Srivastava G. Understanding skin aging: Exploring anti-aging modalities. CosmoDerma 2023;3:109.
Abstract
With ever-improving life expectancy, skin aging has become the first casualty of exuberant health. People are progressively seeking remedies for their age-related skin problems. Understanding the aging process is unfolding newer realms in the molecular biology of the skin, giving us new insights to combat senescence more effectively. Epidermal dysfunction, compromised permeability homeostasis, elevated skin pH, diminished stratum corneum hydration, and dermal extracellular matrix aberrations with changes in its cellular composition are now well understood. Thus, a more scientific approach can be utilized while evolving various specific anti-aging therapies. The present communication attempts to address both the process of skin aging and various therapies to combat it in a concise yet succinct way.
Keywords
Skin aging
Cosmetic dermatology
Aesthetic dermatology
Anti-oxidants
Anti-aging
INTRODUCTION
Skin is the first defense against the external environment from physical and chemical stimuli and infective organisms. It has an immaculate power to shed and regenerate in an orderly time-bound fashion. Genetic factors, sun exposure, occupational activities, and time – all contribute to the aging process of the skin. Although aging changes also occur in other organs/systems of the body, the skin shows visible signs of aging to one and all. As the expectancy of life has increased throughout the world, we are encountering an increasing number of aging populations desperately seeking advice to delay or reverse the signs of aging. The present communication attempts to address these important aspects of both causes and remedies of this natural process.
THEORIES OF AGING
There are two basic theories of aging that have been put forward to explain the complexity beneath – Programmatic theory and Stochastic theory.
The programmatic theory is comprised of telomere shortening and cellular senescence. As the age advances, the human telomere shortens over 30%. Telomeres of patients with premature aging syndromes such as progeria are significantly shorter than their age-matched controls. Cellular senescence denotes a limited capacity of cells to undergo cell division leading to altered differentiation, irreversible growth arrests, and resistance to apoptosis.[1,2] The stochastic theory postulates that a four-pronged process operates in the aging skin [Table 1].
| Biological process |
Features |
|---|---|
| Oxidative stress | Throughout life, cells accumulate a molecular oxidative damage due to decreased efficiency of anti-oxidant defense system. |
| DNA damage | A decreased DNA repair capacity over years can accelerate the aging of the cells. |
| Amino acid racemization | Substitution of the D-amino acids for the L-amino acids within the protein keeps occurring as time passes. This affects the protein function, reducing the deamidation of asparagine and glutamine, thereby reducing the rate of protein degradation. |
| Non-enzymatic glycosylation of proteins | This occurs when sugar aldehydes condense with the protein amino groups, resulting in altered degradation, loss of function, and brown discoloration of cells. |
EXTRINSIC SKIN CHANGES
As age advances, the tell-tale signs of aging start to display on the body gradually, which may vary from individual-to-individual, geographic areas, race, and occupation involving excessive sun exposure, etc. Face, hands, and feet are the first to show chronologic aging and photoaging.[3] Atrophy, wrinkling, laxity, sagging, pigmented blemishes, and dryness can be seen on the skin along with greying of the hairs. With time, they become more pronounced and disturbing; pigmentary changes also develop in sun-exposed areas. The liver spots, senile lentigo, and seborrheic keratoses keep developing and increase in number with time. Hair becomes grey, density may reduce, and the anagen phase of hair follicles progressively shortens. Paradoxically, eyebrows can become bushy in the elderly. In aging females, hirsutism may be a cause of concern – grey terminal hair on the chin even resists the hair removal lasers. Previously full face gives a look of weakening, wrinkling, and reduced facial musculature. The neck shows non-elastic wrinkled sagging skin. The nails may become lusterless and show thickening and longitudinal ridging [Figure 1]. The dry skin of the elderly is a continuous source of irritation and pruritus. Senile people, thus, become prone to xerotic skin conditions like asteatotic dermatitis. Pressure sores can develop over the skin of the pelvis due to loss of subcutaneous fat.[4]

- Extrinsic skin changes seen in the mother (left) compared to her daughter (right).
