Translate this page into:
Tofacitinib in a case of generalized granuloma annulare: A two-year follow-up of relapse and remission

*Corresponding author: Shruthi Pavana Janardhanan, Everything Skin and Hair Medical and Aesthetic Dermatology Clinic, Mumbai, Maharashtra, India. shruthjan@yahoo.com.sg
-
Received: ,
Accepted: ,
How to cite this article: Janardhanan S, Saraogi P. Tofacitinib in a case of generalized granuloma annulare: A two-year follow-up of relapse and remission. CosmoDerma. 2024;4:122. doi: 10.25259/CSDM_137_2024
Dear Sir,
Recent research into the immunopathogenesis of granuloma annulare (GA) has led to new treatments. We share our experience with tofacitinib, a Janus Kinase/signal transducer and activator of transcription (JAK/STAT) inhibitor, in a biopsy-proven case of generalized GA (GGA) over two years of follow-up.
Our patient, a 56-year-old female presented with multiple erythematous papules and annular plaques on her extremities and trunk, diagnosed as GGA for the past year [Figure 1]. She failed previous treatments with topical agents (corticosteroids, tacrolimus), antimicrobials, and hydroxychloroquine (HCQs). She was stressed about the disease reflecting in her dermatology life quality index score of 20. She had no known comorbidities and we performed a biopsy to confirm the diagnosis [Figure 2].
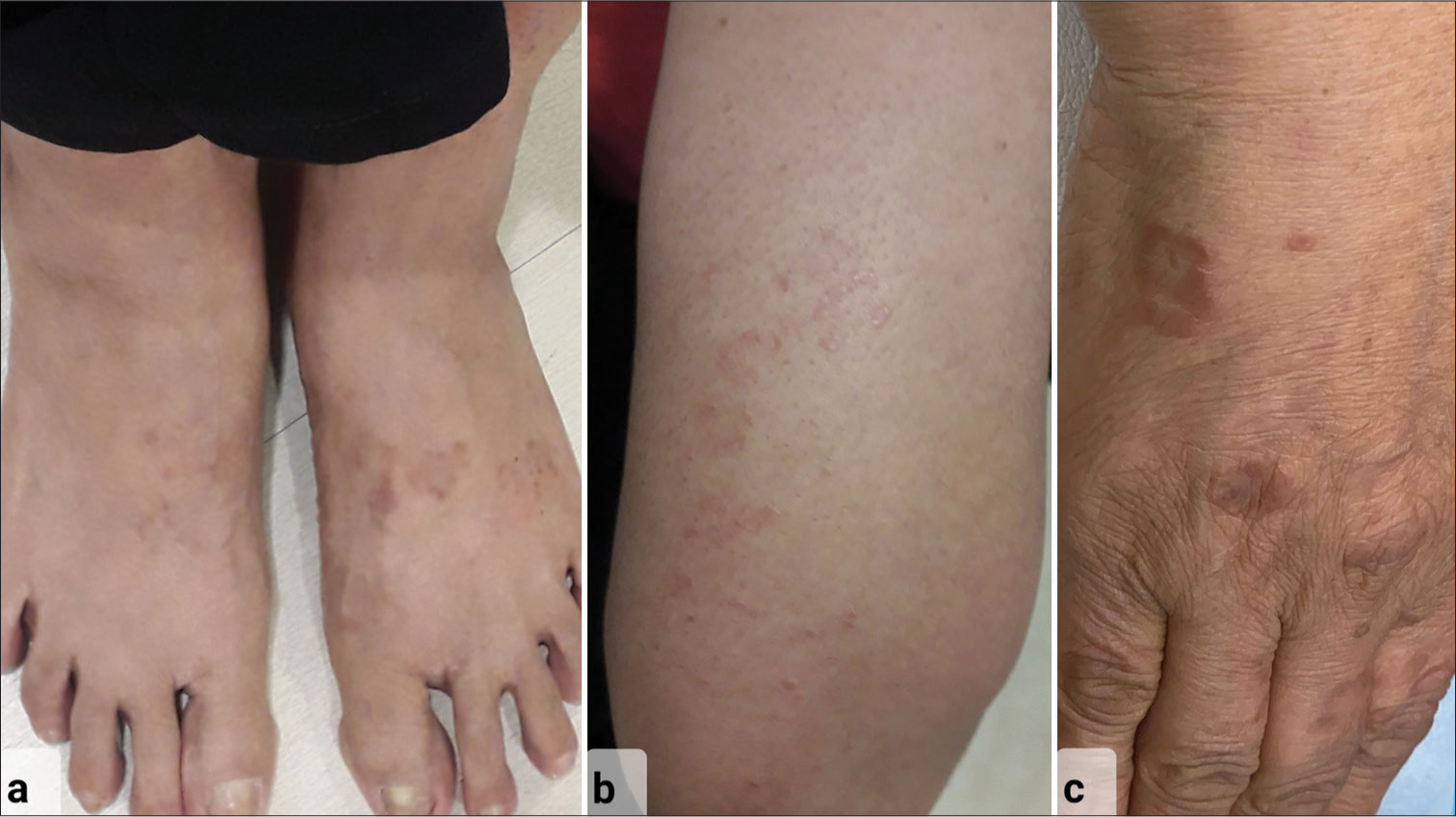
- 56-year-old female with generalized granuloma annulare – Clinical picture showing erythematous annular papules and plaques over (a) both dorsum of feet, (b) extensor aspect of forearm, and (c) dorsum of the right hand at presentation.
