Translate this page into:
Seborrheic dermatitis and pityriasis sicca: A review

*Corresponding author: Bhushan Madke, Department of Dermatology Venereology & Leprosy, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Wardha, Maharashtra, India. drbhushan81@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh R, Madke BS, Bose S. Seborrheic dermatitis and pityriasis sicca: A review. CosmoDerma 2022;2:36.
Abstract
Seborrheic dermatitis (SD) is one of the most common dermatological conditions faced by the general population, regardless of geographic location and ethnicity. Given its widespread existence, it still remains puzzling for dermatologists and the affected individuals due to its varying presentations and recurring nature. We have presented a concise review of seborrheic dermatitis and pityriasis sicca.
Keywords
Seborrheic dermatitis
Dandruff
Pityriasis sicca
Treatment
INTRODUCTION
Seborrheic dermatitis is a common inflammatory condition of so-called seborrheic areas (sebum rich) areas of the body particularly the face, scalp, and upper trunk. Clinically characterized by itchy red areas of inflammation with greasy scaling particularly noticed over nasolabial grooves, nasal ala, scalp, and auricular region. The excess or qualitative difference in sebum production, overgrowth of commensal yeast, and disturbance in innate immunity are the pathogenic events in the causation of seborrheic dermatitis. On the other hand, pityriasis sicca is a common scalp condition referred to as ‘dandruff’ occurring in the adolescent age group.[1,2,3] Management for each condition includes anti-yeast, anti-inflammatory, antiproliferative, and sebum reducing agents. Notably, seborrheic dermatitis can be a chronic relapsing condition that needs either long-term maintenance or intermittent measures.
EPIDEMIOLOGY
Pityriasis sicca or pityriasis simplex capitis commonly referred to as ‘dandruff,’ is the non-inflammatory and mildest manifestation of seborrheic dermatitis limited to the scalp. While the worldwide prevalence of SD is around 5%,[1] SD and dandruff, combined, are estimated to affect half of the adult population at some point in their lives. They are more common in men than in women. In Indians with scalp dermatoses, 18.7% was attributed to SD in one study.[2] Seborrheic dermatitis has a triphasic peak during infancy, puberty, and the 4–6th decade of life. In contrast, dandruff starts at puberty, reaches its peak in the 20th year of life, and becomes less prevalent in the 5th decade.[3]
Etiopathogenesis
Seborrheic dermatitis
The development of seborrheic dermatitis is attributed to various exogenous and endogenous factors. Exogenous factors include skin microbiome, stress, haircare practices, products, environmental factors, medications, and endogenous factors include male gender, increased androgen, sebaceous gland activity, and immune system response.[4]
The most consistent and essential factor implicated in the pathogenesis of SD is the lipophilic yeast Malassezia. M. globosa, M. restricta, and M. furfur are the most prevalent species seen in SD patients in India.[5] Malassezia is a commensal fungus, and it causes SD only in predisposed individuals, probably depending on the immune function, barrier integrity, and sebum composition.[4] Malassezia secretes lipases and phosphates that hydrolyses the sebaceous lipid to release unsaturated free fatty acids like oleic acid and arachidonic acid, which are believed to trigger the inflammation in SD. Many inflammatory markers, like interleukins, TNF-alpha, beta-defensins, histamine, and nitric acid, are increased in SD. In contrast, epidermal markers like ceramides and sphingolipids, which mark the skin barrier integrity, are decreased.[6] This leads to epidermal barrier dysfunction, further disrupting the skin microbiome causing pruritus and erythema. A part from Malassezia, certain bacteria like Acinetobacter, Staphylococcus, and Streptococcus have also been isolated from SD lesions suggesting a possible role.[7]
Recent studies also emphasize the role of oxidative stress in the etiopathogenesis of SD, with SD patients showing higher lesional oxidative stress as compared to the controls.[8]
Dandruff
About 487,000 cells/sq cm get shed physiologically after hair wash, and up to 800,000/sq cm is shed in the ‘dandruff’.[9] Dandruff is thought to be the non-inflammatory manifestation of seborrheic dermatitis. Similar to SD, Malassezia plays a very prominent role in the etiopathogenesis of dandruff. However, some non-microbial factors which irritate the scalp, like frequent washing, over-combing of the scalp, and certain hair products, also exacerbate the scaling, thus contributing to the severity of dandruff.[10]
Clinical features
Dandruff is a cluster of corneocytes and presents as white or yellow, diffuse, mild to dense, non-adherent flaking on the scalp without erythema or inflammation. Itching may be present or absent. Scales are usually noticed falling on the dark clothes. Both the size and abundance of flakes are highly variable, and the course could be recurrent or constant. Fine squames are referred to as pityriasis sicca, and thick seborrheic scales are called steatoid pityriasis, or pityriasis oleosa.[11]
SD runs a chronic and relapsing course in adults and presents with greasy, erythematous, yellowish, scaly patches or plaques on the face [Figure 1a-c], scalp, and upper trunk. Sebum-rich areas like the forehead (especially between the eyebrows), eyelid, eyebrow, beard, perinasal, nasolabial, and retro-auricular region are most commonly affected. On the trunk, the lesions are most commonly seen in the pre-sternal region. Intertriginous sites like the axilla, genital, inguinal, and sub-mammary region may also be involved. ‘Folds’ may show crusting, oozing, and fissures and may get secondarily infected. Pruritus is present in the majority of the cases. An exacerbation is typically seen in the winter months, probably due to disruption in the skin barrier and diminished protective effect of UV rays.
