Translate this page into:
Evaluation of steroids efficacy in preventing and reversing nerve function impairment in multibacillary leprosy cases

*Corresponding author: Ram Malkani, Jaslok Hospital, Room no. 200, Dr. G. Deshmukh Marg, Mumbai – 400026, India. malkanipub@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Malkani R, Ghate SD, Desai M, Wadia, Karmakar S. Evaluation of steroids efficacy in preventing and reversing nerve function impairment in multibacillary leprosy cases. CosmoDerma. 2025;5:58. doi: 10.25259/CSDM_210_2024
Abstract
Objectives:
This study was to assess the utility of steroids to prevent reactions, neuritis and nerve function impairment in multibacillary cases of leprosy and also aimed to see if the use of steroids reversed the nerve function impairment.
Materials and Methods:
The study involved 40 cases with multibacillary leprosy divided into two groups. Group A consisted of prophylactic and therapeutic steroids subgroups. The prophylactic steroid group received WHOMDTMB for 12 months with prednisolone, 20 mg/day for 3 months which was tapered off in the 4 months, while the therapeutic steroid group received MDT-MB with higher doses of prednisolone (40mg per day starting dose, tapered off every 14 days). Group B received only WHOMDT-MB for 12 months. The study assessed clinical parameters to diagnose reactions and nerve function impairment. Silent neuritis was diagnosed when in the absence of clinical symptoms and signs, there was nerve function impairment (NFI). NFI was assessed with touch sensibility test (TST) done with Semmes- Weinstein monofilaments of nylon, voluntary muscle test (VMT) as per modified MRC grade and nerve conduction velocity (NCV) assessments. All the cases were followed up till completion of MDT clinically every month and with TST, VMT and NCV done at 3 monthly intervals. The cases were followed up every 3 months for a further period of 1 year post release from treatment (RFT). A skin biopsy was done in cases presenting with skin lesions. In cases with pure neural leprosy, a cutaneous nerve biopsy was done. The baseline biopsies were done to confirm the diagnosis by histopathology and classify the patient histologically.
Results:
We observed that in Group A, 2 out of 18 patients had neuritis, and both baseline neuritis, while in Group B, 3 out of 22 exhibited a type I reaction along with neuritis. That means the 2 cases in Group A who developed repeat neuritis despite receiving therapeutic dose of prednisone earlier. While majority of them (16/18) didn’t get reaction or neuritis. Occurrence of Type 1 reaction and neuritis was seen in 3/22 cases in the group B. TST and VMT improved in 1 case in Group A while TST deteriorated in 2 cases in Group B suggesting the efficacy of steroids in the steroid group.
Conclusion:
This means our study shows benefits of steroids in preventing reactions/nerve damage in leprosy. It also shows efficacy of steroids in preventing NFI.
Keywords
Corticosteroid
Multibacillary leprosy
Multidrug therapy
Prophylactic steroids
INTRODUCTION
Leprosy is a chronic infectious disease caused by Mycobacterium leprae. It poses significant challenges in both medical treatment and social integration for affected individuals. The disease is characterized by progressive and irreversible damage to the peripheral nerves and tissues, leading to motor and sensory impairments. These physical limitations severely restrict patients’ ability to participate in daily activities and hinder their social engagement.[1] Among the different forms of leprosy, multibacillary leprosy presents a more severe form, characterized by a higher bacterial load and greater risk of complications, including irreversible nerve damage. The effective management of multibacillary leprosy requires not only the prompt administration of multidrug therapy (MDT) but also comprehensive strategies to mitigate nerve damage and preserve neurological function. Corticosteroids are the mainstay of treatment in both type 1 lepra reactions (reversal reactions) and type 2 lepra reactions (erythema nodosum leprosum). These reactions can cause nerve damage, leading to long-term disability if not managed appropriately.[2,3] The nerve function impairment (NFI) can occur without over symptoms and signs that is called silent neuritis. The corticosteroids act by suppressing the T cell-driven inflammatory response to M. leprae antigens in the skin and nerves. Evidence suggests that the appropriate use of steroids can treat NFI and reactions in leprosy with limited nerve function recovery.[1,4] The role of corticosteroids in preventing nerve damage in multibacillary leprosy patients has become a focal point of clinical research, as their immunosuppressive properties may help alleviate inflammation, reduce pain, and ultimately preserve nerve function. Consequently, the World Health Organization (WHO) recommends a standard regimen of corticosteroids, emphasizing both prophylaxis and treatment of NFI as a safe and effective strategy.[4,5] Recent studies on steroid prophylaxis for NFI in leprosy have shown that a 6–8-month regimen is more effective than shorter regimens used in previous studies. However, the optimal dose and duration of prophylactic agents have yet to be determined (van Veen et al., 2016).[6,7] Therefore, we aim to use low-dose steroids with a longer follow-up period to determine the utility of MDT with and without prophylactic steroids in preventing reactions/neuritis, reversing NFI, and preventing silent neuritis over a longer follow-up period.