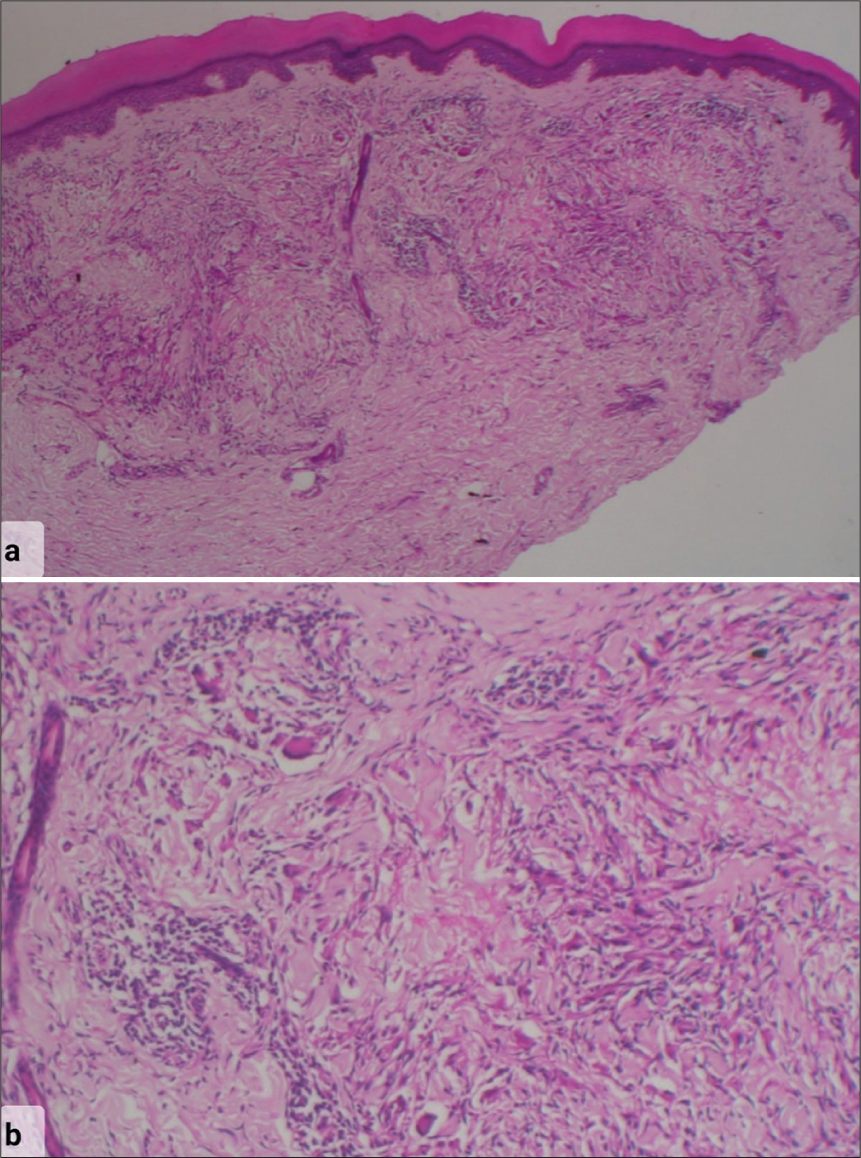
- Histopathology photomicrograph stained with hematoxylin and eosin stain showing (a) normal epidermis with a palisading granuloma in mid reticular dermis around a focus of mucin deposition and incomplete collagen degeneration. The rest of the dermis shows a sparse superficial and deep perivascular lymphocytic infiltrate (×10 Low-power magnification) (b) The granuloma consists of lymphocytes, histiocytes, and occasional giant cells in a semicircular palisade around central mucin deposition and necrobiosis (×40 High-power magnification).
Baseline investigations ruled out known associations of GA. After discussing possible therapy options, we started tofacitinib 5 mg twice daily (BD) with regular monitoring. In three months, she had a significant reduction and no new lesions [Figure 3a and b]. This was continued for two more months and after complete resolution, reduced to once daily (OD) for four months. Three months after stopping tofacitinib, she experienced a mild recurrence on her left forearm that progressed to a near complete relapse with new lesions in another two months. We restarted her on tofacitinib 5 mg BD, which rapidly cleared all lesions in four weeks, and continued once daily for three months with good control [Figure 3c]. Thereafter, she discontinued tofacitinib on her own. At her most recent follow-up, 10 months after stopping treatment, she continues to be disease free [Figure 4]. She had no treatment-related side effects.