INTRINSIC SKIN CHANGES
Epidermis
Stratum corneum has a brick-and-mortar model of compact cellular material. This efficiency maintains selective permeability and controls the transepidermal water loss.[5] Aging progressively impairs this optimum function of stratum corneum. The keratinocyte proliferation declines while the apoptosis increases with age leading to a reduction in the thickness of the epidermis.[6,7] Further, the structural proteins – filaggrin, loricrin, and cornified – envelope proteins markedly decline with aging. Side-by-side the intercellular cementing substance also shows lower production, contributing to the abnormal epidermal permeability.[8] Several other age-related complex enzymatic and biochemical changes including a low level of hyaluronic acid, further, contribute to altered epidermal function.[5-10] Other aging changes include elevation of skin surface pH beginning at 55 years of age, caused by a declining sebum content resulting in reduced triglycerides in the stratum corneum.[5] Other factors contributing to a higher pH include reduced levels of sodium hydrogen exchanger 1, secretory phospholipase 2, and filaggrin.[5,11-14] Finally, stratum corneum hydration gets significantly reduced with age [Table 2]. The underlying mechanisms include reduced contents of natural humectants and their metabolites, sebum, and glycerol. Aquaporin 3 levels decrease in the aging skin as well, further contributing to reduced epidermal hydration.[5,15,16]
| Changes | Effect of epidermis | Clinical effect on skin |
|---|---|---|
| Decreased sebum, NHE1, Filaggrin, and sPLA2 | Increased skin pH | Cutaneous inflammation and pruritus |
| Decreased sebum, Filaggrin, AQP3, and stratum corneum lipids | Decreased stratum corneum hydration | Pruritus and xerosis |
| Decreased stratum corneum lipids, NHE1, sPLA2, and differentiated related protein | Decreased/Altered permeability barrier | Cutaneous inflammation and increased allergic reactions |
NHE1: Sodium hydrogen exchanger 1, sPLA2: Secretory phospholipase 2, AQP3: Aquaporin 3
Dermis
In contrast to the dense cellular texture of the epidermis, the dermis is primarily composed of extracellular matrix (ECM). The components of the dermis include collagen fibers, elastic fibers, fibroblasts, immune cells, and skin appendages. With aging, there is a gradual reduction in both the quality and quantity of collagen and elastic fibers, clinically reflected in skin wrinkles and loss of elasticity [Table 3].[17-26]
| Components | Features |
|---|---|
| Collagen[5,17-22] | • Collagen deficiency due to quantitative and structural changes. • Generation of ROS causing increased MMP expression and inhibition of TGF-β. This leads to decreased collagen biosynthesis and increased fragmentation. • Fibroblasts which normally adhere to ECM in young skin gets separated and reduced in size. • Aged fibroblasts produce more ROS which in turn aggravates the effects of increased MMP and inhibition of TGF-β. |
| ECM components[10,23-26] | • Elastic fibers, responsible for skin compliance and resilience (elasticity) get structurally changed and decreased in number. • In extrinsic aging and photoaging, there is paradoxical increase in abnormal and non-functional elastic fibers accumulated in dermis. • GAGs, a major component of ECM, are both sulfated (five types) and non-sulfated (One type – hyaluronic acid). They maintain the water content in tissues. Aging reduces their function and abnormal accumulation. • Proteoglycans, a family of conjugated proteins which impart mechanical strength to the skin, also become abnormal and less functional. |
| Appendages[5,17] | • Hair follicle changes vary greatly according to the site and gender. Hair cycles get shortened with greying of the hair, starting with scalp. The eyebrows of elderly become bushy and there is hirsutism in the elderly women with reduction of scalp hair. • Sebaceous and apocrine glands reduce in size, quantity, and function. |
ROS: Reactive oxygen species, MMP: Matrix metalloprotein, TGF-β: Transforming growth factor-β, ECM: Extracellular matrix, GAGs: Glycosaminoglycans
ANTI-AGING MODALITIES
The scientific, in depth knowledge of the sophisticated aging and photoaging processes has led us to rational research, both for preventing and delaying the process of aging. Several approaches have been advocated to reverse the aging process to some extent. Everyday, new pharmacological, noninvasive, and laser/surgical intervention is being added to the ever-expanding basket of anti-aging therapy. It is worthwhile to briefly recount them [Figure 2].
