Translate this page into:
The efficacy, safety, and application of platelet-poor plasma gel as an autologous dermal filler for esthetic enhancements and facial rejuvenation – A pilot study

*Corresponding author: Shivani Bhardwaj, Department of Dermatology, Venereology and Leprosy, Pacific Medical College and Hospital, Udaipur, Rajasthan, India. shivani00444@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nethaji B, Bhardwaj S, Deora MS, Vaishampayan S, Patra A, Sharma M, et al. The efficacy, safety, and application of platelet-poor plasma gel as an autologous dermal filler for esthetic enhancements and facial rejuvenation – A pilot study. CosmoDerma. 2025;5:42. doi: 10.25259/CSDM_221_2024
Abstract
Objectives
This study aims to evaluate the efficacy, safety, and application of platelet-poor plasma gel (PPP gel) as an autologous dermal filler for esthetic enhancements and facial rejuvenation. The primary objective is to assess the subjective esthetic improvement using the global esthetic improvement (GAI) scale and to evaluate the safety profile. The secondary objective is to evaluate the patients’ satisfaction scores to highlight both the psychological and esthetic benefits.
Materials and Methods
Thirty patients, who presented with various facial concerns such as tear trough deformity, facial aging, facial asymmetry, scars like acne scars, post-burn scar and post-varicella scar, and facial rejuvenation, who attended the dermatology and venereology outpatient department at our tertiary care hospital received the treatment after taking a written and informed consent. Each treatment involved a thorough patient evaluation, preparation of the PPP gel from the patient’s own blood, and precise injections according to their individual requirements. They were assessed clinically before treatment sessions and at the end of the follow-up period of two months. Statistical analysis was performed to confirm the significance of this study.
Results
Most of the patients had a significant clinical improvement immediately post-procedure that was maintained until the end of the follow-up period in 53.3% of the patients. This finding was confirmed by the statistically significant difference in the GAI scores of pre- and post-treatment and P < 0.05, proving that autologous PPP gel is efficacious. Mild erythema, pain, and burning sensation were noted immediately post-procedure but these were short lived. None of the patients had any other side effects or serious complications during the follow-up period. The overall satisfaction score (using a Likert scale) of 3.52 indicates a high level of patient contentment with the PPP gel procedure, reflecting positive experiences and outcomes.
Conclusion
This article introduces a novel aspect of esthetic dermatology, highlighting the potential of autologous PPP gel as an innovative, biocompatible, and cost-effective alternative to synthetic biofillers. By promoting collagen synthesis and skin rejuvenation while minimizing hypersensitivity risks, PPP gel presents a promising advancement in regenerative esthetics. However, its temporary volumizing nature and lack of immediate reversibility, if injector-induced complications are encountered, necessitate further clinical exploration. Future research on long-term efficacy and safety, comparative trials, and hybrid formulations may refine the standardization of preparation and properties of PPP gel. As esthetic medicine advances, integrating PPP gel biofillers could enhance cost-effectiveness, personalization, and treatment outcomes.
Keywords
Biofiller
Dermal filler
Facial rejuvenation
Global esthetic improvement scale
Platelet poor plasma gel
INTRODUCTION
Facial rejuvenation procedures have become increasingly popular in recent years, driven by a growing desire for esthetic enhancement and the correction of skin imperfections. As the demand for non-surgical interventions rises, practitioners are continuously seeking effective and safe options that cater to diverse patient needs. Among various modalities, autologous dermal fillers have gained prominence due to their biocompatibility, cost-effectiveness, and lower risk of adverse hypersensitivity reactions. Notably, platelet-poor plasma gel (PPP gel) has emerged as a valuable alternative, offering a cost-effective solution for facial augmentation and rejuvenation.[1]
The history of dermal fillers dates back to the early 20th century with materials such as paraffin and silicone, which, despite their innovation, often caused complications. The introduction of collagen-based fillers in the 1980s improved biocompatibility, but the demand for longevity led to the rise of hyaluronic acid (HA) fillers in the late 1990s, valued for their safety and reversibility. The shift toward autologous fillers, such as PPP gel, reflects the field’s evolution toward natural approaches.[2] Activated by thermal treatment, PPP gel forms a stable, fibrin-rich material with enhanced consistency and volume, offering a promising alternative in regenerative esthetics.[3]
The aim of this study is to evaluate the efficacy and safety profile of PPP gel in facial rejuvenation along with patients’ satisfaction. By utilizing the global esthetic improvement scale (GAIS) and assessing patient satisfaction scores with the Likert scale, we aim to provide a comprehensive understanding of the psychological and esthetic benefits of this innovative treatment.
MATERIALS AND METHODS
A total of 30 patients were enrolled in our study as per the convenience sampling method. Patients between 20 and 65 years of age with facial volume loss, scars, or requiring facial rejuvenation were included in our study excluding patients with unrealistic expectations, chronic illnesses, and who have undergone any other cosmetic procedures within the preceding two months. All patients were subjected to full history taking and dermatological examination.
Outcome measures
The primary outcome measures were the improvement in facial volume, contour, skin texture, reduction in fine lines and wrinkles, and improvement in scars, assessed using standardized photographic analysis, and the GAIS for assessment. The analysis of the results was performed by a double analyst approach where the primary author and a blinded analyst independently evaluated the results to prevent subjective observer bias. Inter-rater agreement was measured using Cohen’s kappa to ensure the reliability of subjective assessment.
The secondary outcome measure will be the patient satisfaction assessed using standardized questionnaires and a Likert scale.
Safety assessment
Immediate side effects, late adverse events, and complications were monitored throughout the study period and any adverse events or complications were documented.
Evaluation parameters
High-quality digital-colored photographs were taken for each participant before, immediately post-procedure, and at the end of the two-month follow-up period. Digital image analysis of the photographs was done to determine the percentage of improvement according to GAIS, as mentioned in Table 1. GAIS is a subjective assessment tool that rates the overall improvement in the appearance of the treated area on a 5-point scale, ranging from “worse” to “much improved”. A standardized questionnaire was used to assess the patient’s esthetic and psychological satisfaction using a Likert scale to rate the responses, ranging from “very unsatisfied” to “very satisfied.”
| 5 (worse) | Appearance is worse than the initial condition |
| 4 (no improvement) | Appearance is essentially the same as the original condition. |
| 3 (improved) | Slight improvement |
| 2 (much improved) | Marked improvement, but not completely optimal |
| 1 (very much improved) | Optimal cosmetic result. |
GAIS: Global esthetic improvement scale
Ethics
Approval by the Institute Ethical Committee, Pacific Medical College and Hospital, Udaipur was obtained before the commencement of the study.
Date of issuance: June 01, 2023.
Reference no.: PMU/PMCH/IEC/PG/2023/244
Informed and written consent for the study and procedure was explained. Consent for use of their clinical photographs for publishing purposes was obtained from all patients.
PPP gel preparation
Ten milliliters of the patient’s blood is drawn and mixed with 1 mL anticoagulant acid citrate dextrose. A two-staged centrifuging is performed using a Digital 8R Dermafuge Centrifuge device (Derma India, Chennai). It is rotated at 2400 rotations per minute (RPM) for 5 min in the centrifuge machine. The plasma solution is withdrawn and centrifuged again at 2600 RPM for 10 min. The upper part (upper 2/3rd) that is platelet-poor plasma (PPP) is withdrawn in a tuberculin syringe or 2 mL syringe as per requirement and the lower part (lower 1/3rd) that is Platelet-rich plasma (PRP) is withdrawn in tuberculin/insulin (1 mL) syringes (which can be used for rejuvenation if required). Calcium gluconate (activator) is added to the PPP in the proportion of 0.1 mL/1 mL of PPP. It is incubated in hot water [Figure 1] (heated in a metal container/hot water bath) at 60°C–100°C (gradually reducing to 98°C) for 3–5 min and then in a cold water bath in a metal container with initial temperature 7°C and gradually increasing to 10°C for 10 min. The temperatures are measured by a digital thermometer. The viscous gel obtained is the PPP gel, a bio-filler [Figure 2].