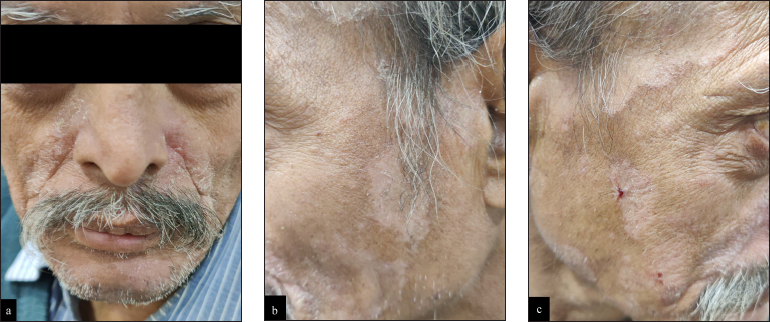
- Erythematous and scaly patches and plaques on so-called sebum-rich areas of the face.
Even though seborrheic dermatitis affects the sebum-rich area, it does not have a direct association with the degree of sebum production. Not all individuals with seborrhea suffer from SD, and in turn, patients with SD may have a normal sebum production.
SD in infants commonly presents as cradle cap – greasy yellow plaque on the vertex and can also involve other sites the nasolabial, retro-auricular area, eyelid, eyebrow, neck, inguinal, and axilla.
An extensive and exacerbated manifestation of SD is usually seen in older and immunocompromised patients, especially with HIV infection. SD is one of the rare but known causes of erythroderma.
Dandruff, seborrheic dermatitis, and alopecia
Dandruff and seborrheic dermatitis may precede, or accompany telogen effluvium.[12] This is due to the underlying inflammation of the disease process. The inflammation alters the anchoring of hair fiber in the follicle, increasing the proportion of telogen and dysplastic anagen hair.[13] In some cases, the effluvium can be due to alteration in the teloptosis phase.[11] Pitney et al. proposed that chronic microinflammation on the scalp can also lead to some degree of permanent hair follicle damage and subtle cicatricial alopecia.[14] In addition, oxidative stress due to lipid peroxidation has a negative impact on hair growth.[15]
The role of SD and dandruff in hair loss is further corroborated by the finding that the treatment of dandruff and SD with anti-dandruff shampoos like ketoconazole, and zinc pyrithione, or piroctone olamine attenuates hair shedding due to androgenic alopecia.[16] SD and dandruff, therefore, should always be kept in mind in the case of hair loss, even if it is not the primary diagnosis.
Dandruff and hair texture
Scalp receives very little attention when it comes to the aesthetic of the hair shaft. However, conditions affecting scalp health also directly affect the quality of hair. Hair emerging from the scalp with dandruff and SD shows altered cuticle with rigidity, pitting, roughness, reduction in shine, and breakage of hair.[15]
Associations
SD is more common in immunosuppressed individuals like transplant recipients, HIV patients, lymphoma, chronic pancreatitis, and hepatitis. SD in HIV is an early manifestation and is already seen with CD4 counts of 200–500 cells/mm3.[17] SD can be seen in up to 83% of immunocompromised patients as compared to less than 5% in the general population.[18]
SD is also associated with neurological disorders like Parkinson’s disease, stroke, depression, tardive dyskinesia, and genetic conditions like Down’s syndrome and Hailey-Hailey disease.[3,18]
Differential diagnosis
Depending on the location, the differential diagnoses of SD include psoriasis (overlap of SD and psoriasis called sebopsoriasis can also be seen on the scalp), pityriasis amiantacea, tinea capitis, rosacea, lupus erythematosus, atopic dermatitis, pemphigus foliaceous, intertrigo, amongst many others.