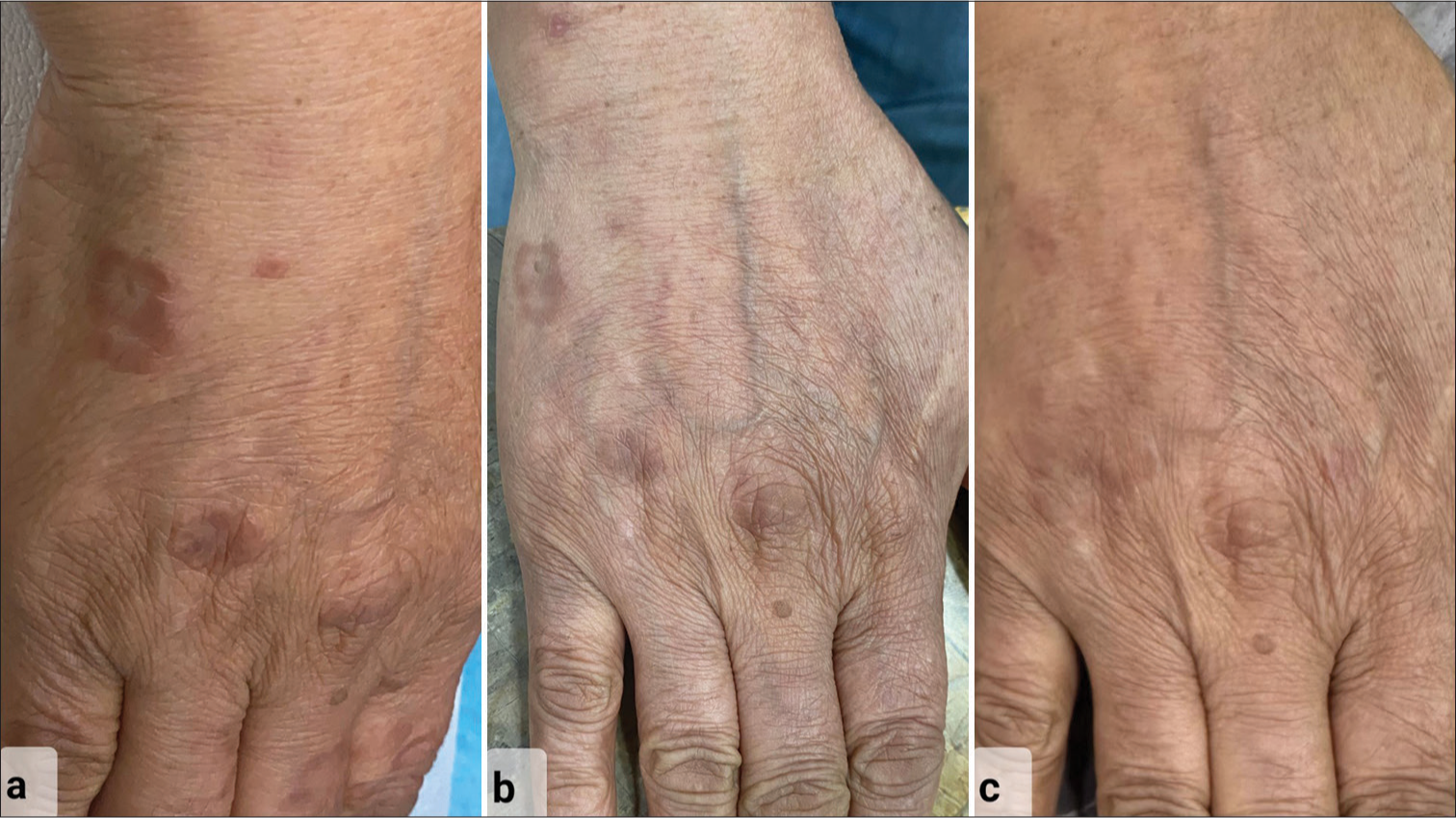
- Clinical pictures showing improvement over dorsum of the right hand of patient with (a) classical erythematous annular papules and plaques at presentation, (b) partial resolution after treatment initiation with tofacitinib at 3-month follow-up with decrease in erythema and flattening of skin lesions, and (c) complete resolution of skin lesions with no sequelae as seen at 2-year follow-up.
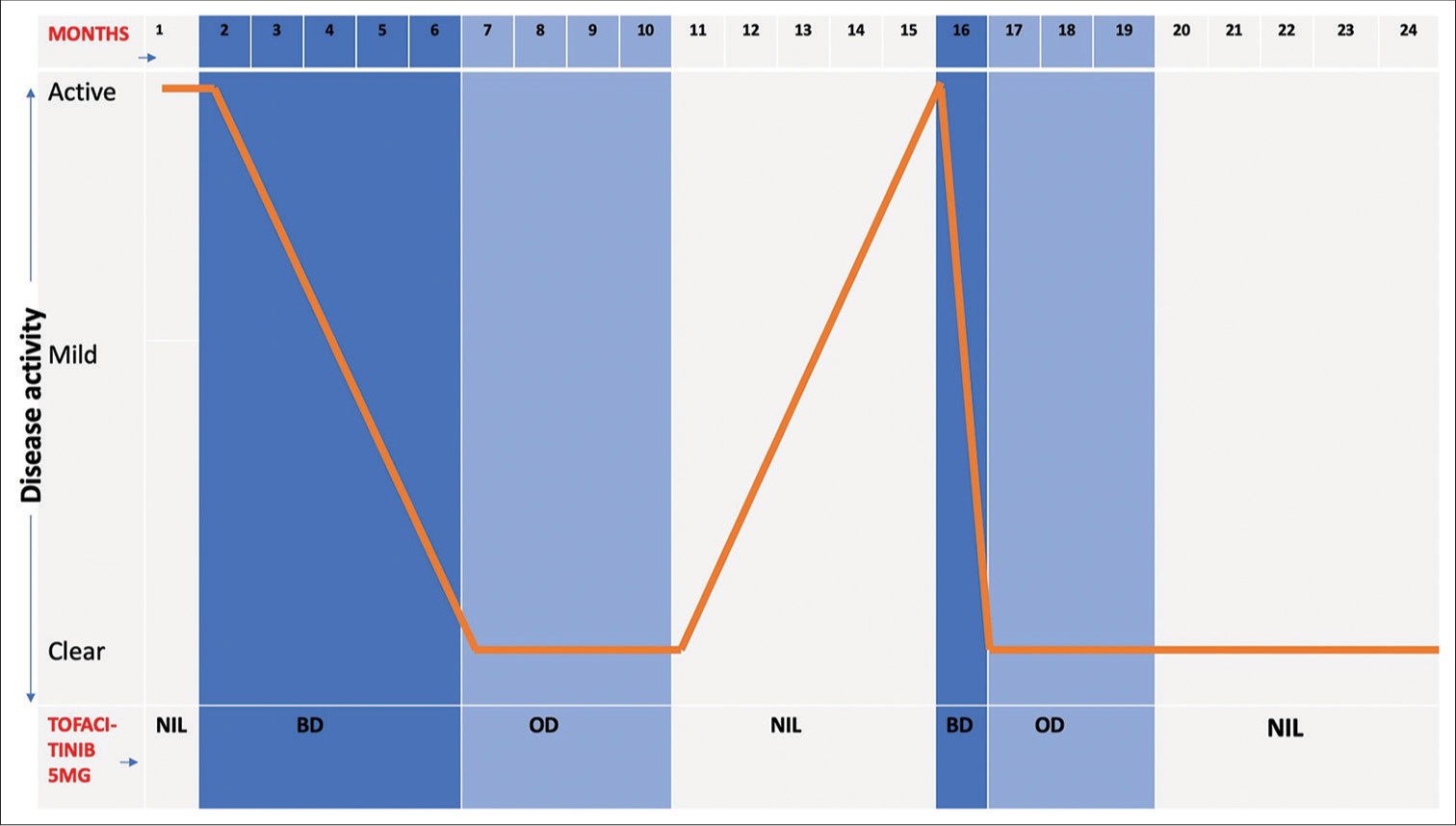
- Graphical representation of clinical history summary showing treatment given to patient corresponding with disease activity and response over 2 years. OD: Once daily, BD: Twice daily.
GGA with ≥10 widespread lesions is a benign inflammatory granulomatous disorder of unknown etiology. Its known associations are diabetes mellitus, thyroid disease, mild trauma, hyperlipidemia, infections (Epstein-Barr virus, human immunodeficiency virus, and Varicellazoster virus), vaccines, malignancy, and certain drugs (tumor necrosis factor [TNF]-alpha inhibitors).[1]
Unlike localized GA, GGA tends to be recalcitrant, has lower therapy success rates and less chance of spontaneous remission. HCQ therapy is variably successful in GGA with a response rate ranging from 35% to 79.6% and may be considered as a first-line treatment.[2] Other treatments with promising but inconsistent results are apremilast, dapsone, doxycycline, methotrexate, and pentoxifylline.