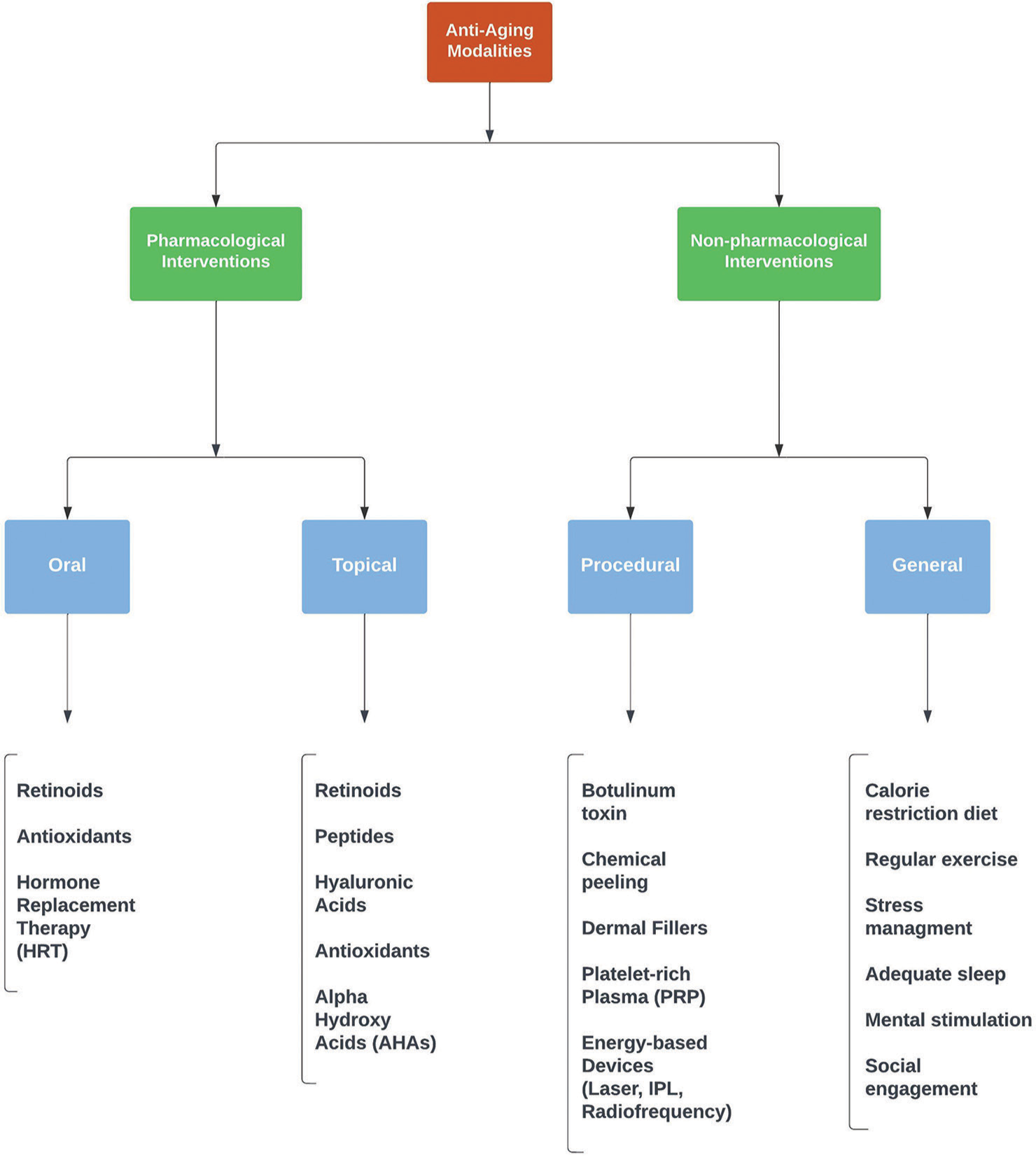
- Broad classification of anti-aging modalities.
PHARMACOLOGICAL AGENTS
Both oral and topical pharmacologic agents have been advocated for preventing, tackling, and delaying natural photoaging. It is believed that the prevention, especially that of ultraviolet radiation exposure from early childhood can go a long way in reducing extrinsic skin aging. Wearing protective clothing and using physical and chemical sunscreens, along with avoidance of sunlight, form a substantial denominator of any anti-aging treatment.