In infants, the conditions that need to be differentiated from seborrheic dermatitis include—atopic dermatitis, candidiasis, diaper dermatitis, Langerhans cell histiocytosis, and acrodermatitis enteropathica.
Investigation
Both seborrheic dermatitis and dandruff are clinically diagnosed with a history and physical examination. Rarely, a biopsy is needed to rule out other differential diagnoses. Histopathology of the acute lesion shows spongiosis with a superficial perivascular and perifollicular lymphocytic infiltrate, and shoulder parakeratosis around hair follicles. Chronic lesions show parakeratosis and psoriasiform hyperplasia. Neutrophils may be found in the scale crust along the margins of follicular ostia. Malassezia yeasts may be seen surrounding the parakeratotic cells.[3]
Dandruff shows histological features akin to SD although lymphocytes and NK cells (inflammatory cells) are seen in higher numbers in SD as compared to dandruff. There may be little or no neutrophil infiltration in dandruff.[6]
Treatment
The ideal treatment of SD and dandruff should mainly focus on disease clearance, along with a fast reduction of pruritus followed by maintenance of long-term remission. The pathologic agent being Malassezia, and inflammation secondary to the proliferation of the same, the most common agents used are topical antifungals and anti-inflammatory agents.
Topical therapy
Topical antifungal agents:
These include ketoconazole (2% shampoo, cream, gel, or foam), miconazole (2% cream, gel, or powder), bifonazole (1% shampoo, cream, or ointment), selenium sulfide (2.5% shampoo, lotion, cream, foam, and suspension), zinc pyrithione (1% shampoo), ciclopirox olamine (1.5% shampoo, 1% cream, gel or lotion). Ketoconazole, miconazole, and bifonazole come under the azole group of antifungals and work by inhibiting fungal cell wall synthesis. Selenium sulfide acts as a cytostatic and keratolytic agent. It is known to have fungicidal properties against Pityriosporum ovale. Zinc pyrithione acts by increasing the cellular levels of copper which in turn interferes with the iron-sulfur cluster of proteins essential for fungal growth and metabolism.[19] Ciclopirox olamine acts by inhibiting metal-dependent enzymes such as cytochromes, catalase, and peroxidase by chelating with metal cations such as ferric and aluminum. This results in the disruption of multiple cellular activities of the fungal cell.[20]
Topical antifungals reduce Malassezia proliferation hence preventing the subsequent inflammatory response. Shampoos should be used twice to thrice a week for four weeks depending on the severity of dandruff, followed by once a week for maintenance. The most common side effect noted with all the topical antifungal agents is irritant contact dermatitis, ranging from <1–10% of patients, most commonly with bifonazole (10%) use. Selenium sulphide can cause an orange-brown discoloration of the scalp, especially in children.[3] It should be kept in mind that this should not be confused with Langerhans cell histiocytosis. This discoloration is reversible and can be easily removed with the help of an isopropyl alcohol swab.[18]
Treatment failure to azole antifungals has been noted in clinical practice due to resistance of strains of M. globosa and M. restricta (the most common etiological agent associated with dandruff and SD).[21]
Topical steroids:
Various steroids such as hydrocortisone (1% cream), fluocinolone (0.01% shampoo, lotion, or cream), betamethasone dipropionate (0.05% lotion), desonide (0.05% lotion) have been used. They act as a potent anti-inflammatory agent but carry the risk of atrophy of skin and hypopigmentation, telangiectasia, hypertrichosis, and folliculitis with prolonged use.
Topical keratolytic agents:
Topical urea and propylene glycol have keratolytic effects whereas lactic acid has keratolytic as well as hydrating properties. All of them are known to inhibit the growth of bacteria and/or fungi. A study by Emtestam et al. in 88 patients in two randomized, double-blind, multicentre trials showed that a combination containing topical urea, propylene glycol, and lactic acid when applied daily for four weeks in cases with mild to severe SD of the scalp showed significant improvement in erythema and desquamation (p < 0.05).[22]
Topical calcineurin inhibitors:
Tacrolimus (0.03% and 0.1% ointment) and pimecrolimus (1% cream) have been used for treating SD. They inhibit cytokine production by T-lymphocyte. Both have shown comparable efficacy with topical corticosteroids as well as topical antifungal agents.[23,24] Tacrolimus, though has been shown to have more prolonged remission compared to topical betamethasone.[23] Prolonged use of these immune modulators can lead to skin malignancy and lymphomas; hence they should be used with caution.