[2] Biologicals such as TNF-alpha inhibitors (adalimumab, etanercept, and infliximab) which are also known to paradoxically induce GA can be equally effective in treatment with a response rate of 79.3%.[2] Due to their high cost, they may be reserved for patients who have failed all other therapies. Among phototherapy modalities, photodynamic therapy and ultraviolet A1 therapy are more effective than Narrow-band ultraviolet B therapy but due to affordability and accessibility, these remain out of reach to most patients.[2]
Managing diabetes and hyperlipidemia may indirectly treat GA by reducing the T-cell dysregulation central to its pathogenesis. Two recent studies investigating the immunopathogenesis of GA arrived at conflicting reports. Min et al. revealed upregulation of interleukin (IL)-4 and IL-31 messenger RNA sequences in the Th2 pathway whereas Wang et al. found nearly no expression of IL-4 and instead reported activation of Th1 and JAK/STAT pathways.[3,4]
Damsky et al. pioneered the research of small molecule JAK inhibitors in granulomatous disorders including GA. They correlated the clinical efficacy of tofacitinib, a JAK1/3 inhibitor to the immunohistochemical analysis of skin biopsy samples showing the downregulation of proximal JAK/ STAT-dependent cytokines and other pathways critical to the formation and maintenance of granuloma such as interferon-gamma and TNF-alpha.[5]