Oral agents
Antioxidants – Carotenoids, Vitamin C, Vitamin E, selenium, proanthocyanidins, and astaxanthins – have all been used orally for variable period of time for their anti-aging effects [Table 4].[27-30]
| Endogenous defense (innate) | Exogenous defense (dietary) | |
|---|---|---|
| Enzymatic • Superoxide dismutase • Glutathione peroxidase • Catalase |
Non-enzymatic • Albumin • Bilirubin • Thiol • Glutathione • Uric acid • Nutritional factors |
• Ascorbic acid (Vitamin C) • Tocopherol (Vitamin E) • Carotenoids • Copper • Selenium |
Topical agents
A wide variety of topical agents, both chemical and herbal have flooded the market in the past two decades. Scientific data are available on a variety of topical antioxidants (vitamins, flavonoids, and polyphenols). They all reduce the burden of free radicals in the tissues through regular applications. Another researched entity includes the cell regulators (retinol, peptides, and growth factors) which show a positive effect on collagen metabolism as well as production[27] [Table 5]. A combination of antioxidant and cell regulators in the same cream has been known to multiply the anti-aging potential of these agents.[31-50]
| Agents | Preparation | Regimen | Efficacy |
|---|---|---|---|
| Retinol[33,34] | 0.04–1% | 1–3 times/week, up to 6 weeks |
Improvement in epidermal thickening, wrinkling with histopathological confirmation. |
| Retinoic acid[35] | 0.01–0.1% | 1–2 times/week, up to 3 months |
Clinical improvement in wrinkling, pigmentation, sallowness, and texture. |
| Retinaldehyde[36] | 0.05–0.1% | 1–2 times/week, 1–3 months |
Better tolerated, significant improvement in profilometric score. |
| Glycolic acid[37] | 25–50% | Once a week, up to 4 weeks | Reduction in fine wrinkles and solar elastosis. Improves skin texture. |
| Lactic acid[38] | 5–12% | Twice daily, up to 3 months | Increased firmness of epidermis and dermis. Clinical improvement in skin texture, fine lines, and wrinkles. |
| Mandelic acid[39] | 4–6% | Twice a day for 4 weeks | Significant increase in skin firmness and elasticity; reduced fine lines. |
| α-lipoic acid[40] | 5% | Twice a day for 12 weeks | Mean epidermal and dermal thickness improvement; significant reduction in photoaging. |
| Hyaluronic acid[41] | 0.1% | Twice daily for 2 months | Significant improvement in skin hydration, elasticity and reduction of wrinkle depth. |
| Niacinamide[42] | 4–5% | Once daily for 8-12 weeks | Significant improvement in skin texture and wrinkling. |
| L-ascorbic acid[43] | 5% | Once daily for 6 months | Decrease in deep furrows with microscopic evidence of neocollagenesis. |
| Vitamin E[44] | 1% with ferulic acid and vitamin C | Daily for 4 days, followed by UV irradiation | Increased photoprotection to solar simulated UV radiation. |
| CoQ10[45] | 1% | Twice daily for 3 months | Reduction in wrinkle score. |
| Phenols (Green tea, Berry)[46] |
10% cream+300 mg oral | Twice daily for 8 weeks | Significant improvement in elastin content of the skin. |
| Ferulic acid[44,47] | 0.5% with vitamin C | Daily for 4 days, followed by UV irradiation | Increased photoprotection to solar simulated UV radiation. |
| Signal peptide[31,48] | 5% niacinamide, pal-KTTS cream | Daily for 8 weeks | Significant improvement in wrinkles in comparison to retinoic acid. |
| GEGK cream | Daily for 8 weeks | Increased formation of procollagen, hyaluronic acid, and fibronectin. | |
| Carrier peptide[49] | GHK-Cu cream | Daily for 8 weeks | Significant improvement in wrinkles, reduction in fine lines, and increase in skin density. |
| Neurotransmitter inhibitor peptides[50] | Acetyl hexapeptide-3 with tripeptide-10 cream | Daily for 60 days | Significant reduction in wrinkles. |
CoQ10: Coenzyme Q10, KTTS: Lysine-threonine-threonine-lysine-serine, GEGK: Gly-Glu-Lys-Gly, GHK: Glycil-L-histidyl-L-lysine, UV: Ultraviolet
CHEMICAL PEELS
Another substantial therapy widely used for age diminishing is different varieties of chemical peels. They have shown promising results when used either alone or with combination of different agents [Table 6].[51,52]
| Type | Agents | Effect |
|---|---|---|
| Superficial peel | α-β-, lipohydroxy acid, trichloroacetic acid (10–30%) | Exfoliation of epidermal layer without going to basal layer. |
| Medium depth peel | Trichloroacetic acid (30–50%) | Exfoliation up to upper reticular dermis. |
| Deep peels | Trichloroacetic acid (>50%), phenol | Penetrate lower reticular dermis |
ENERGY-BASED DEVICES
A selective heat-induced denaturalization of dermal collagen which results in subsequent reactive synthesis is the principle behind various types of light devices. Laser, intense pulsed light, and radiofrequency can be effectively employed for rejuvenation, resurfacing, and tightening of the skin. Laser resurfacing has been shown to be very encouraging in reversing the photoaging by causing extensive dermal remodeling, regeneration of cellular organelles, and stimulation of neocollagenesis.[53-55]
DERMAL FILLERS AND INJECTABLE SKIN REJUVENATORS
Dermal fillers can be injected within or beneath the skin to improve the blemishes and to cause soft tissue augmentation. They very useful in treating permanent wrinkles like crow’s feet and prominent nasal labial furrows[27,56] [Table 7].