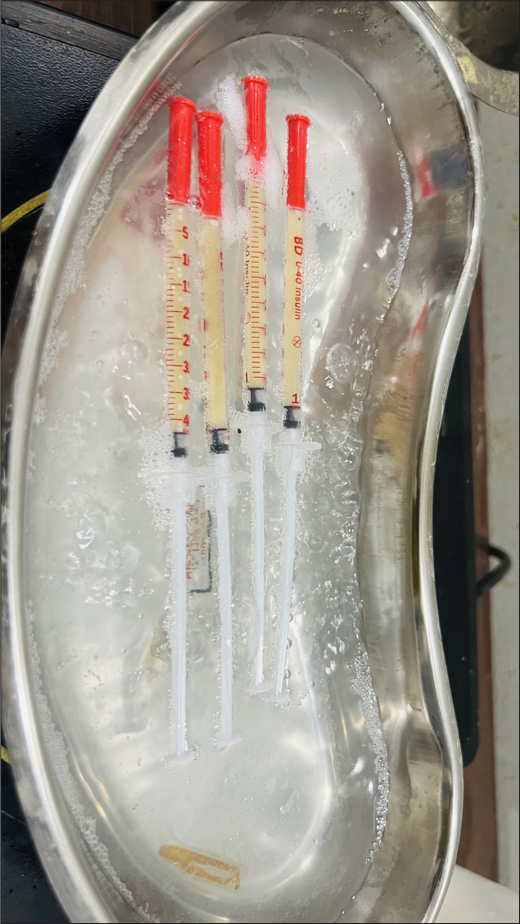
- Platelet-poor plasma gel preparation by thermal treatment with monitoring of temperature using a digital thermometer.
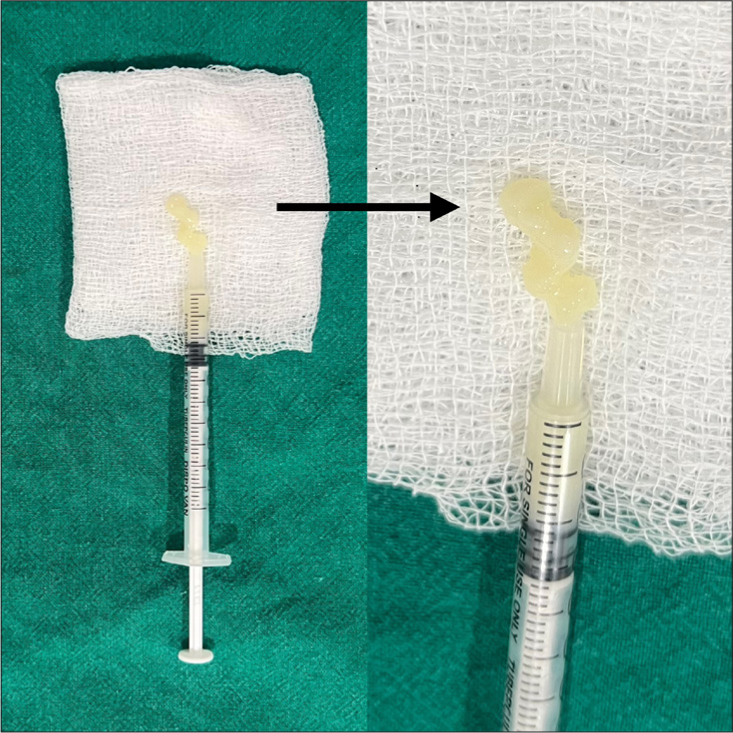
- Black arrow shows the prepared platelet-poor plasma gel.
Treatment procedure
After informed and written consent, topical 2.5% lidocaine plus 2.5% prilocaine anesthetic cream is applied over the patient’s face on the areas to be treated for 45 min (if required). Ten milliliters of the patient’s blood is drawn and mixed with 1 mL anticoagulant acid citrate dextrose. PPP gel is prepared as mentioned above. After cleaning the topical anesthetic cream, PRP is injected at multiple points over the forehead, cheeks, and chin through insulin syringes (This step is only done if required for rejuvenation to prevent wastage of PRP prepared). Then, PPP gel filler is injected in the deep dermis with tuberculin syringes with 25 G/27 G needle or 50 mm/38 mm blunt tip microcannula [Figure 3a and b] as per requirement at the desired site with appropriate needles and precautions. It is injected linearly along the desired site depositing the bio-filler while withdrawing the needle for lip augmentation (linear threading technique). Injections are placed away from blood vessels with facial anatomy kept in mind. The sites are gently massaged to allow uniform distribution of the injected plasma gel to maintain the contour of the surrounding tissues.
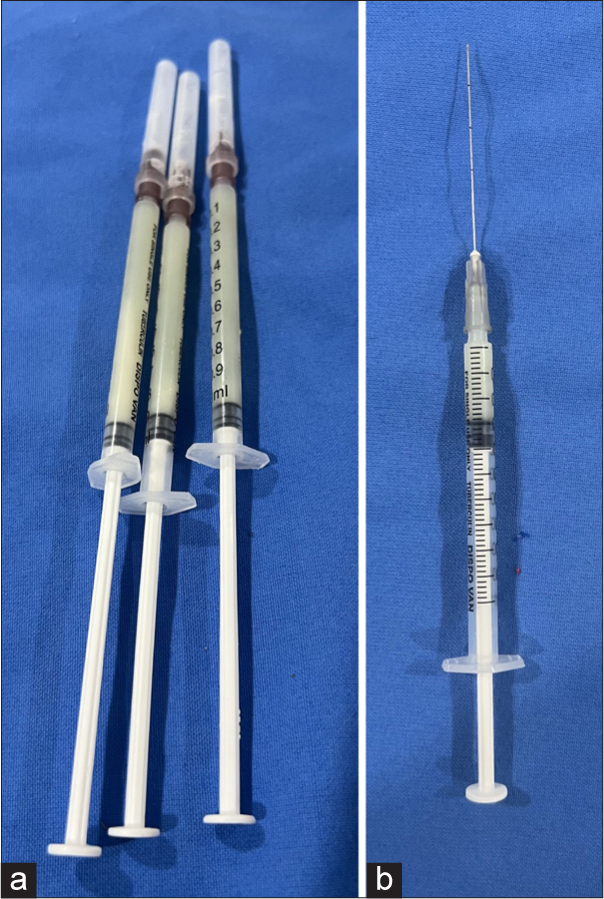
- (a) Platelet-poor plasma gel prepared after activation and thermal treatment in tuberculin syringes. (b) Microcannula of 50 mm attached to tuberculin syringe.
Post-session advice and follow-up
Following PPP gel treatment, patients are advised to adhere to post-treatment care guidelines to optimize results and minimize potential complications. It is recommended to avoid massaging or applying pressure to the treated area for 1 week and to refrain from strenuous physical activity for 24–48 h. The application of makeup or skincare products should be avoided for the first 24 h, while excessive sun exposure should be minimized if required with the use of a topical sunscreen. Patients should also refrain from manipulating the treated area and may apply a cold compress to alleviate swelling or bruising. Scheduled follow-up appointments were advised to be maintained to evaluate the treatment efficacy and monitor for any adverse reactions.
Statistical analysis
Statistical analysis for the assessment of the efficacy and safety of PPP gel as an autologous dermal filler for facial rejuvenation included both descriptive and inferential statistics.
