Translate this page into:
Pigmented contact dermatitis: A brief review

*Corresponding author: Keshavamurthy Vinay, Department of Dermatology, Venereology & Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh, India. vinay.keshavmurthy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Subburaj K, Vinay K, Bishnoi A, Kumaran MS, Parsad D. Pigmented contact dermatitis: A brief review. CosmoDerma 2022;2:43.
Abstract
Hyperpigmentation is one of the common pigmentary complaints that brings the patient to dermatology services. Though there are multiple etiologies for hyperpigmentation, pigmented contact dermatitis (PCD) remains a common diagnosis. The cosmetics containing dyes, preservatives, fragrances, bactericidal, emulsifiers/surfactants, and vehicles are the potential sources, and paraphenylenediamine, benzyl salicylate, brilliant lake red R, thiomersal and gallate mix are some of the most commonly implicated allergens.
The clinical manifestation includes diffuse or patchy brown to blue-black pigmentation of the cheeks, the outer surface of ears, preauricular region, temporal area, nape of the neck, and upper back. Patch testing plays a pivotal role in the diagnosis of PCD and the testing series has to be selected according to the representative population. Given the chronicity of the disease, counseling patients against the use of cosmetics is challenging as the pigmentation tends to persist for longer durations inspite of stopping cosmetics. The pillars of treatment in PCD include recognition of the culprit allergen and preventing further exposure along with pharmacological therapy. This review provides a brief overview and an insight into the etiopathogenesis and management of PCD.
Keywords
Acquired dermal macular hyperpigmentation
Allergens
Cosmetics
Paraphenylenediamine
Pigmented contact dermatitis
Pigmented cosmetic dermatitis
INTRODUCTION
Pigmented contact dermatitis (PCD) (Syn: Riehl’s melanosis, pigmented cosmetic dermatitis) is a non-eczematous presentation of contact dermatitis (CD), characterized by increased pigmentation with minimal or no inflammation. It is commonly seen among Asians and Latin Americans (skin phototype IV-VI) and presents as blotchy or reticulate slate-gray as well as brown hyperpigmentation without symptoms. The disease is common among middle-aged females and the face is the most common site of involvement.[1] The frequently detected allergens in PCD are aniline dyes, bactericides, and fragrances.[2] However, in the Indian scenario, paraphenylenediamine (PPD) and other hair dye-associated allergens are often the common culprits.[1] The incidence of PCD and the commonly implicated allergens vary as per the cultural practices in different countries. Its occurrence also depends on regulations governing the sale and labeling of cosmetics. Such regulations may not be stringent in developing countries, leading to products with poor quality and allergic potential. A concise review was lacking in the literature and this review provides a brief overview of PCD.
HISTORY
After the end of World War, with improvement in economic status, industrialization, and trade, more women had access to cosmetics. This resulted in a proportionate increase in facial pigmentation and was referred to by various names including Riehl’s melanosis’ and lichen ruber planus cum pigmentatione.[3] Riehl from Vienna was the first to describe Riehls’ melanosis during the first World War. He reported multiple patients with hyperpigmentation of the face in both sexes. Riehl could not explain the etiology but hypothesized that it could be due to wartime food substitutes.[4] Minami and Noma proposed the entity to be a novel disease and framed the term “melanosis faciei feminae.”[5] Later in the 1960s, melanosis facei feminae was hypothesized to be secondary to an allergic reaction to cosmetics. Pigmented cosmetic dermatitis is a variant of PCD was initially reported in Japan around 1970 by Nakayama.[3] Saito in 1964 did patch testing using multiple cosmetics in a cohort of patients with facial pigmentation and found that 18 of 57 patients tested positively.[6]
The common difficulty faced in the 60s when patch test was performed with cosmetics “as is” was that the contents of these cosmetics were unknown. Following the reports from Nakayama and Saito, there has been a steady decrease in the incidence of PCD, when strong contact sensitizers were removed by leading cosmetic enterprises.[3] Recently the conditions lichen planus pigmentosus, Riehl’s melanosis, erythema dyschromicum perstans/ashy dermatosis have been described with the umbrella term acquired dermal macular pigmentation (ADMH).[1]
EPIDEMIOLOGY
Though PCD was initially described in Japan, it is more commonly reported in regions of the world with a dark-skinned population like Asia, Africa, and Latin America. The allergen profile and patch test positivity also vary from country to country. The effect of ethnicity on results of patch tests has been reported by an American study wherein they found a higher frequency of positive reactions to PPD in blacks and to fragrances and formaldehyde in whites.[7] The Consumer Product Safety Commission identifies five allergens as “strong sensitizers” which include PPD and Europe and USA have legislation against the use of PPD on this basis.[8] However, developing countries do not enforce such legislation resulting in the inadvertent use of allergens in cosmetics.