Tofacitinib is well-tolerated with the most commonly reported adverse effect being upper respiratory tract infection and nasopharyngitis in over 10% of patients. The other adverse effects to be monitored are increased risk of infections, raised liver enzymes, hyperlipidemia, hypothyroidism, bone marrow suppression, and adverse cardiovascular events.[3,4]
There have been multiple isolated case reports across the world for tofacitinib use in GGA along with a recent case series by Dev et al. with an average follow-up duration of 7.5 months, in which two patients were non-responders.[6] The average duration of therapeutic resolution is 12–15 weeks for topical therapy and around six months for oral therapy.[7] Durgin et al. reported a patient of GGA who used topical 2% tofacitinib gel over one arm, but achieved resolution over lesions in other areas of the body as well giving promise to those patients who do not have oral therapy options.[8] Slater et al.’s case report of treatment-resistant GGA showing rapid resolution with upadacitinib (selective JAK1 inhibitor) adds to the growing body of literature on the potential role of JAK/ STAT inhibitors but the high cost and ease of availability in India is currently a limiting factor.[9]
In conclusion, immunohistochemical analysis has enabled the use of JAK/STAT inhibitors in dermatology for treating localized/GGA. The availability of generic tofacitinib in India has made this affordable. We documented the successful use of oral tofacitinib for biopsy-proven GGA, unresponsive to standard treatment, with diligent follow-up over two years, monitoring improvement, relapse, and resolution.