Descriptive statistics involve measures of central tendency such as mean and median, as well as measures of variability. These statistics were used to summarize the characteristics of the sample population.
Inferential statistics, on the other hand, involves hypothesis testing and determining the statistical significance of the results. This included the calculation of the Wilcoxon signed-rank test (ordinal data) using SPSS and Excel software to compare pre- and post-treatment outcomes and to assess the efficacy of the treatment.
P < 0.05 was considered significant.
RESULTS
A total of 30 patients were included in our study with a male-to-female ratio of 1:1.6. The treated conditions included twelve patients for under-eye rejuvenation, eight patients with acne scars, four patients for chin augmentation, two patients each for lip augmentation and post-varicella scars, and one patient each with chemical scarring and facial hemiatrophy.
GAIS score point reduction in pre- and post-treatment scores are summarized in Graph 1. The majority of the patients had a two- to three-point reduction in GAIS. The conditions that exhibited the most significant four-point GAIS reduction post-treatment were facial hemiatrophy [Figure 4], lip augmentation [Figure 5], and chin augmentation [Figure 6]. Few cases of under-eye rejuvenation [Figures 7 and 8] and chemical scarring [Figure 9] also demonstrated substantial improvements with GAIS reductions of three to four points. The mean GAIS score significantly improved from 4.4 (pre-treatment) to 2 (post-treatment), reflecting an overall improvement of 54%. Effect size according to Cohen’s d was approximately 1.8 indicating a large treatment effect. The inter-rater agreement calculated using Cohen’s kappa shows that the chance-corrected agreement was 0.8, meaning their agreement was almost perfect.
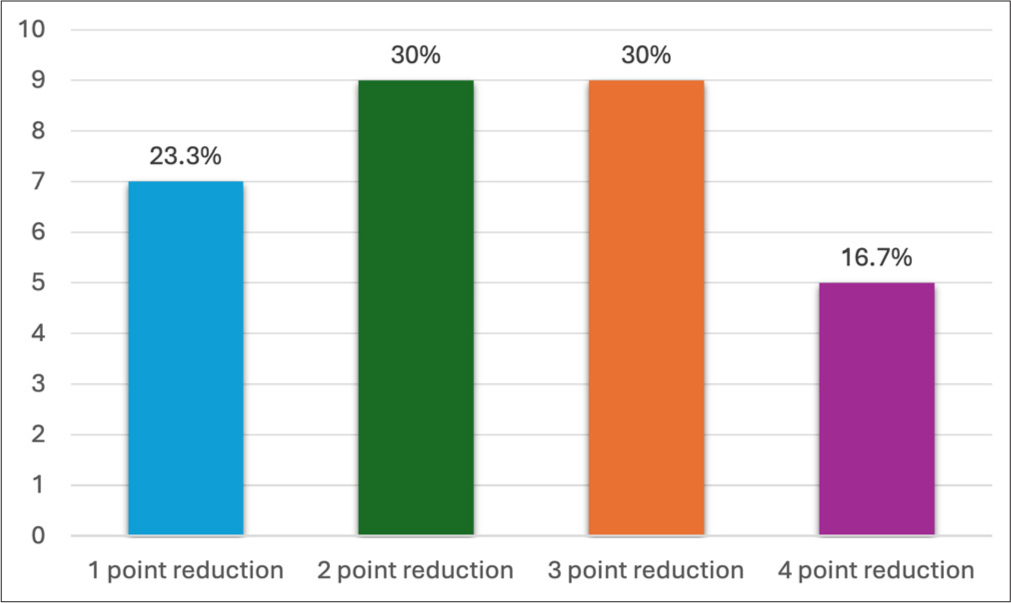
- Summarizes the global esthetic improvement scale (GAIS) point reduction percentage in study participants. X-axis shows the number of patients and Y-axis shows GAIS point reduction.
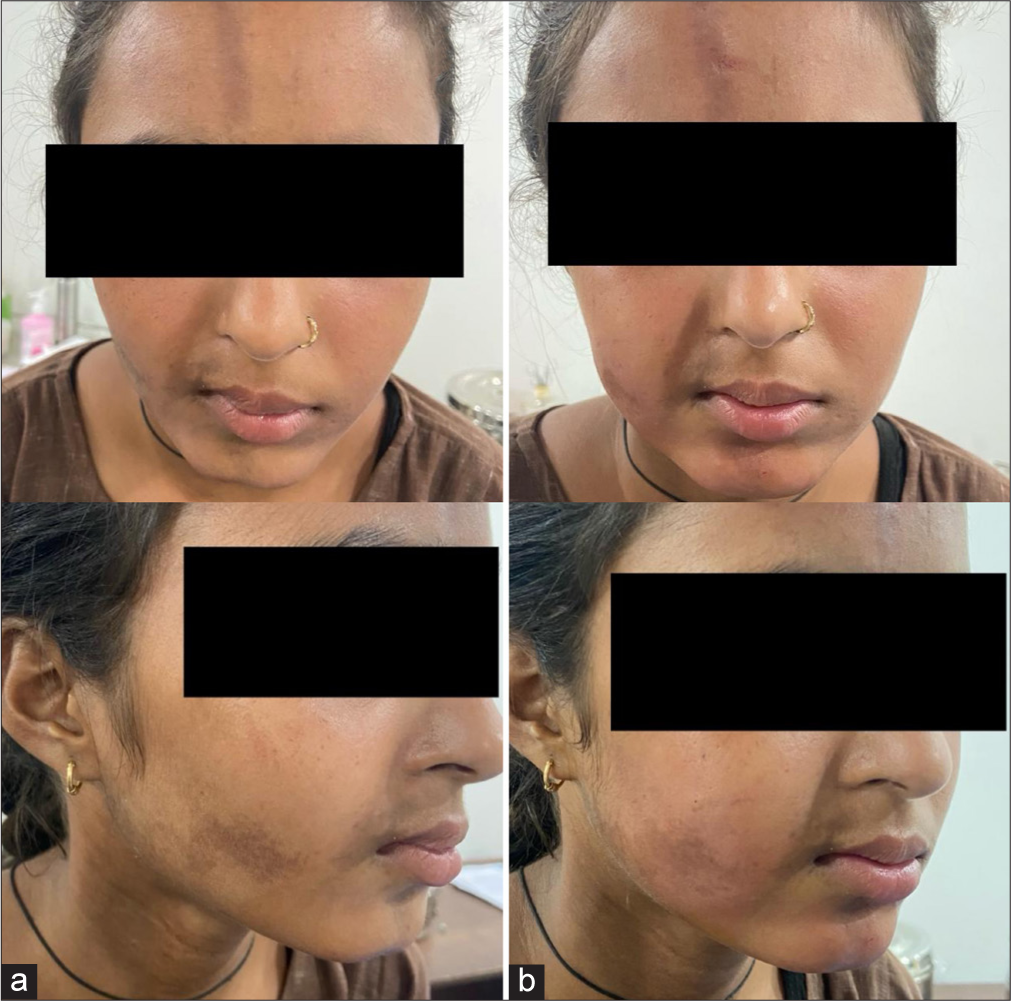
- A 23-year-old female with facial hemiatrophy due to morphea (a) pre-treatment and (b) post-treatment.