ETIOLOGY
Various cosmetics, sensitizers, and allergens have been implicated in causing PCD. However, there seems to be a strong pigment-genetic interaction, given the higher incidence of PCD in patients with a dark complexion.[9] The differences between PCD and allergic contact dermatitis have been described in Table 1.
| Pigmented contact dermatitis (PCD) | Allergic contact dermatitis (ACD) |
|---|---|
| Asymptomatic blotchy or reticulate slate-gray and brown hyperpigmentation involving the face | Dermatitis manifesting as eczematous oozy lesions, erythematous plaques |
| The allergen in PCD could be revealed only on patch testing in most cases | The causative allergen is usually evident in cases of ACD to cosmetics |
| Cetrimonium, gallate mix, and thimerosal are the common allergens causing PCD. | Methylchloroisothiazolinone methylisothiazolinone, fragrances, acrylates, toluene-2,5 diamine sulphate and paraphenylene diamine are the most common causes of ACD to cosmetics |
| Predominant finding in histopathology is pigment incontinence | Predominant finding in histopathology is spongiosis |
Optical whiteners
Osmundsen[10] reported an epidemic of PCD in Copenhagen secondary to optic whiteners in washing powder. A mixture of two pyrazoline derivatives, Tinopal CH 3566 was found to be responsible for the pigmentation. Similarly, Pinol-Aguade et al.[11] reported optic whitener as a cause of PCD in nearly half of their patients.
Azo dyes
Naphthol AS, an azoic dye used in textiles was reported to be the culprit allergen in an epidemic outburst of PCD.[12] Patients with relatively darker complexion developed PCD whereas classical eczema was seen in fair-skinned individuals. Sudan I, vaccine red,[13] and brilliant lake red R[14] are few dyes reported to induce PCD. Isolated cases with exposure to insoluble cutting oils,[15] PPD,[16] and other substances are also described.[2]
Fragrances
Nakayama[17] reported fragrances like benzyl salicylate, hydroxy-citronellal, geraniol, and cinnamic alcohol and essential oils (e.g., ylang-ylang and Jasmine absolute)[18] to induce PCD. Mathias[19] studied a patient with recurrent facial dermatitis with subsequent facial hyperpigmentation and chromium hydroxide in commercial toilet soap was found to be the allergen. Prurigo pigmentosa-like contact dermatitis has also been reported secondary to chrome in detergent, though the link is not well established.[20]
Kwong et al., reported limonene, a terpene derived from citrus fruits used for its fresh lemon aroma, to be the allergen responsible for the antiperspirant-led PCD of the axilla. Though limonene on its own has low sensitization potential, hydroperoxides formed from its oxidation are potent sensitizers.[21] PCD with airborne contact allergens has been reported with whitening dyes, packaging adhesive tapes, and musk ambrette present in incense.[18]
Formaldehyde
Pigmented cosmetic dermatitis was reported in a 51-year-old white woman who wore a leather watch strap and paratertiary-butylphenol-formaldehyde resin was found to be the culprit allergen in the strap.[22]
Cosmetics
In a recent study by Sharma et al.,[8] cosmetics were found to be the commonest culprit for PCD in India. Out of the 152 relevant patch test reactions with cosmetic allergens, the most frequent culprits were preservatives (n = 71, 46.7%), hair dye ingredients (n = 47, 31%), fragrances (n = 25, 16.4%), emulsifiers/surfactants (n = 5, 3.2%), and vehicles (n = 2, 1.3%).