| Types | Agents |
|---|---|
| Autologous | • Fat • Cultured human fibroblasts |
| Collagen | • Bovine-derived • Human-derived (from tissue culture) |
| Hyaluronic acid | • Non-animal stabilized • Viscoelastic (from bacterial fermentation) |
| Synthetic/ Pseudosynthetic |
• Silicon • Polymethacrylate microsphere suspended in aqueous polysaccharide gel • Alkyl-imide gel polymer |
BOTULINUM TOXIN AND PLATELET-RICH PLASMA (PRP)
Although it has an insignificant effect on skin aging or skin texture on a molecular level, the botulinum toxin is often prescribed for slowing down the visible aging process by acting on dynamic wrinkles.[57] It is used in various dilutions at various sites and repeated after variable intervals of several months. PRP derived from fresh whole blood of the subject has a very high concentration of platelets which harbor various growth factors including platelet-derived growth factors, transforming growth factors, vascular endothelial growth factors, and insulin-like growth factors. All these growth factors are known to regulate processes including cell migration, attachment, proliferation, and differentiation; promoting ECM accumulation by binding to specific cell surface receptors. This in turn induces the synthesis of collagen and other matrix components by stimulating the activation of fibroblasts, resulting in skin rejuvenation.[27,58]
ANTI-AGING DIET
Calorie restriction has remained a longstanding advice that delays age-associated diseases and extends the lifespan of an individual. Time and again, claims of intermittent fasting, ketogenic diet, protein restriction, time-restricted feeding, diet restriction of certain amino acids, and fast-mimicking diets have been advocated in natural anti-aging regimens. Although the effects seem to be highly variable and inconsistent, some anti-oxidant properties of such diets causing the elimination of reactive oxygen species may be beneficial to some extent.[59]
HORMONE REPLACEMENT THERAPY (HRT)
The progressive decrease of hormone synthesis with age makes it logical to use HRT in select individuals – both males and females, with overall benefit in the quality of life and halting signs of aging.[27,60] Melatonin is one such example, other being estrogen and progesterone. Obese men with Type-2 diabetes mellitus have a low testosterone level and can be advised a controlled testosterone therapy, improving their stamina as well as skin tone.[61]
CONCLUSION
With increasing longevity, improved comforts, better standard of living, and abundant social interactions, the humankind is getting more concerned about their physical appearance and youthful personality. The first blemish on the face or a fine line on the forehead can be a significant cause for concern for any self-conscious socialite person. Understanding the aging mechanism has translated into a plethora of modalities available for preventing and delaying aging. Social media has also increased awareness about the use of beautifying creams promising a glowing, youthful skin. Thus, an ever-increasing number of people approach skin specialists for various procedures and cosmeceuticals to prevent their skin from signs of senescence. Sunscreen common to all age-defying agents can also be used prophylactically to prevent photoaging, the spectrum of which has now included visible light, infrared rays, and anti-pollutants. The future is getting more and more promising in the field of senescence and skin rejuvenation. An accurate understanding of the aging process up to genomic levels has made it possible to identify novel targets for better prevention, maintenance, and reduction of chronological aging and photoaging.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Demographics of aging and skin disease. Clin Geriatr Med. 2001;17:631-41, v
- [CrossRef] [PubMed] [Google Scholar]
- Focus on the contribution of oxidative stress in skin aging. Antioxidants (Basel). 2022;11:1121.