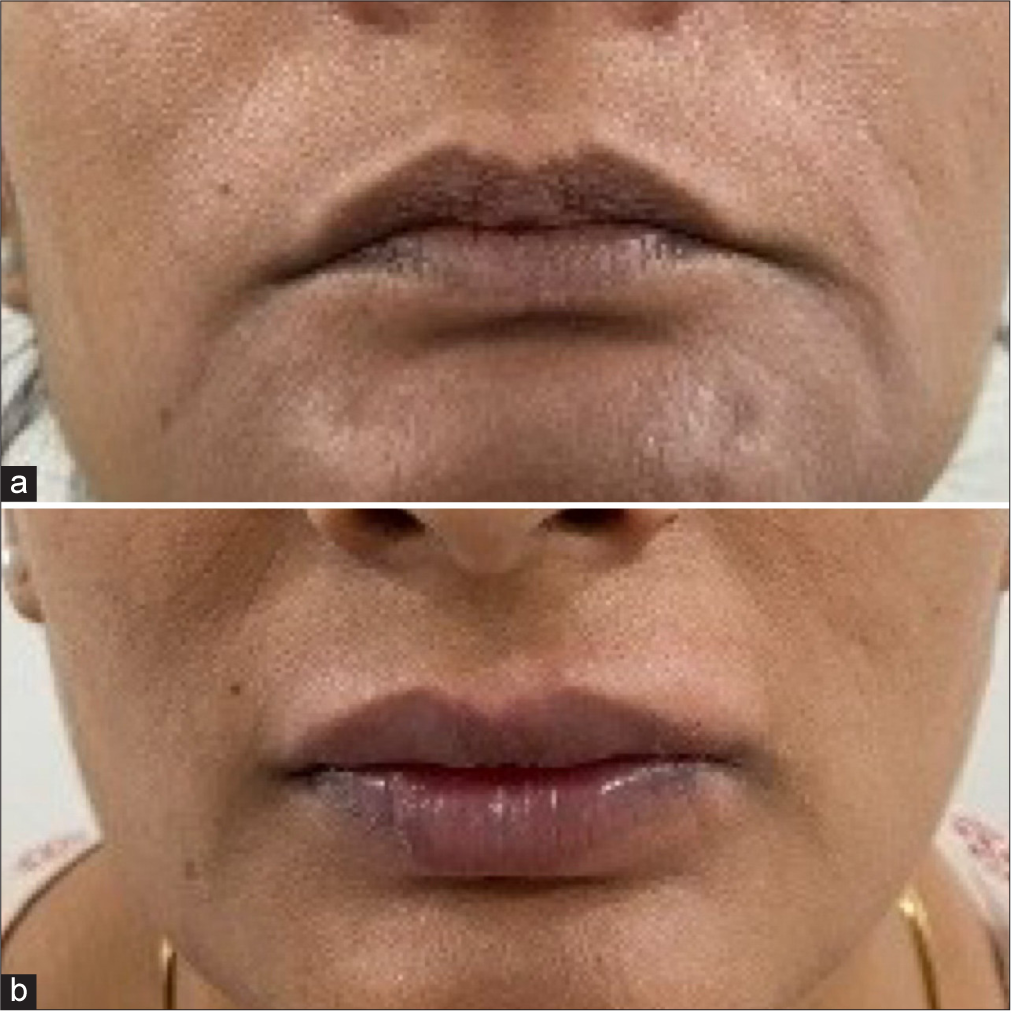
- A 32-year-old female for lip augmentation (a) pre-treatment and (b) post-treatment.
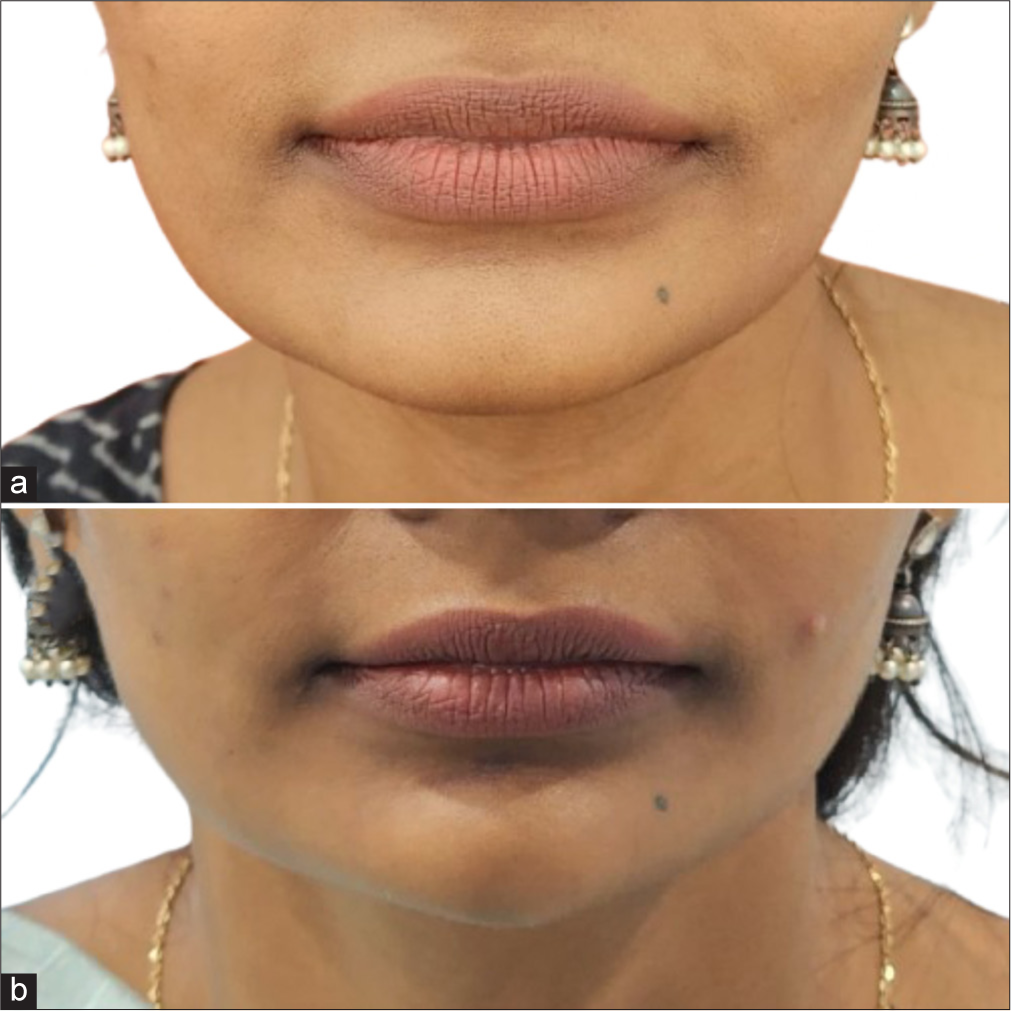
- A 26-year-old female for chin augmentation (a) pre-treatment and (b) post-treatment.