Various individual components in cosmetics have been reported to result in PCD in the literature. A solvent, benzyl salicylate used as odorant, fixative, and sunscreen leads to PCD which usually takes a longer time to resolve (sometimes more than 1 year).[23] PCD to lipsticks has been reported with coal tar dyes like CI 15800 brilliant lake red and other 1-phenylazo-2-naphthol derivatives. Other allegens reported causing lip PCD to include ester gum,[24] isopalmityl diglyceryl sebacate,[25] ricinoleic acid[26] and dipentaerythritol fatty acid ester (dipentaerythritolesters with hexahydroxy stearate and rosinate).[27] Musk moskene produces a sweet fragrance with a creamy powder note in cosmetics. Hayakawa et al., reported a patient with slate brown pigmentation after using a cheek rouge containing musk moskene.[18]
Many cultural practices followed by specific population can result in PCD. ‘Kumkum’ is usually applied by women to the center of the forehead or on the frontal hair parting to represent the marital status in India. Among patients of kumkum dermatitis, Nath and Thappa[28] reported 76% and 24% of patients to have PCD and allergic contact dermatitis, respectively.[29] Clinically either brown or slate gray colored pigmentation suggestive of lichen planus pigmentosus was found without overt dermatitis. “Kumkum” commonly contains brilliant lake red R, Sudan I, aminoazobenzene, canaga oil, fragrances, groundnut oil, tragacanth gum, turmeric powder, thimerosal, gallate mix, PPD, Kathon CG, benzotriazol, tert-butyl hydroquinone, parabens, azo dyes, mercury, and lead sulphide.[30]
The incidence of hair dye-induced skin reactions is increasing as many people dye their hair periodically for cosmetic reasons. PPD was reported to be the most frequent sensitizer in a retrospective study by Samanta et al.[31] In a Korean study by Woo et al.,[31] henna was found to be associated with slate-greyish dyspigmented patches over the hairline. Patch testing performed in patients with ACD and PCD following hair dye use revealed that ACD was associated with PPD whereas natural henna powder was associated with PCD.[33] In spite of being considered safe, henna can also lead to immediate and delayed-type hypersensitivity reactions.[34] In a recent study by Bishnoi et al.,[35] it was found that in a total of 108 patients with acquired dermal macular hyperpigmentation (ADMH), the frequency of positive patch tests was higher within "own hair colors" than that with PPD in Indian standard series. There are several components in the hair dyes like primary intermediates or precursors (PPD, toluene-2,5-diamine), developers (hydrogen peroxide, which may contain ammonia), and couplers (resorcinol, 3-aminophenol, and 4-aminophenol). It has been reported that though PPD is not an allergen on its own but the oxidation products of PPD like 40-nitroaniline and 4,40-azodianiline are strong sensitizers.
Next to hair dyes, one of the common cosmetics used to cause PCD among patients with skin of color is the fairness cream. Lavender absolute, musk mix, cetyl alcohol, thimerosal, sorbic acid, germall 11, and benzyl salicylate are the most common allergens in fairness creams.[8]
Medications turned into allergens
Inui et al.,[36] studied hyperpigmentation occurring as a complication of diphenylcyclopropene in alopecia areata totalis (AAT) and alopecia areata universalis (AAU). About 11 (5.91%) of the 186 patients had hyperpigmentation and all these patients had severe AA. The patients with pigmentation showed poor response to contact immunisation. Garcia-Gavin et al., reported paradoxical hyperpigmentation in a patient who applied kojic acid for lightening of lentigines on arms, and a patch test with kojic acid 1% aqua was positive.[37] Single case reports of PCD secondary to minoxidil and green tea have also been reported.[38] Common allergens implicated in PCD have been listed in Table 2.