- [CrossRef] [PubMed] [Google Scholar]
- Skin ageing In: Rook's Textbook of Dermatology (9th ed). West Sussex: Wiley Blackwell; 2010. p. 155, 1-9
- [Google Scholar]
- Aging-associated alterations in epidermal function and their clinical significance. Aging (Albany NY). 2020;12:5551-65.
- [CrossRef] [PubMed] [Google Scholar]
- Age-dependent variation in cytokines, chemokines, and biologic analytes rinsed from the surface of healthy human skin. Sci Rep. 2015;5:10472.
- [CrossRef] [PubMed] [Google Scholar]
- Aging of human epidermis: Reversal of aging changes correlates with reversal of keratinocyte fas expression and apoptosis. J Gerontol A Biol Sci Med Sci. 2004;59:411-5.
- [CrossRef] [PubMed] [Google Scholar]
- Age-related changes in the composition of the cornified envelope in human skin. Exp Dermatol. 2013;22:329-35.
- [CrossRef] [PubMed] [Google Scholar]
- Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta. 2014;1841:280-94.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo. Exp Dermatol. 2011;20:454-6.
- [CrossRef] [PubMed] [Google Scholar]
- Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847-56.
- [CrossRef] [PubMed] [Google Scholar]
- Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001;117:44-51.
- [CrossRef] [PubMed] [Google Scholar]
- NHE1 regulates the stratum corneum permeability barrier homeostasis, Microenvironment acidification assessed with fluorescence lifetime imaging. J Biol Chem. 2002;277:47399-406.
- [CrossRef] [PubMed] [Google Scholar]
- Filaggrin deficiency leads to impaired lipid profile and altered acidification pathways in a 3D skin construct. J Invest Dermatol. 2014;134:746-53.
- [CrossRef] [PubMed] [Google Scholar]
- Stratum corneum lipids: The effect of ageing and the seasons. Arch Dermatol Res. 1996;288:765-70.
- [CrossRef] [PubMed] [Google Scholar]
- Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc Natl Acad Sci U S A. 2003;100:7360-5.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019;20:2126.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J Invest Dermatol. 2010;130:415-24.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J Invest Dermatol. 2013;133:1362-6.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress and aging: Beyond correlation. Aging Cell. 2002;1:117-23.
- [CrossRef] [PubMed] [Google Scholar]
- Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Investig Dermatol. 2013;133:658-67.
- [CrossRef] [PubMed] [Google Scholar]
- Age-associated reduction of cell spreading induces mitochondrial DNA common deletion by oxidative stress in human skin dermal fibroblasts: Implication for human skin connective tissue aging. J Biomed Sci. 2015;22:62.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization and mechanisms of photoageing-related changes in skin. Damages of basement membrane and dermal structures. Exp Dermatol. 2016;25(Suppl 3):14-9.
- [CrossRef] [PubMed] [Google Scholar]
- Elastin structure and its involvement in skin photoageing. Int J Cosmet Sci. 2017;39:241-7.
- [CrossRef] [PubMed] [Google Scholar]
- Glycosaminoglycan and proteoglycan in skin aging. J Dermatol Sci. 2016;83:174-81.
- [CrossRef] [PubMed] [Google Scholar]
- Intrinsic aging-and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J Dermatol Sci. 2011;62:192-201.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of antioxidant supplementation on the aging process. Clin Interv Aging. 2007;2:377-87.
- [Google Scholar]
- Active oxygen species generation and cellular damage by additives of parenteral preparations: Selenium and sulfhydryl compounds. Nutrition. 1999;15:651-5.
- [CrossRef] [PubMed] [Google Scholar]
- Ferulic acid stabilizes a solution of Vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol. 2005;125:826-32.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, controlled comparative study of the wrinkle reduction benefits of a cosmetic niacinamide/peptide/ retinyl propionate product regimen vs. a prescription 0.02% tretinoin product regimen. Br J Dermatol. 2010;162:647-54.
- [CrossRef] [PubMed] [Google Scholar]
- Topical over-the-counter antiaging agents: An update and systematic review. Dermatology. 2021;237:217-29.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480-6.
- [CrossRef] [PubMed] [Google Scholar]
- Improvement of naturally aged skin with Vitamin A (retinol) Arch Dermatol. 2007;143:606-12.
- [CrossRef] [PubMed] [Google Scholar]
- Isotretinoin improves the appearance of photodamaged skin: Results of a 36-week, multicenter, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2000;42:56-63.