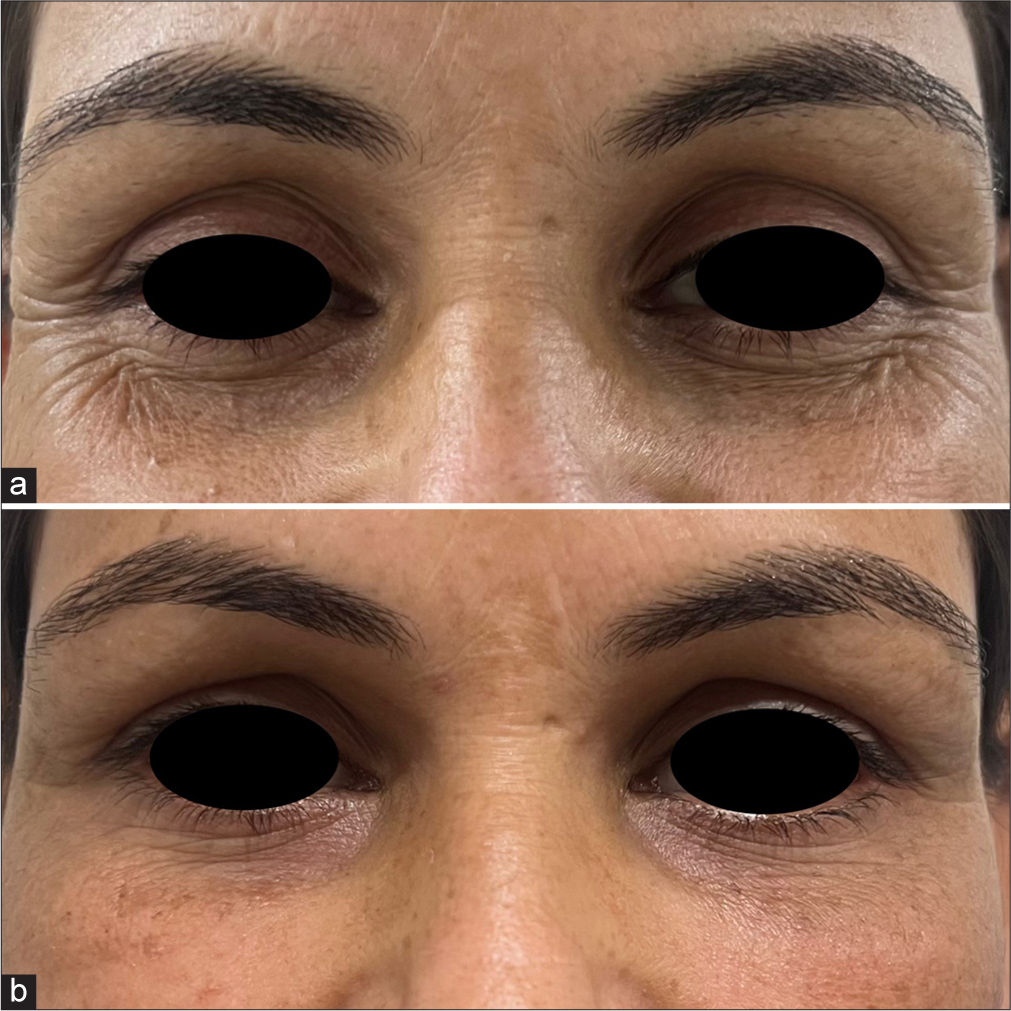
- A 37-year-old female with tear trough deformity and crow’s feet (a) pre-treatment and (b) post-treatment.
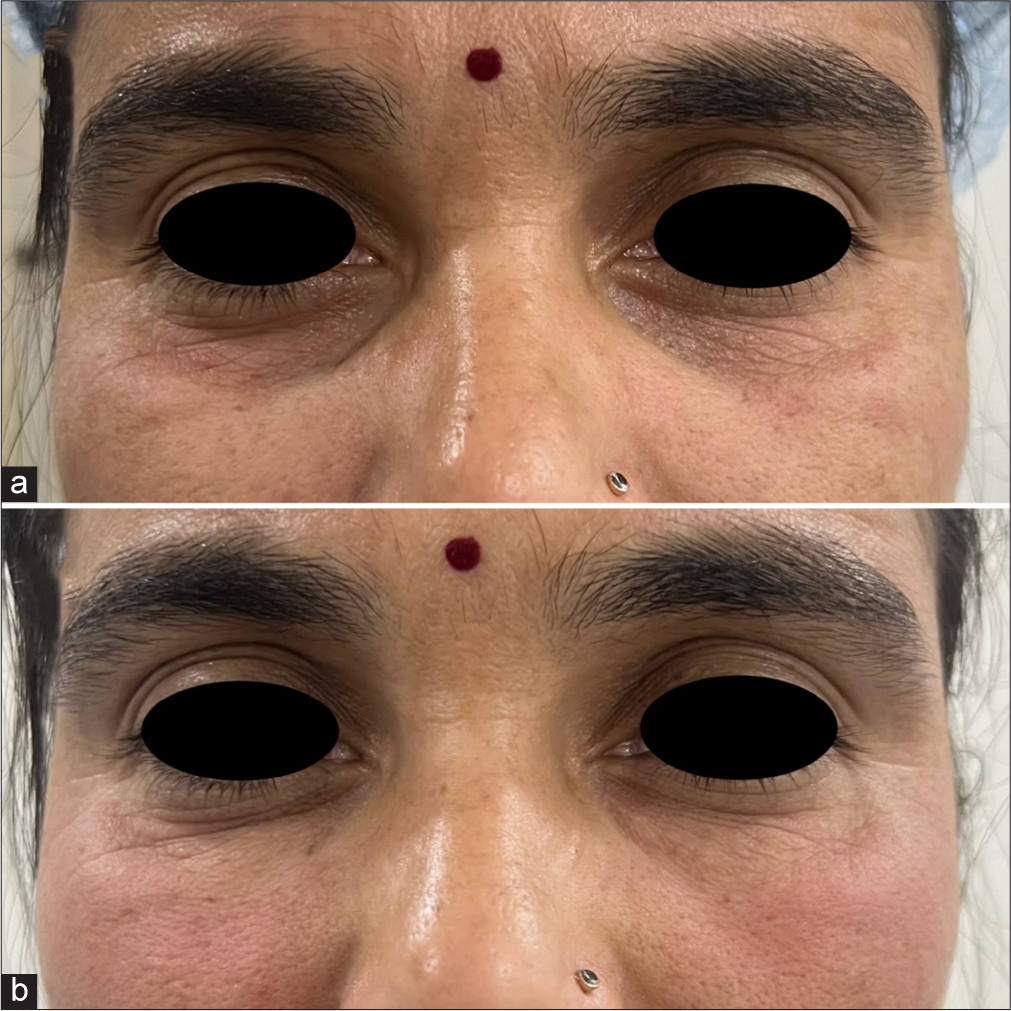
- A 34-year-old female with tear trough deformity (a) pre-treatment and (b) post-treatment.
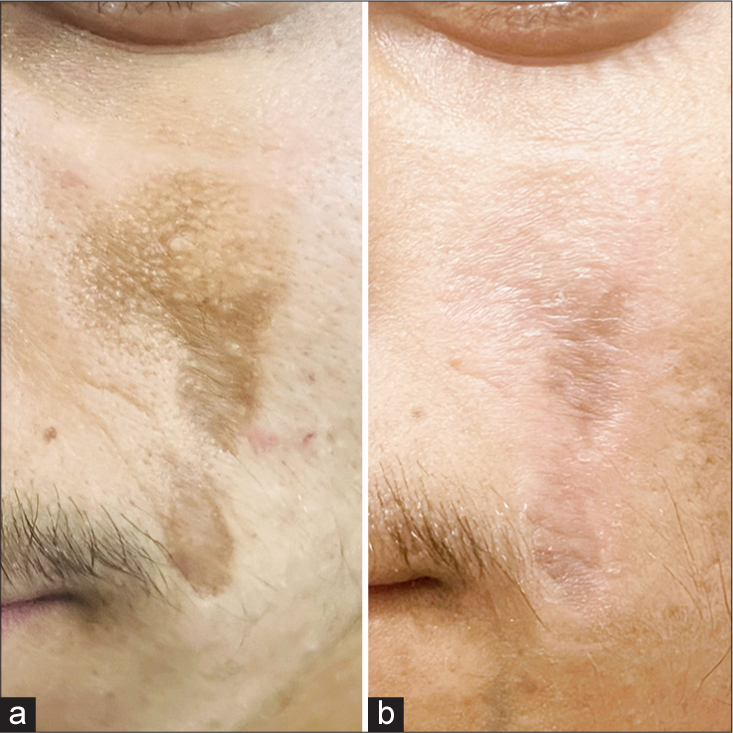
- A 20-year-old male with chemical burn scar due to choona application (a) pre-treatment and (b) post-treatment.