| Optical whiteners | Tinopal CH 3566 |
|---|---|
| Dyes | Naphthol AS |
| Sudan I | |
| Brilliant lake red | |
| Vacanceine red | |
| Solvent orange 8 | |
| Cosmetics pigments | Pigment orange 3 |
| Pigment red 3 | |
| Pigment red 49 | |
| Pigment red 53 | |
| Pigment red 64 | |
| Azoic solvents | Solvent orange 2 |
| Solvent orange 8 | |
| Fragrances | Jasmine |
| Hydroxycitronellal | |
| Ylang-ylang | |
| Patchouli | |
| Cananga | |
| Antiseptics | Carbanilide |
| Miscellaneous | Formaldehyde |
| Nickel | |
| Rubber | |
| Primula obconica | |
| Musk ambrette |
PATHOGENESIS
In patients of PCD, repeated exposure to low levels of allergens produces a type IV hypersensitivity reaction resulting in basal cell vacuolization with melanin incontinence. The involvement of photo-exposed sites and the property of photosensitization by few allergens suggests ultraviolet light exposure as an important attributing factor.[1]
In a recent study by Woo et al.,[40] the expression of estrogen receptor (ER) β and progesterone receptor (PR) was analyzed in the lesional and the surrounding skin biopsies of Riehl’s melanosis cases and controls. There was an elevated dermal ERβ immunostaining intensity, dermal ERβ, and dermal PR expressions as well as ERβ and PR mRNAs expression in the lesional skin of patients. This abnormal expression strengthens the fact that receptors of estrogen and progesterone are involved in the pathomechanism of RM.
Clinical features
The onset of pigmentation in PCD is insidious and usually with no preexisting inflammation. The clinical symptoms are usually mild and because of the slower clearance of dermal pigment the lesions persist for a longer time in spite of the withdrawal of the allergen. The skin manifestations can be diffuse or patchy brown hyperpigmentation on the cheeks, ears (outer surface, helix, and lobule), temporals and preauricular regions, nape of the neck, and the upper half of the back, inframammary area, and arms [Figure 1].[41] The pigmentation can be of different shades of black, purple, or blue-black. Erythematous macules or papules, slight erythema, and exfoliation suggest a mild contact dermatitis can be observed rarely.[3] Sometimes the allergen responsible can produce pigmentation where the contact is maximal like PCD secondary to textiles commonly involve the front of the thighs and axillae (without involving the vault, Figure 2). The clinical manifestations can overlap with entities such as lichen planus pigmentosus and ashy dermatosis.[1]
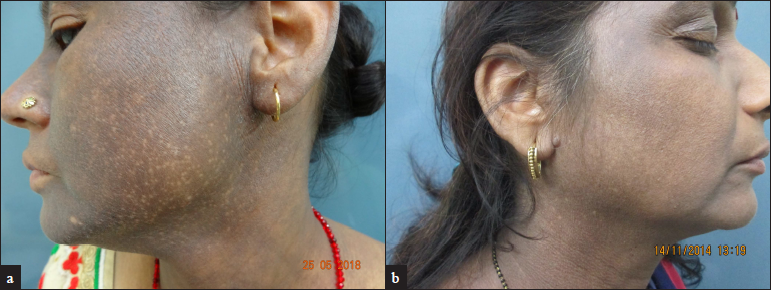
- Clinical image of pigmented contact dermatitis showing diffuse bluish-black pigmentation of the face. (a) Involvement of the helix and ear lobule can be appreciated; (b) Prominent involvement of forehead, temples, ear and neck with relative sparing of central face.