Larger randomized controlled studies are needed to confirm tofacitinib’s efficacy, assess long-term safety, establish tapering protocols, and compare it to conventional GA treatments.
Ethical approval
The Institutional review board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Granuloma annulare: A clinical update. Curr Dermatol Rep. 2024;13:183-97.
- [CrossRef] [Google Scholar]
- Therapeutic options for granuloma annulare: An updated review. J Pharm Res Int. 2023;35:1-10.
- [CrossRef] [Google Scholar]
- Granuloma annulare skin profile shows activation of T-helper cell type 1, T-helper cell type 2, and Janus kinase pathways. J Am Acad Dermatol. 2020;83:63-70.
- [CrossRef] [Google Scholar]
- Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol. 2021;147:1795-809.
- [CrossRef] [Google Scholar]
- Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82:612-21.
- [CrossRef] [Google Scholar]
- Therapeutic success of tofacitinib in granuloma annulare: A retrospective case series of 15 patients. Indian J Dermatol Venereol Leprol 2024 Ahead of print:1-6
- [CrossRef] [Google Scholar]
- Treatment of granuloma annulare with tofacitinib. Australas J Dermatol. 2022;63:400-3.
- [CrossRef] [Google Scholar]
- Generalized granuloma annulare: A widespread response to limited application of compounded 2% topical tofacitinib. JAAD Case Rep. 2020;6:1113-5.
- [CrossRef] [Google Scholar]
- A case of generalized granuloma annulare treated with upadacitinib. JAAD Case Rep. 2023;34:12-4.
- [CrossRef] [Google Scholar]