The mean patient satisfaction score was 3.52/5 with an overall satisfaction rate of 70.4%. The highest satisfaction rates were observed in patients who underwent chin augmentation, under-eye rejuvenation, facial hemiatrophy correction, and lip augmentation.
Wilcoxon signed-rank test results demonstrated a statistically significant difference between pre- and post-treatment GAIS scores where Wilcoxon test statistic (W) was 465.0 and the calculated p-value was ~1.86 × 10−9, that is <0.05, which strongly suggests autologous PPP gel to be efficacious.
Follow-up was done for a duration of two months post-procedure. There was a gradually progressing decrease in the filling and volumetric effect of the autologous PPP gel and 53.33% of the patients sustained the same overall response until the end of two months of follow-up duration. The majority of the patients with acne scars [Figure 10] and post-varicella scars [Figure 11] had a reduction in effect after one month of treatment. Patients who underwent under-eye rejuvenation, lip and chin augmentation, and facial hemiatrophy correction showed better maintenance of the overall results. Three patients – two cases of acne scars and one case of post-varicella scars were lost to follow-up probably due to the low overall improvement seen post-procedure.
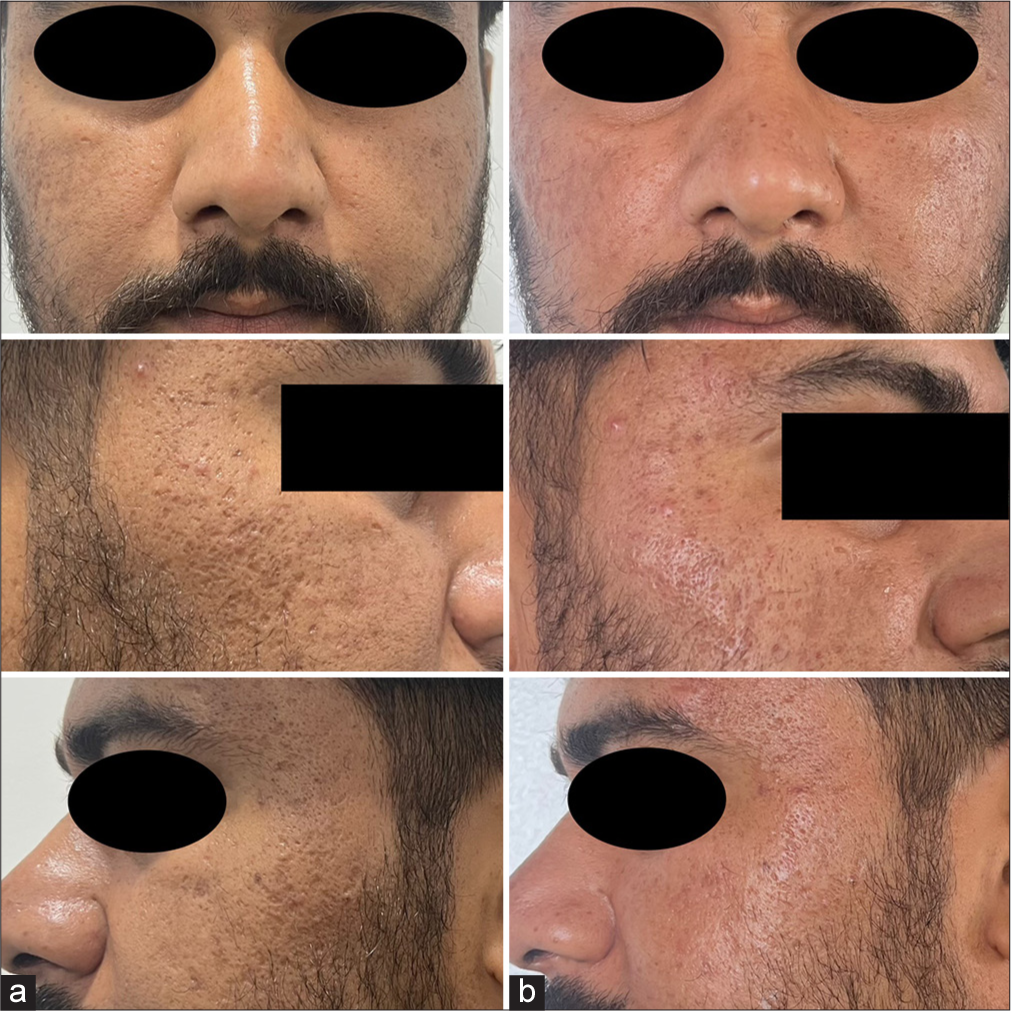
- A 27-year-old male with acne scars (a) pre-treatment and (b) post treatment.
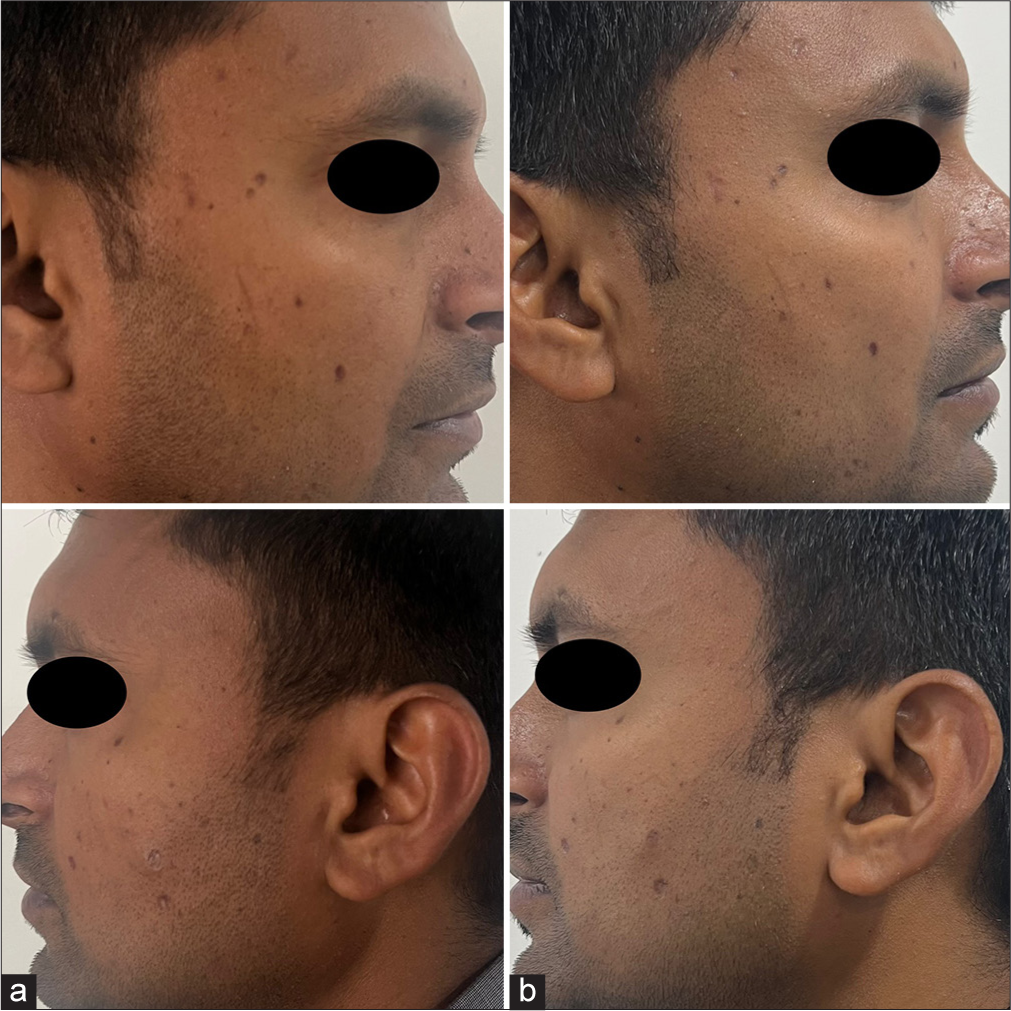
- A 28-year-old male with post post-varicella scars (a) pre-treatment and (b) post treatment.