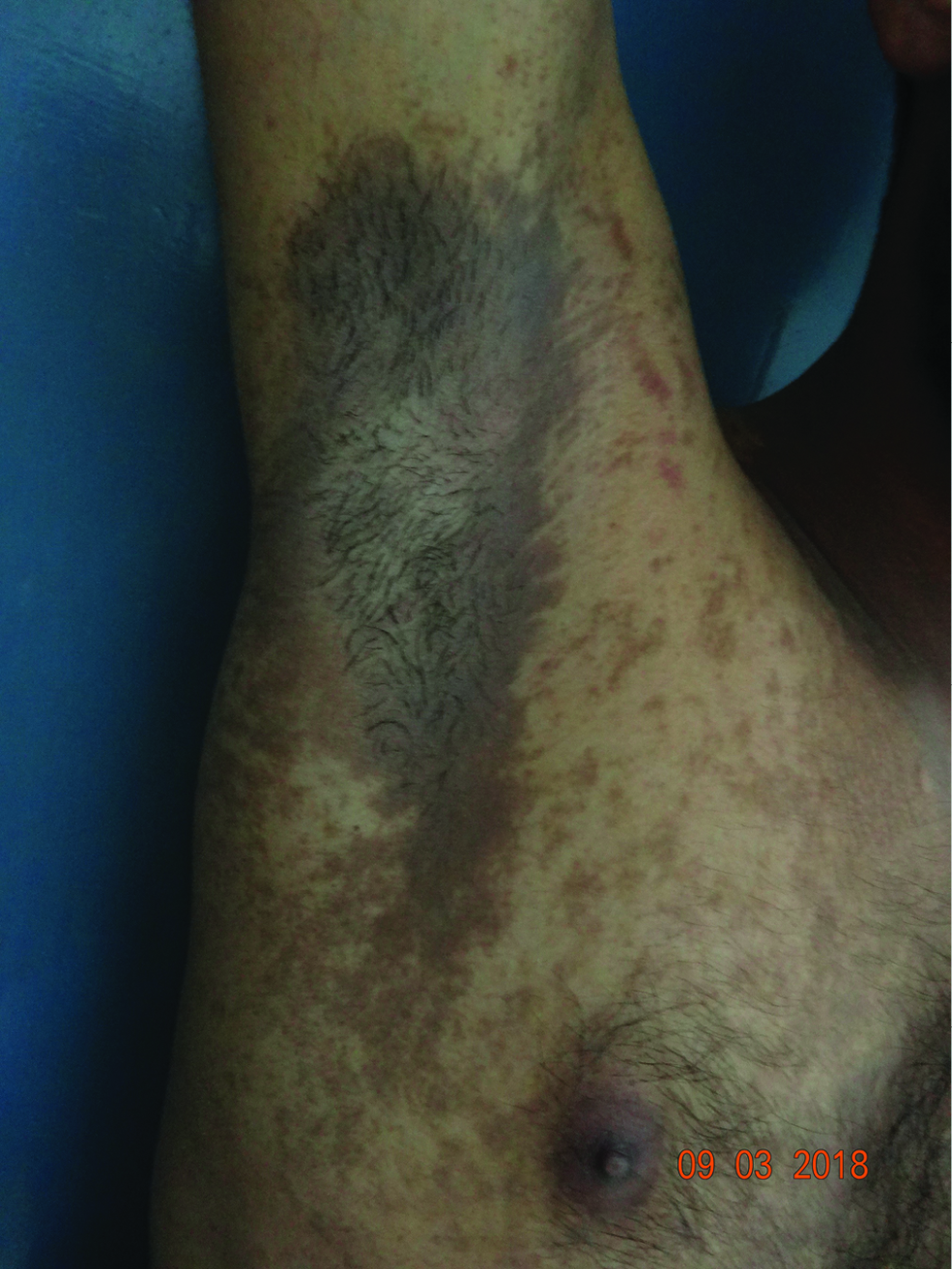
- A case of pigmented contact dermatitis to textile dyes showing prominent involvement of axilla with central sparing.