MATERIALS AND METHODS
We conducted a study on 40 patients with multibacillary leprosy, each presenting with 5 or more skin lesions and 2 or more affected nerve trunks. The inclusion criteria were all naïve cases of leprosy presenting with the specified number of lesions and patients or their guardians consenting to the study, investigations, and follow-up assessments. Exclusion criteria included cases with contraindications for steroid use such as diabetes or acid peptic disease, G6PD deficiency, cases presenting with a relapse, or those unwilling to consent. The patients were divided into two groups, A and B. Group A included patients who were either given prophylactic steroids (A1) or those who presented with reactions or neuritis and were given a therapeutic dose of steroids (A2). Patients in the prophylactic steroid group received MDT-multibacillary (MB) for 12 months along with prednisolone 20 mg/day for the first 3 months, which was then tapered off in the 4th month. Those in the therapeutic steroid group, presenting with reaction/neuritis, received WHO MDT-MB for 12 months along with prednisolone in doses of 40 mg, 30 mg, 20 mg, 15 mg, 10 mg, and 5 mg, each dose lasting for 2 weeks. Group B (the control group) received WHO MDT-MB for 12 months only.
Monthly analysis was conducted on the cases for 12 months using standard criteria to diagnose reactional episodes and neuritis. Touch sensibility tests (TSTs) were performed using Semmes-Weinstein monofilaments of nylon, and voluntary muscle tests (VMTs) were graded by the modified Medical Research Council scale. Nerve conduction velocity (NCV) was measured at baseline and 3-month intervals. The nerves assessed included the ulnar, median, and radial cutaneous nerves in the upper extremities and the common peroneal, posterior tibial, and sural nerves in the lower extremities. Blue filament with a force value of 200 mg was considered normal for the palms, and 2 g was considered normal for the soles. A VMT score of 5 was deemed normal, with any score below that considered abnormal. To confirm the diagnosis, a punch skin biopsy was performed on cases with skin lesions, and a cutaneous nerve biopsy was performed on cases without skin lesions.
RESULTS
The study group (Group A) included 18 out of 40 patients, comprising 15 males and 3 females. Among the 18 patients treated with prednisolone, 14 received prophylactic steroids, while 4 received therapeutic steroids. The ages of the patients ranged from 8 to 60 years, with one child being 8 years old.
The control group (Group B) consisted of 22 patients, including 13 males and 9 females. The ages of these patients ranged from 18 to 60 years, and there were no children in this group.
It was observed that in Group A, 2 out of 18 patients had neuritis. Both these cases had baseline neuritis. In Group B, 3 out of 22 exhibited a type I reaction along with neuritis. Two cases of silent neuritis (NFI) were identified. Both belonged to Group B, while in 1 case, both TST and VMT improved, in 1 case in Group A.
It was found that erythematous patches were identified as the most common clinical manifestation, followed closely by hypo-pigmented patches in both groups of patients. Among the cases examined, six were diagnosed with pure neural leprosy, with four belonging to Group A and two to Group B [Figure 1].