The most commonly observed side effect was transient erythema [Figure 12], followed by injection site edema [Figure 13], pain, and burning sensation. These side effects were self-limiting which resolved within a few hours post-procedure. No severe adverse events or complications were reported during the two-month follow-up period. Almost all patients experienced injection site pain and erythema while the least common side effect noted was asymmetry due to overfilling on one side in an under-eye rejuvenation patient [Figure 14], which was corrected by manual manipulation. There were no reported complications or adverse effects such as vascular occlusion, ecchymosis, infection, nodules, foreign body granuloma formation, or tissue necrosis.
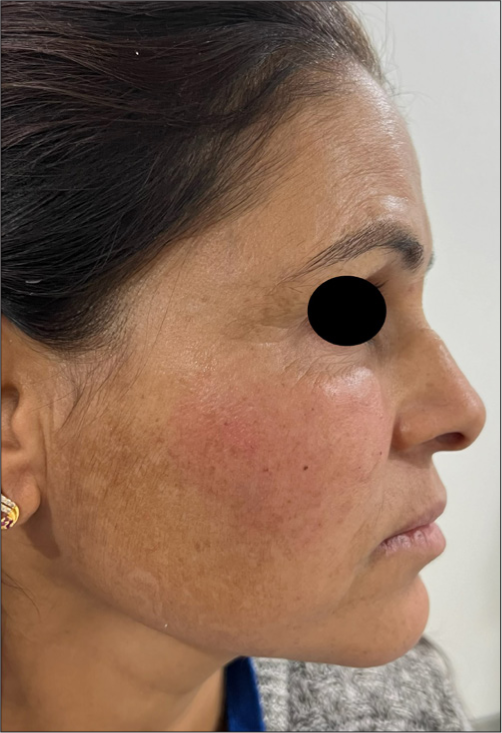
- Transient erythema immediately post-procedure.
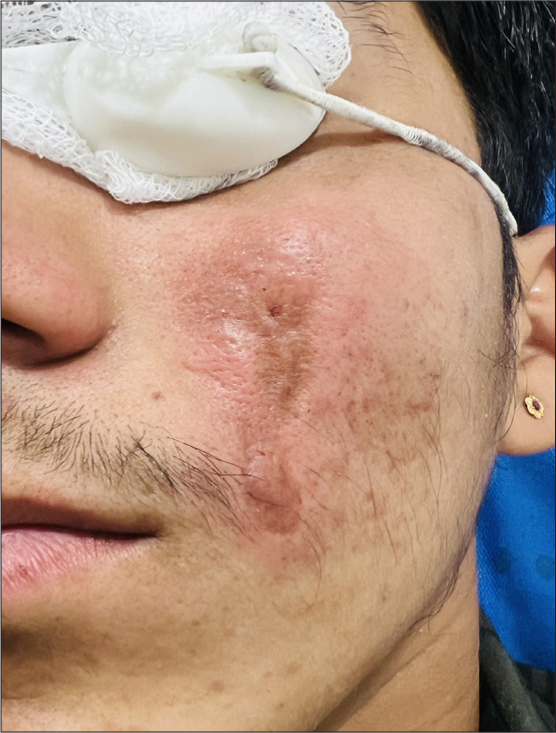
- Erythema with localized edema immediately post-procedure.
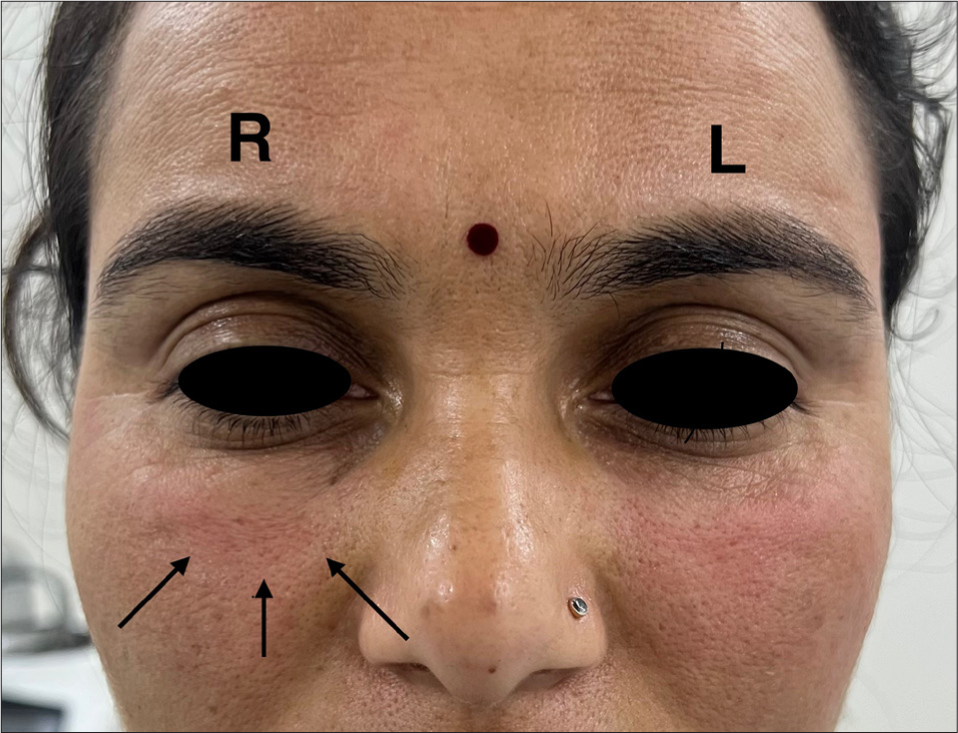
- Asymmetry due to overfilling of platelet-poor plasma gel over right side (R) in comparison to the left side (L). Black arrows shown point the area overfilled.