DERMOSCOPY
Dermoscopy is a non-invasive tool to assist in the diagnosis of PCD. The most common findings are gray dots or globules, which are arranged in an arcuate, semi-arcuate, or hexagonal fashion. Exaggerated pseudo network with a background blue-grey hue and telangiectatic vessels can also be seen.[41]
Reflectance confocal microscopy show pigment incontinence, mildly refractive cells (lymphocytes), dilated blood vessels and obscured papillary rims, and total or partial obliteration of the ring-like structures around the dermal papillae indicating basal layer vacuolization.[42]
Patch and photo-patch testing in PCD
Patch testing is done with standard series, cosmetic series, and fragrance series comprised of indigenous allergens to which the local population is more susceptible. The readings are to be taken at 48 hrs and on the 7th day. The International Contact Dermatitis Research Group (ICDRG) scoring system is helpful in interpreting the results as given in Table 3. In photo-patch testing, two sets of patch tests would be applied over the upper back and after 24 hours the readings will be recorded. Later only one set will be exposed to 14 J/cmsq of UVA. The second reading would be taken at 48 hours following UVA exposure (day 3). According to the standard photo-patch criteria, the photoallergic reaction is labeled when only the UVA exposed side has a positive result. If there is patch test positivity on both the sides but with an equal grading or with UVA exposed site having >1+ positivity then a diagnosis of contact dermatitis or contact dermatitis with photoaggravation is given respectively [Figure 3]. In the case of PCD, delayed brown pigmentation can also be seen on patch testing. Based on the history or examination of the patient the clinical relevance of a patch test positivity can be labeled as per no/past/present relevance. Samanta et al.,[30] performed patch testing in PCD patients with Indian cosmetic series. About 79% of patients showed patch test positivity with significance in 71%. The colorant PPD was reported as the most common allergen (37%), followed by fragrances (18%), preservatives (15%), anti-microbial (11%), and emulsifier and anti-oxidants (each 8%). In a retrospective analysis by Sharma et al.,[8] cetrimonium bromide, gallate mix, thimerosal, and skin lightening creams were found to be the common allergens. The studies done in patch testing of PCD have been summarized in Table 4.