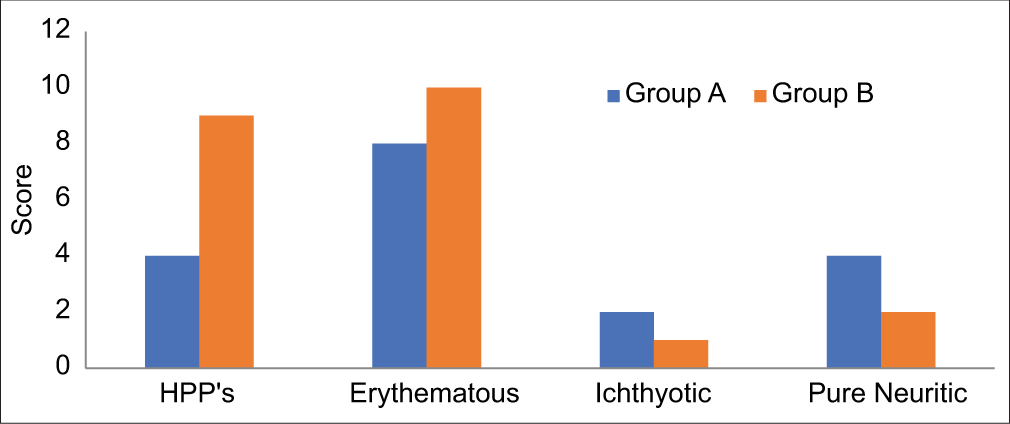
- The comparison of clinical features reveals that erythematous patches represent the most prevalent manifestation across both patient groups, followed closely by hypopigmented patches. In addition, a total of six cases of pure neuritic leprosy were identified, with four cases classified under Group A (study group) and two cases under Group B (control group). HPP’s: Hypo-pigmented patches.
The predominant histopathological finding was Borderline Tuberculoid leprosy, with additional cases classified as Midborderline, Borderline Lepromatous, and Indeterminate. Notably, two patients from each group did not undergo a biopsy [Figure 2].
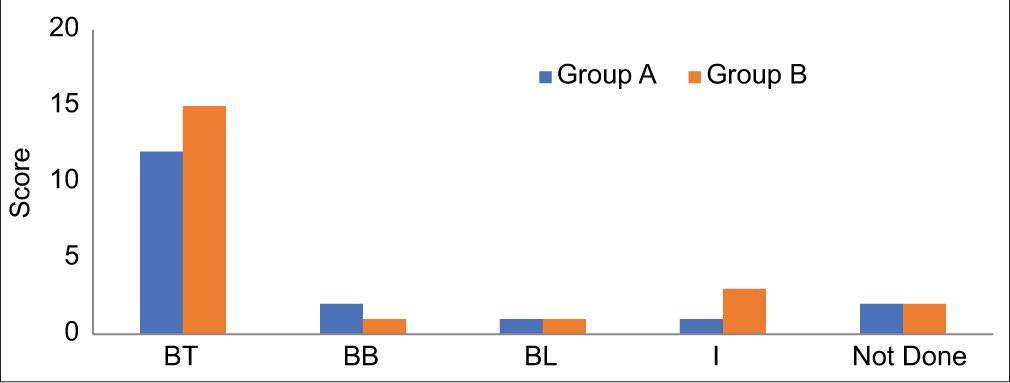
- In the comparative analysis of histopathological findings, borderline tuberculoid leprosy (BT) emerged as the most prevalent diagnosis. In addition, other classifications such as midborderline (BB), borderline lepromatous (BL), and indeterminate (I) were also identified. It is noteworthy that two patients from each of these categories did not undergo a biopsy. Group A: Study group, Group B: Control group.
At baseline, abnormal TST results were recorded in eight cases from Group A and three from Group B. By the final assessment, abnormal TST findings decreased to seven in Group A and increased to five in Group B [Figure 3].