Other agents:
Metronidazole gel (0.75%, applied twice daily for four weeks): Metronidazole works as an anti-inflammatory agent by inhibiting free radical species, in turn preventing oxidative tissue damage. In a randomized controlled trial, metronidazole gel has been shown to have similar efficacy and safety profile to ketoconazole 2% cream.[25]
Coal tar 4% shampoo, to be applied on the scalp once or twice a week has anti-inflammatory, keratolytic as well as antifungal properties. It reduces sebum production. Scalp folliculitis and irritant contact dermatitis on fingers are commonly reported side effects.
Lithium gluconate/succinate (8% ointment or gel applied twice daily for 8 weeks). It acts as an anti-inflammatory agent and increases IL-10 while reducing TLR2 and TLR4 in keratinocytes.[26]
Narrowband UVB Phototherapy (three times a week for 8 weeks or until clearance of lesions, with a cumulative dose of 9.8 J/cm2). It acts via inhibition of cell proliferation.[27]
Licochalcone (0.025%): An extract from Glycyrrhiza inflate, licochalcone has been found to be effective in infantile SD due to its anti-inflammatory and antimicrobial effects.[28]
Antimicrobial peptides (AMPs): They are part of the innate defense of the epithelium and act well against M. furfur. Cathelicidins, cecropin A (CA), and magainin 2 (MA) are a few known AMPs. A modified synthetic AMP, P5, has shown potent antifungal action which was three to four times more potent than ketoconazole or itraconazole against M. furfur.[18]
Systemic therapy
Itraconazole (100 mg twice a day for seven days): It acts via inhibition of the enzyme lanosterol 14α demethylase which is required for fungal cell wall synthesis. Itraconazole acts as an anti-inflammatory agent by inhibiting the synthesis of 5-lipoxygenase metabolites, known to be involved in various inflammatory disorders such as SD.[29]
Das et al. found a dose of 100 mg twice a day for seven days followed by 200 mg once a day for two days a month for two months, effective in 83.3 % of cases treated with seborrheic dermatitis. Although oral ketoconazole (200 mg daily for four weeks) has also been used for treating SD, it is more hepatotoxic as compared to itraconazole. As oral terbinafine is not effective against Malassezia, hence it is not used in the treatment of SD or pityriasis versicolor.[30]
Low dose isotretinoin: A randomized, comparative clinical trial, on 45 patients conducted over six months showed that isotretinoin given at a dose of 10 mg every other day is quite effective in moderate to severe seborrhoea as well as seborrheic dermatitis, particularly involving the scalp and face. There was a significant reduction in sebum secretion on the face and scalp within three months of initiating therapy, hence showing the effectiveness of isotretinoin in controlling sebum production, which in turn reduces the colonization of M. furfur.[31] Abraham and Piguet reported two cases successfully treated with oral isotretinoin at 20 mg/day.[32]
CONCLUSION
Seborrheic dermatitis and its non-inflammatory counterpart pityriasis sicca are one of the most common dermatoses across the globe. Fungal agents (Malassezia species) and immune dysfunction are the pathologic events in the causation of seborrheic dermatitis. Seborrheic dermatitis can have a chronic relapsing course while pityriasis sicca may show seasonal worsening. During the active phase of the condition, treatment consists of systemic and topical antifungal, oral and topical anti-inflammatory agents. Intermittent treatment during relapses is frequently needed and long-term maintenance may be needed to sustain remission.
Declaration of patient consent
Patient consent is not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Seborrheic dermatitis: Lifetime detection rates. J Eur Acad Dermatol Venereol. 2012;26:524-6.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical spectrum of scalp dermatoses in adults presenting to a tertiary referral care centre. Int J Biol Med Res. 2014;5:4434-9.
- [Google Scholar]
- Seborrheic dermatitis and dandruff: A comprehensive review. J Clin Investig Dermatol. 2015;3
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An update on the microbiology, immunology and genetics of seborrheic dermatitis. Exp Dermatol. 2020;29:481-9.
- [CrossRef] [PubMed] [Google Scholar]
- Association of malassezia species with dandruff. Indian J Med Res. 2014;139:431-7.
- [PubMed] [PubMed Central] [Google Scholar]
- A comprehensive pathophysiology of dandruff and seborrheic dermatitis—towards a more precise definition of scalp health. Acta Derm Venereol. 2013;93:131-7.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive pyrosequencing analysis of the bacterial microbiota of the skin of patients with seborrheic dermatitis. Microbiol Immunol. 2016;60:521-6.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress in patients with scalp seborrheic dermatitis. Acta Dermatovenerol Croat. 2013;21:80-5.