| Symbol | Morphology | Interpretation |
|---|---|---|
| − | No lesion | Negative |
| ? | Faint macular erythema only | Doubtful |
| + | Erythema, infiltration and possibly papules | Weak positive reaction |
| ++ | Erythema, infiltration, papules and vesicles | Strong positive reaction |
| +++ | Bullous lesions | Extreme positive reaction |
| IR | Irritant reaction | Irritant reaction |
| Study | Sample size | Most common allergens in the study |
|---|---|---|
| Trattner et al.[51] 1999, Israel | 29 | Perfume mix (n = 6), Fragrance mix (n = 4) Nickel (n = 3), Formaldehyde (n = 2), Potassium dichromate (n = 1) |
| Nath and Thappa[28] 2007, India | 35 | Thiomersal (n = 18), Gallate mix (n = 12), Kumkum (n = 7), PPD (n = 1), MI/MCI (n = 1) |
| Tienthavom et al.[52] 2014, Thailand | 10 | Nickel (n = 8), Fragrance mix 2 (n = 3), Cobalt (n = 2) |
| Sharma et al.[8] 2018, India | 74 | Cetrimonium bromide (n = 20), Thiomersal (n = 16) Skin lightening creams (n = 14), Gallate mix (n = 13) |
| Samanta et al.,[31] 2019, India | 38 | Paraphenylenediamine (n = 14), Cetrimide (n = 3), Lavender absolute (n = 2), Musk mix (n = 1), Thiomersal (n = 1) |
| Bishnoi et al.,[35] 2019, India | 108 (ADMH) | Paraphenylenediamine (n = 53), Fragrance (n = 6), Biodyes and red henna (n = 6) |
| Sharma et al.,[53] 2017, India | 50 (LPP) | Para-phenylenediamine (n = 5,) Nickel sulphate (n = 3),Colophony (n = 2), Perfume mix (n = 2) and Fragrance mix (n = 2) |
PPD- Paraphenylene diamine, MI/MCI- Methylisothiazolinone, ADMH- Acquired dermal macular hyperpigmentation, LPP- Lichen planus pigmentosus
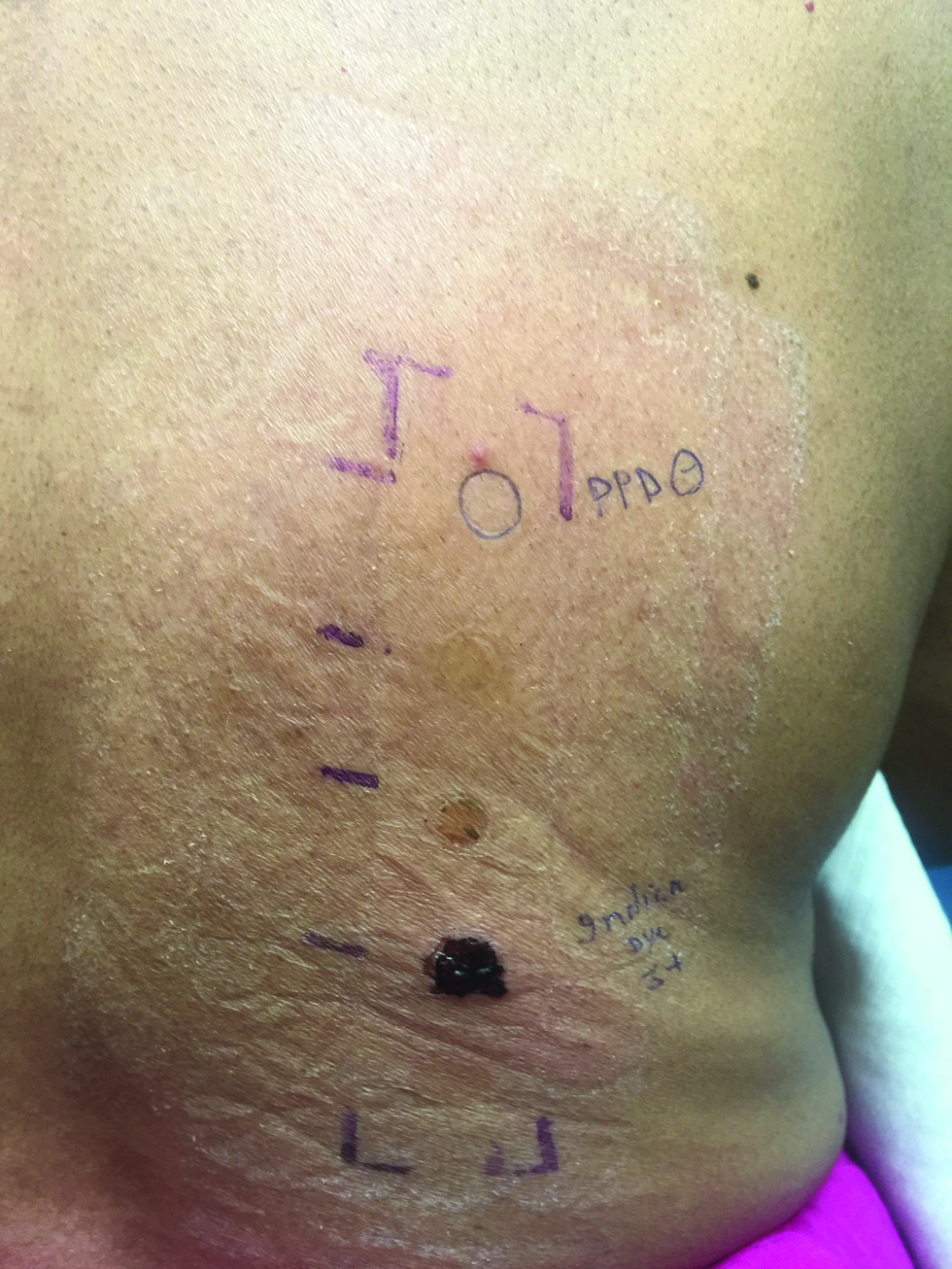
- Photo-patch testing in a case of pigmented contact dermatitis showing increased positivity (3+ with blistering) over irradiated site compared to the patch tested site (2+) to hair dye suggestive of photoaggravation. Patch and photo-patch testing with PPD shows negative results in comparison to own hair colors.