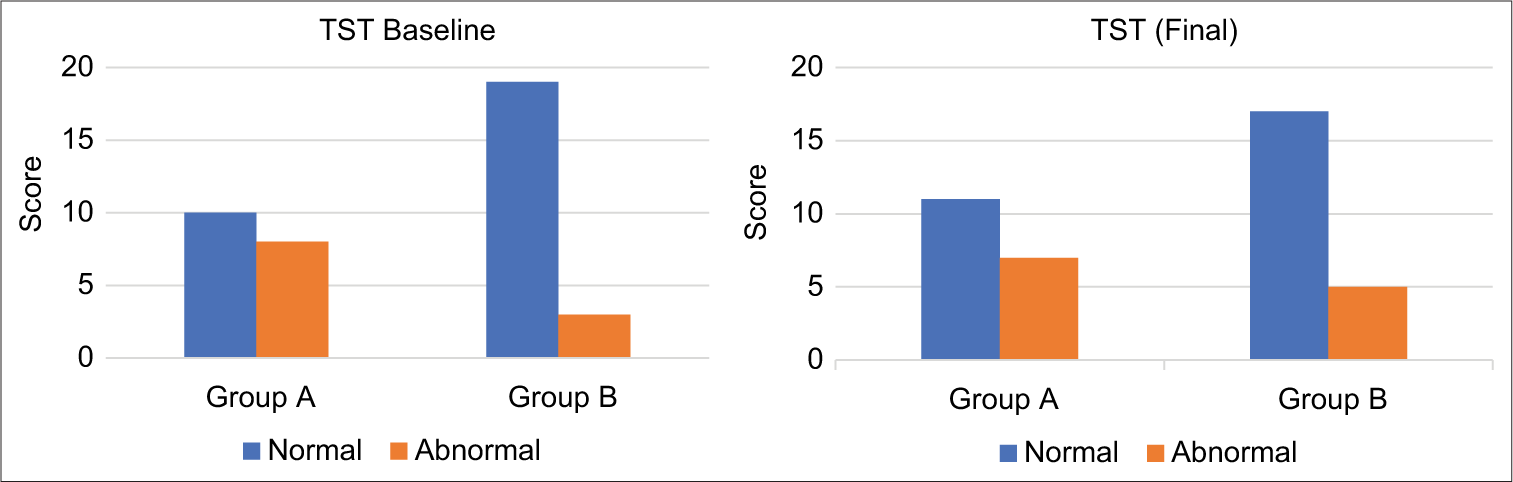
- The initial assessment of the touch sensibility test (TST) revealed that eight participants in Group A (study group) and three participants in Group B (control group) exhibited abnormal results. Upon final evaluation, the number of participants displaying abnormal TST results decreased to seven in Group A, while Group B demonstrated an increase, with five participants now recording abnormal results.
Similarly, at baseline, abnormal VMT was noted in eight cases from Group A and two from Group B, with the final counts showing a reduction to seven in Group A, while Group B maintained two [Figure 4].
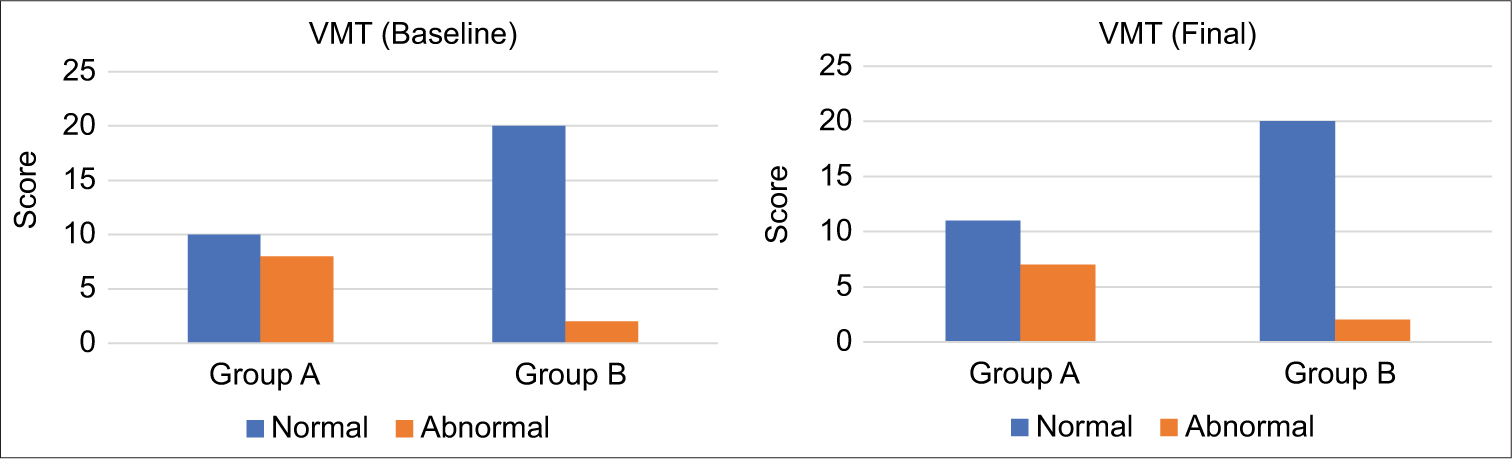
- The visual motor test (VMT) results indicate a concerning pattern in both groups studied. Initially, baseline abnormal VMT results were observed in eight cases within Group A (study group) and in two cases within Group B (control group). Upon concluding the assessment, final abnormal VMT results were recorded in seven cases in Group A, while Group B maintained a consistent two cases of abnormal results.