DISCUSSION
The evolution of dermal fillers has progressed significantly over the past century. In the early 20th century, paraffin and liquid silicone were among the first substances used for facial augmentation. By the 1960s, collagen-based fillers derived from animal sources (bovine collagen) became popular, though they had limitations in longevity and potential allergic reactions. The 1990s saw the introduction of HA fillers, which were biocompatible and naturally integrated into the skin and are still being routinely used worldwide. The 2000s brought longer-lasting fillers, including cross-linked HA, calcium hydroxyapatite, and poly-L-lactic acid, offering enhanced durability and volume restoration. In the 2010s, the focus shifted to customization and non-surgical facelifts followed by advancements in fat transfer and biostimulatory fillers. The 2020s have been marked by the rise of longer-lasting HA fillers, PRP integration, and personalized treatments, along with the emergence of biofillers utilizing PPP, representing the latest innovation in regenerative esthetics.[2]
Biofillers being natural dermal fillers can be classified into synthetically prepared HA fillers, collagen-based fillers, and those derived from natural substances or autologous fillers. Autologous dermal fillers are products that are derived from the patient’s own tissues, primarily used for facial volume restoration and skin rejuvenation. Unlike synthetic fillers, these fillers utilize materials such as fat or blood components taken from the patient, ensuring high biocompatibility and minimizing the risk of allergic reactions. Common types of blood-derived fillers such as PRP, PPP or platelet-rich fibrin harness the regenerative properties of growth factors in the blood. The use of autologous materials allows for natural-looking results while promoting healing and stimulating collagen production, making them a popular choice in esthetic medicine.[4]
PPP although contains a lower concentration of platelets, is composed of 90% water, contains essential proteins (albumin, fibrinogen, and immunoglobulins), electrolytes (sodium, potassium, and calcium), gases (oxygen and carbon dioxide), essential nutrients (glucose, amino acids, and lipids), and hormones. Key growth factors such as transforming growth factor-β, fibroblast growth factors, platelet-derived growth factors, epidermal growth factors, and insulin-like growth factors in PPP play a crucial role in volumizing and rejuvenation. In addition, hepatocyte growth factor enhances tissue healing, and interleukins regulate inflammation and tissue recovery making PPP a valuable tool in regenerative esthetics.[5]
Preparation of PPP using a double spin method in our study was done considering the rotor radius of our centrifuge machine. Activation of the prepared PPP by adding a clotting cascade activator such as calcium gluconate, calcium chloride, or thrombin, followed by thermal treatment, gives rise to the formation of a gelatinous material, PPP gel [Figure 1]. The thermal treatment can either be done in a hot water bath with a thermometer or an incubator fixed at the advised temperature. The duration of the heating process is directly proportional to the viscosity or the G prime of the PPP gel. That is, the more the duration, the higher the G prime.[6]
The primary mechanism of action for PPP gel involves volumization and stimulation of collagen production. On injection, PPP gel occupies space beneath the skin, filling in lines and hollows while simultaneously triggering a biological response that promotes the body’s own collagen synthesis due to fibrin bundles that provide a scaffold for platelets trapping them in the treatment area to serve as a source for sustained release of growth factors over time.[3,6] The gel gets absorbed gradually by the body over a period of time making it a temporary option for a natural volumizing effect.
PPP gel offers multiple advantages in esthetic treatments providing a natural volumizing effect similar to that of synthetic HA biofillers. As an autologous, biocompatible material, it eliminates the risk of allergic reactions and adverse hypersensitivity reactions. Its ability to stimulate collagen production enhances skin rejuvenation along with a long-term structural and textural improvements. In addition, PPP gel is well-tolerated, with minimal downtime, making it an effective alternative to traditional dermal fillers. Furthermore, its cost-effectiveness reduces the need for expensive synthetic biofiller, offering a sustainable and economical solution for people seeking esthetic enhancements.[1,3]
While biofillers are generally considered safe, it is crucial to acknowledge potential side effects and adverse reactions. Commonly reported side effects include transient erythema, localized edema, bruising, pain, and burning sensation at the injection site, which are typically mild and temporary. Although, there were no reports of any serious complications in this study, complications such as vascular occlusion and allergic reactions have been documented and require immediate attention.[7] Practitioners with a high level of expertise and training have significantly reduced the incidence of these complications. Awareness and appropriate management of these risks are vital for ensuring patient safety and satisfaction. By prioritizing thorough training and patient education, practitioners can help mitigate risks leading to a more positive overall experience for patients considering PPP gel.
PPP gel biofillers offer several advantages primarily due to their easy accessibility, biocompatibility, and cost-effectiveness. However, it is of utmost importance to understand that they are non-dissolvable in contrast to HA fillers that can be lysed with hyaluronidase and provide a better safety profile for serious complications.[8]
In comparison, HA fillers are often preferred over PPP gel due to their immediate volumizing effect, predictable results, and longer-lasting outcomes. HA fillers offer instant correction with reversible outcomes, making them a safer and more versatile option in esthetic treatments. In addition, HA fillers have Food and Drug Administration approval and extensive clinical research backing their efficacy and safety.[8]
The increasing societal emphasis on idealized beauty standards has placed significant pressure on the current generation to conform to specific esthetic norms, driving a growing demand for esthetic procedures as individuals seek to enhance their appearance and align with these expectations. Research indicates that improvements in physical appearance can lead to enhanced self-esteem and quality of life. Patients frequently report increased confidence and greater social engagement following treatment.[9] The patient satisfaction scores in this study illustrate this trend, highlighting the importance of addressing both the physical and emotional aspects of dermatological care.
As the field of esthetic medicine evolves, ongoing research into biofillers continues to yield innovative formulations. For example, hybrid fillers that combine different types of biofillers are being explored to enhance efficacy and longevity.[10] The versatility of autologous dermal fillers and their application across various dermatological and surgical contexts are yet to be explored. Future studies may explore the potential use of PPP gel and other biofillers in reconstructive surgery, post-trauma repair, and other medical applications beyond esthetic enhancements. Further researches and explorations in near future will likely lead to innovative formulations and expanded uses, reinforcing the significance of biofillers in both esthetic and therapeutic settings.
Limitations
This study on PPP gel as a dermal filler has limitations, including a short follow-up period that restricts the assessment of long-term effects and durability, as well as a small sample size that limits the generalizability of the outcomes. The lack of direct comparison with standard fillers such as HA fillers makes it difficult to determine their relative efficacy and safety. In addition, variations in PPP gel preparation may affect the uniformity in results and the absence of a reversal agent poses challenges in managing complications. The subjective nature of esthetic outcomes further underscores the need for standardized imaging and volumetric analysis. Future research should focus on long-term follow-ups, larger randomized trials, and comparative studies with HA fillers to establish standardized protocols and optimize the clinical application of PPP gel in esthetic medicine.
CONCLUSION
Autologous PPP gel proved to be an effective, well-tolerated, and economical simple clinic procedure for facial rejuvenation and esthetic enhancements, demonstrating significant improvements in patient outcomes. The procedure was associated with high satisfaction rates and a favorable safety profile. Although the maintenance period of plasma gel is relatively short, initial results are favorable and encouraging.
Acknowledgment
A sincere appreciation and gratitude to each team member for their invaluable contribution. In addition, we are grateful for the unwavering support provided by our institution.
Ethical approval
The research/study was approved by the Institutional Review Board at the Institutional Ethical Committee, Pacific Medical College and Hospital, number ECR/808/Inst/RJ/2017, dated June 01, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Pacific Medical College and Hospital.
References
- Assessment of the efficacy and safety of platelet-poor plasma gel as autologous dermal filler for facial rejuvenation. Menoufia Med J. 2022;35:25.
- [Google Scholar]
- The history of injectable facial fillers. Facial Plast Surg. 2009;25:67-72.
- [CrossRef] [PubMed] [Google Scholar]
- Bio-filler: An effective facial rejuvenation tool-easy on pocket. J Cutan Aesthet Surg. 2020;13:243-6.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous platelet concentrates for facial rejuvenation. J Appl Oral Sci. 2022;30:e20220020.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma in regenerative medicine. Biomed Res Ther. 2014;1:25-31.
- [CrossRef] [Google Scholar]
- Understanding the structure and function of platelet-poor plasma biofiller, an electron microscopic and fourier transform infrared spectroscopy (FTIR)-based analysis. Indian Dermatol Online J. 2024;15:534-6.
- [CrossRef] [PubMed] [Google Scholar]
- Review of the adverse effects associated with dermal filler treatments: Part I nodules, granuloma, and migration. Diagnostics (Basel). 2024;14:1640.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluating hyaluronic acid dermal fillers: A critique of current characterization methods. Dermatol Ther. 2022;35:e15453.
- [CrossRef] [PubMed] [Google Scholar]
- Multimodal facial aesthetic treatment on the appearance of aging, social confidence, and psychological well-being: HARMONY study. Aesthet Surg J. 2022;42:NP115-24.
- [CrossRef] [PubMed] [Google Scholar]
- Facial rejuvenation with the new hybrid filler HArmonyCa™: Clinical and aesthetic outcomes assessed by 2D and 3D photographs, ultrasound, and elastography. J Cosmet Dermatol. 2023;22:2186-97.
- [CrossRef] [PubMed] [Google Scholar]






