Translate this page into:
Hand-foot syndrome secondary to sorafenib in a case of breast carcinoma

*Corresponding author: Shilpi Tyagi, Department of Dermatology, All India Institute of Medical Sciences, Rajkot, Gujarat, India. tshilpi94@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Sihag Y, Tyagi S, Singh A. Hand-foot syndrome secondary to sorafenib in a case of breast carcinoma. CosmoDerma. 2024;4:89. doi: 10.25259/CSDM_111_2024
A 70-year-old female on sorafenib for the treatment of carcinoma breast (left side) presented with acute-onset painful erythematous macules over the dorsal as well as palmar aspect of hands [Figure 1a]. Simultaneously, the patient developed large painful erythematous scaly plaques with multiple superficial erosions involving the genitals and extending over the medial aspect of both thighs[Figure 1b]. Her quality of life was significantly affected as the lesions interfered with dayto-day activities. The patient was diagnosed as a case of hand-foot syndrome (HFS) secondary to sorafenib. She was started on topical corticosteroids and emollients with cessation of sorafenib and responded well with lesions resolving in 8–10 days. Later, chemotherapy was resumed, with regular application of emollients, without another episode of recurrence.
HFS, also known as palmar-plantar erythrodysesthesia or Burgdorf syndrome, is a painful reversible cutaneous adverse effect noted with oral multikinase inhibitors such as sorafenib. It presents as painful erythema, papules, plaques, desquamation, blisters or ulceration over hands and feet and rarely over the genitals.[1,2]
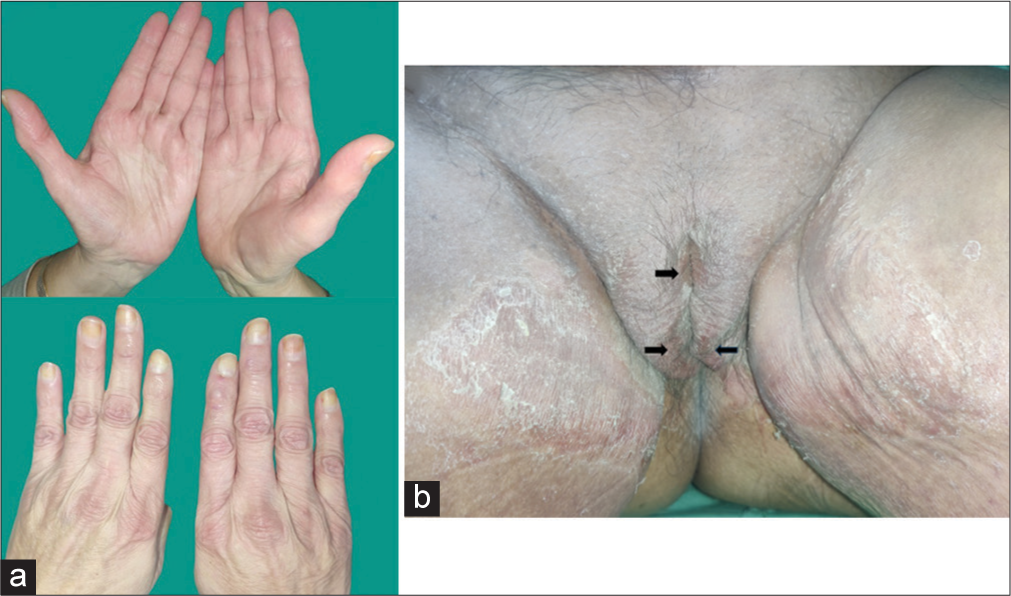
- (a) Tender erythematous macules over the palmar and dorsal aspect of both hands and (b) well-defined erythematous plaques with overlying scaling and erosions (arrow) over the genitals (involving labia majora and mons pubis) and medial aspect.
The pathogenesis of hand-foot syndrome is not known. It is hypothesized to occur due to the local accumulation of chemotherapeutic drugs that bring about degeneration and necrosis of eccrine glands of hands and feet.[3] It is not a life-threatening condition but severely impairs the quality of life in these patients.[2] It can be managed by emollients, topical corticosteroids, and cold compresses along with dose reduction or cessation of the chemotherapeutic drug until remission, generally resolving in two weeks.[3] A close mimicker of HFS is hand-foot skin reaction that occurs secondary to multikinase inhibitors [Table 1].[4,5]
| HFS | HFSR | |
|---|---|---|
| Site | Acral area (palms>soles), rarely genital area | Intertriginous and pressure bearing areas (soles>palms) |
| Clinical Presentation | Erythema, edema, fissuring, and desquamation | Blisters with reddish halo, followed by appearance of hyperkeratosis |
| Drugs | Chemotherapeutic drugs (e.g., Methotrexate, capecitabine, cytarabine, and 5-fluorouracil) | Multikinase inhibitors (e.g., Sorafenib) |
Sorafenib at dose <300 mg twice a day is infrequently associated with HFSR, higher doses such as 300–600 mg twice a day are associated with increased severity of HFSR, and dose limiting toxicity was noted at doses higher than 600 mg twice a day. Meta-analysis has shown relatively higher frequency of HFS secondary to sorafenib as compared to HFSR secondary to sorafenib. HFS: Hand-foot syndrome, HFSR: Hand-foot skin reaction
The present case shows the involvement of the palms and the genitals, the latter being a rare presentation of HFS.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Hand-foot skin reaction induced by sorafenib in a hepatocellular carcinoma patient. Postgrad Med J. 2020;96:495.
- [CrossRef] [PubMed] [Google Scholar]
- Management of cytotoxic chemotherapy-induced hand-foot syndrome. Oncol Rev. 2020;14:442.
- [CrossRef] [PubMed] [Google Scholar]
- Do you know this syndrome? Hand-foot syndrome. An Bras Dermatol. 2017;92:131-3.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: Experience in breast cancer. Oncologist. 2011;16:1508-19.
- [CrossRef] [PubMed] [Google Scholar]
- Sorafenib-associated hand-foot syndrome treated with topical calcipotriol. JAAD Case Rep. 2017;3:354-7.
- [CrossRef] [PubMed] [Google Scholar]