- [PubMed] [Google Scholar]
- Dandruff: The most commercially exploited skin disease. Indian J Dermatol. 2010;55:130-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- From axioms to new insights into dandruff. Dermatology. 2000;200:93-8.
- [CrossRef] [PubMed] [Google Scholar]
- Piérard GE. Revisiting dandruff. Int J Cosmet Sci. 2006;28:311-8.
- [CrossRef] [PubMed] [Google Scholar]
- Dandruff-associated smouldering alopecia: A chronobiological assessment over 5 years. Clin Exp Dermatol. 2006;31:23-6.
- [CrossRef] [PubMed] [Google Scholar]
- Trichogram findings in psoriasis and seborrheic dermatitis patients. Turk Klin Dermatoloji. 2017;27:61-8.
- [CrossRef] [Google Scholar]
- Is seborrhoeic dermatitis associated with a diffuse, low-grade folliculitis and progressive cicatricial alopecia? Australas J Dermatol. 2016;57:e105-7.
- [CrossRef] [PubMed] [Google Scholar]
- Scalp condition impacts hair growth and retention via oxidative stress. Int J Trichology. 2018;10:262-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Nudging hair shedding by antidandruff shampoos. A comparison of 1% ketoconazole, 1% piroctone olamine and 1% zinc pyrithione formulations. Int J Cosmet Sci. 2002;24:249-56.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of mucocutaneous manifestations of HIV/AIDS infection with CD4 counts and disease progression. Int J Dermatol. 2007;46:14-8.
- [CrossRef] [PubMed] [Google Scholar]
- Seborrheic dermatitis: Etiology, risk factors, and treatments: Facts and controversies. Clin Dermatol. 2013;31:343-51.
- [CrossRef] [PubMed] [Google Scholar]
- The antifungal mechanism of action of zinc pyrithione. Br J Dermatol. 2011;165:9-12.
- [CrossRef] [PubMed] [Google Scholar]
- Topical ciclopirox olamine 1%: Revisiting a unique antifungal. Indian Dermatol Online J. 2019;10:481-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Susceptibility testing of malassezia species using the urea broth microdilution method. Antimicrob Agents Chemother. 2000;44:2185-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Treatment of seborrhoeic dermatitis of the scalp with a topical solution of urea, lactic acid, and propylene glycol (K301): Results of two double-blind, randomised, placebo-controlled studies. Mycoses. 2012;55:393-403.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical efficacies of topical agents for the treatment of seborrheic dermatitis of the scalp: A comparative study. J Dermatol. 2009;36:131-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pimecrolimus 1% cream for the treatment of seborrheic dermatitis: A systematic review of randomized controlled trials. Expert Rev Clin Pharmacol. 2012;5:91-7.
- [CrossRef] [PubMed] [Google Scholar]
- Metronidazole 0.75% gel vs. ketoconazole 2% cream in the treatment of facial seborrheic dermatitis: A randomized, double-blind study. J Eur Acad Dermatol Venereol. 2007;21:345-50.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-inflammatory effects of lithium gluconate on keratinocytes: A possible explanation for efficiency in seborrhoeic dermatitis. Arch Dermatol Res. 2008;300:215-23.
- [CrossRef] [PubMed] [Google Scholar]
- Narrow-band ultraviolet B (ATL-01) phototherapy is an effective and safe treatment option for patients with severe seborrhoeic dermatitis. Br J Dermatol. 2000;143:964-8.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized, double-blind, split-side comparison study of moisturizer containing licochalcone vs. 1% hydrocortisone in the treatment of infantile seborrhoeic dermatitis. J Eur Acad Dermatol Venereol. 2012;26:894-7.
- [CrossRef] [PubMed] [Google Scholar]
- Oral itraconazole for the treatment of severe seborrhoeic dermatitis. Indian J Dermatol. 2011;56:515-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18:13-26.
- [CrossRef] [PubMed] [Google Scholar]
- Low-dose oral isotretinoin for moderate to severe seborrhea and seborrheic dermatitis: A randomized comparative trial. Int J Dermatol. 2017;56:80-85.
- [CrossRef] [PubMed] [Google Scholar]
- An unusual presentation of malassezia dermatosis. Dermatology. 2006;212:4-6.
- [CrossRef] [PubMed] [Google Scholar]