- [CrossRef] [PubMed] [Google Scholar]
- Profilometric evaluation of photodamage after topical retinaldehyde and retinoic acid treatment. J Am Acad Dermatol. 1998;39:960-5.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical improvement of photoaged skin with 50% glycolic acid. A double-blind vehicle-controlled study. Dermatol Surg. 1996;22:455-60.
- [CrossRef] [PubMed] [Google Scholar]
- Epidermal and dermal effects of topical lactic acid. J Am Acad Dermatol. 1996;35:388-91.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of topical mandelic acid treatment on facial skin viscoelasticity. Facial Plast Surg. 2018;34:651-6.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized, placebo-controlled, double blind study on the clinical efficacy of a cream containing 5% alpha-lipoic acid related to photoageing of facial skin. Br J Dermatol. 2003;149:841-9.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-wrinkle creams with hyaluronic acid: How effective are they? MMW Fortschr Med. 2016;158(S4 Suppl 4):1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of anti-wrinkle effects of a novel cosmetic containing niacinamide. J Dermatol. 2008;35:637-42.
- [CrossRef] [PubMed] [Google Scholar]
- Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. placebo. Exp Dermatol. 2003;12:237-44.
- [CrossRef] [PubMed] [Google Scholar]
- A topical antioxidant solution containing Vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J Am Acad Dermatol. 2008;59:418-25.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of inhibitory effects of CoQ10 on UVB-induced wrinkle formation in vitro and in vivo. Biofactors. 2008;32:237-43.
- [CrossRef] [PubMed] [Google Scholar]
- Double-blinded, placebo-controlled trial of green tea extracts in the clinical and histologic appearance of photoaging skin. Dermatol Surg. 2005;31:855-60. discussion 860
- [CrossRef] [PubMed] [Google Scholar]
- Protective effects of a topical antioxidant mixture containing Vitamin C, ferulic acid, and phloretin against ultraviolet-induced photodamage in human skin. J Cosmet Dermatol. 2008;7:290-7.
- [CrossRef] [PubMed] [Google Scholar]
- Bioactive tetrapeptide GEKG boosts extracellular matrix formation: In vitro and in vivo molecular and clinical proof. Exp Dermatol. 2011;20:602-4.
- [CrossRef] [PubMed] [Google Scholar]
- Skin Care Benefits of Copper Peptide Containing Eye Creams In: American Academy of Dermatology Meeting, February 2002. Abstracts P68, P69
- [Google Scholar]
- The efficacy study of the combination of tripeptide-10-citrulline and acetyl hexapeptide-3. A prospective, randomized controlled study. J Cosmet Dermatol. 2017;16:271-8.
- [CrossRef] [PubMed] [Google Scholar]
- Chemical peels in aesthetic dermatology: An update 2009. J Eur Acad Dermatol Venereol. 2010;24:281-92.
- [CrossRef] [PubMed] [Google Scholar]
- Visible light treatment of photoaging. Dermatol Ther. 2005;18:191-208.
- [CrossRef] [PubMed] [Google Scholar]
- Update on non-ablative light therapy for rejuvenation: A review. Lasers Surg Med. 2003;32:120-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of facial rhytides with a high-energy pulsed carbon dioxide laser. Plast Reconstr Surg. 1996;98:791-4.
- [CrossRef] [PubMed] [Google Scholar]
- Injectable soft-tissue fillers: Clinical overview. Plast Reconstr Surg. 2006;118:98e-106e.
- [CrossRef] [PubMed] [Google Scholar]
- Cosmetic use of botulinum toxin Type A in the elderly. Clin Interv Aging. 2007;2:81-3.
- [CrossRef] [PubMed] [Google Scholar]
- Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol. 2011;23:424-31.
- [CrossRef] [PubMed] [Google Scholar]
- Antiaging diets: Separating fact from fiction. Science. 2021;374:eabe7365.
- [CrossRef] [PubMed] [Google Scholar]
- Putting cancer to sleep at night: The neuroendocrine/circadian melatonin signal. Endocrine. 2005;27:179-88.
- [CrossRef] [PubMed] [Google Scholar]
- Hormone therapy and anti-aging: Is there an indication? Internist (Berl). 2008;49:572-6, 578-9
- [CrossRef] [PubMed] [Google Scholar]