The higher prevalence of baseline reactional episodes in Group A likely contributed to the increased abnormalities observed in TST, VMT, and NCV metrics. Notably, one case in Group A demonstrated improvement in both TST and VMT, whereas TST deteriorated in two cases from Group B, suggesting a potential prophylactic benefit of steroid treatment. Furthermore, NCV improved in one case within Group A, while it worsened in three cases in Group B, further supporting the utility of prophylactic steroids in managing leprosy-related complications [Figure 5].
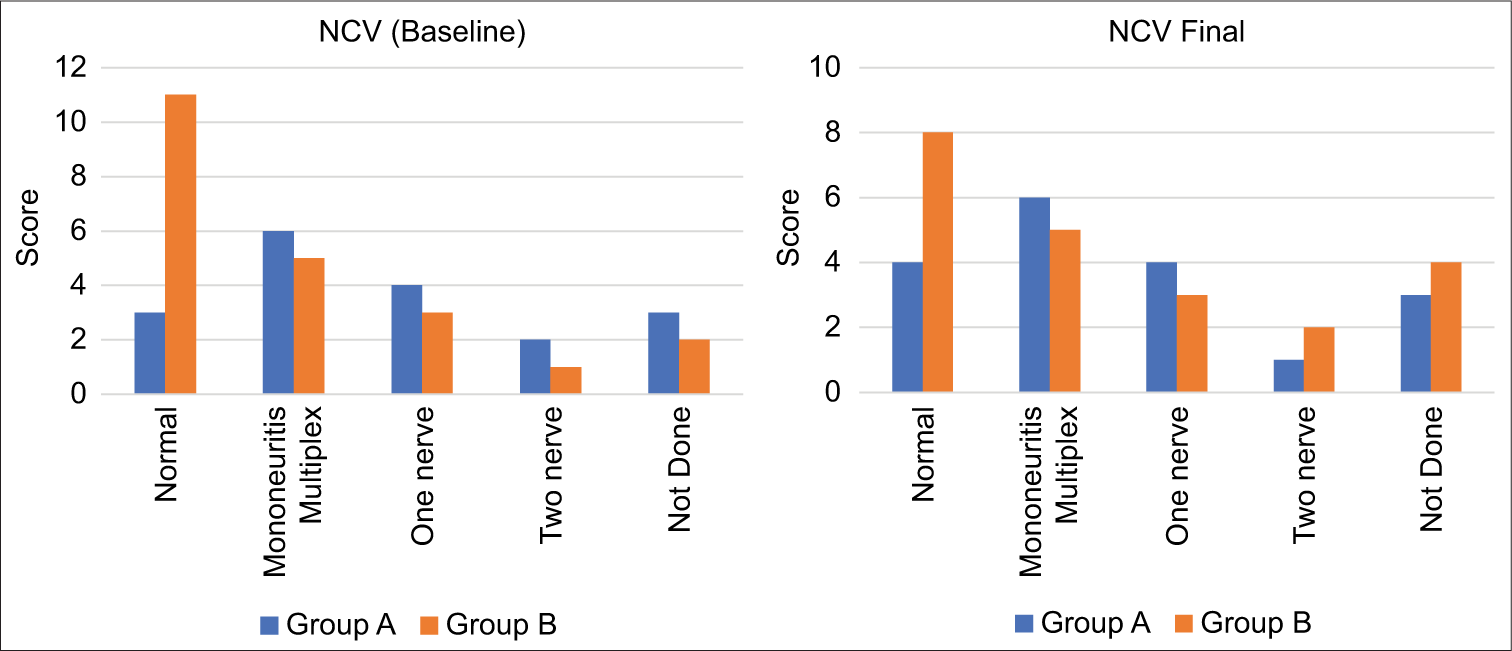
- The nerve conduction velocity (NCV) results indicate a concerning pattern in both groups studied. Initially, baseline NCV results were observed within Group A (study group) and Group B (control group).
DISCUSSION
NFI is a consequence of leprosy, mostly in cases of type 1 reactions. It affects peripheral nerves, causing sensory, motor, and autonomic loss of function. Nerve damage can occur before, during, or after effective antibiotic anti-leprosy treatment.[8] Several large cohort studies have shown that most leprosy patients have demonstrable nerve damage.[9,10] The use of steroids in the management of leprosy, particularly to prevent new reactions and mitigate NFI during MDT has garnered attention in recent literature. A notable study by Pai et al. (2012) provided a thorough review of the effects of steroids administered at various dosages and durations for the treatment of NFI associated with leprosy.[11] Specifically, a randomized control trial known as TRIPOD 1 examined the efficacy of low-dose prednisolone (20 mg) as prophylaxis during the initial 4 months of MDT. The results indicated a reduction in the incidence of new reactions and NFI in the short term; however, this beneficial effect was not sustained beyond 1 year. This observation raises critical questions regarding the timing and duration of steroid administration in conjunction with MDT. Smith et al. (2004) highlighted that the presence of NFI at diagnosis may influence the overall response to low-dose steroids, suggesting a complex interplay between disease progression and therapeutic intervention.[1] We sought to evaluate the prolonged impact of low-dose steroid prophylaxis over 24 months in MB cases. Our findings affirm that prophylactic steroid use enhances nerve function among patients, in sharp contrast with the deterioration observed in those who did not receive such prophylaxis. These results underscore the need for further research with a larger sample population to systematically explore the implications of extended low-dose steroid prophylaxis combined with MDT. The potential to improve long-term outcomes in patients with leprosy through refined treatment protocols remains a critical area of inquiry in the field of infectious diseases. Ultimately, these studies may pave the way for more effective strategies for managing leprosy and its associated complications, thereby enhancing the quality of care for the affected individuals.
Limitations
The small sample size was a limitation in this study.
CONCLUSION
In this study, the positive effects observed with prophylactic steroids in improving nerve function in patients with leprosy. Further, larger studies are needed to validate our findings.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Steroid prophylaxis for prevention of nerve function impairment in leprosy: Randomised placebo controlled trial (TRIPOD 1) BMJ. 2004;328:1459.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive assessment of multibacillary leprosy: A prospective study at a tertiary care center in Chennai. J Cardiovasc Dis Res. 2024;15:184-9.
- [Google Scholar]
- Leprosy: Treatment and management of complications. J Am Acad Dermatol. 2020;83:17-30.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment and evaluation advances in leprosy neuropathy. Neurotherapeutics. 2021;18:2337-50.
- [CrossRef] [PubMed] [Google Scholar]
- Prednisolone adverse events in the treatment and prevention of leprosy neuropathy in two large double blind randomized clinical trials. Lepr Rev. 2021;92:236-46.
- [CrossRef] [Google Scholar]
- Effect of steroid prophylaxis on nerve function impairment in multi-bacillary leprosy patients on MDT-MB. Indian J Lepr. 2015;87:133-43.
- [Google Scholar]
- Corticosteroids for treating nerve damage in leprosy. Cochrane Database Syst Rev. 2016;2016:CD005491.
- [CrossRef] [PubMed] [Google Scholar]
- Nerve damage in leprosy: A continuing challenge to scientists, clinicians and service providers. Int Health. 2012;4:77-85.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a self-help intervention to promote the health and wellbeing of marginalised people including those living with leprosy in Nepal: A prospective, observational, cluster-based, cohort study with controls. BMC Public Health. 2021;21:873.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic aspects of leprosy-neglected but important. Trans R Soc Trop Med Hyg. 2019;113:813-7.
- [CrossRef] [PubMed] [Google Scholar]
- A study of standardized regimens of steroid treatment in reactions in leprosy at a referral centre. Indian J Lepr. 2012;84:9-15.
- [Google Scholar]