If patch testing is negative or equivocal, due to the low concentration of the allergen in cosmetic and fragrance series there is a role for repeated open application test (ROAT). In case of an equivocal result in ROAT done on the forearm, the test can be repeated with the cosmetic of the patient over affected areas or face.[9]
HISTOPATHOLOGY
The histological findings in PCD include basal cell vacuolar degeneration along with the involvement of pilosebaceous units accompanied by melanophages in the papillary dermis. Similar findings were observed from sites of positive allergic patch tests. The penetration of allergen into the hair follicles can be seen by an extension of inflammation into the upper pilosebaceous units. The site of the positive patch test has a predominance of CD4+ cells over CD8+ cells, however, in chronic skin lesions, they were equal with a mild increase in CD8+ cells.[43] An interesting observation of an enhanced melanization of the epidermis (presence of melanin throughout epidermis above the basal layer) along pigment incontinence in the dermis has been reported in ADMH.[41]
Quantitative assessment and quality of life in PCD
Acquired dermal macular hyperpigmentation area and severity index (DPASI), is a tool utilized in the assessment of disease severity in ADMH quantitatively.[44] The face and the neck are divided into six different segments and using dermoscopy the severity is graded and multiplied by the surface area involved with a maximum score of 40.
Cosmetic disfigurement in PCD can lead to significantly low self-esteem, anxiety and depression, and social stigma. In a cross-sectional study of 52 patients, it was found that the mean score of the Dermatology Life Quality Index (DLQI) and Melasma Quality of Life Scale (MELASQOL) were high in patients with PCD upon comparison with melasma and healthy controls. The lower educational status and long duration of the disease were associated with poor quality of life.[45] Dabas et al., reported that in a total of 100 patients with ADMH, 18.7%, 24.1%, and 14.3% had anxiety, depression, and somatoform disorder respectively along with a positive correlation between severity and the point prevalence of anxiety and depression.[46]
TREATMENT
The patients should be adequately counseled regarding the causative role of cosmetics or textiles and that the hyperpigmentation can persist despite cessation of their usage. The general measures in the treatment of PCD include avoidance of the causal allergen, broad-spectrum sunscreens, and sun-protective behavior.
Treatment options include cosmetic camouflage, topical agents including hydroquinone, topical corticosteroids, retinoids, vitamin C, azelaic acid, with or without light to medium depth chemical peels (trichloroacetic acid, glycolic acid) or light-based therapies.[47] A recent study showed the effectiveness of mid-fluence Q-switched Nd:YAG 1064-nm laser targeting the deep pigmentation of PCD.[48] Bhari et al., reported only a modest improvement with Q-switched Nd:YAG in clinical and histopathological aspects of lichen planus pigmentosus.[49] This discrepancy may be due to variation in the laser parameters.
A combination of one or more treatment options should be tried in resistant cases. A significant improvement in PCD has been reported with a triple combination of salicylic acid peels, oral glycyrrhizin compound, and vitamin C.[47] Another study used a combination of therapies including low-fluence, 1064-nm, Q-switched Nd:YAG laser, hydroquinone cream, and oral tranexamic acid and reported success in the majority of patients.[50] Long term management and prevention of PCD can be assured by legislation prohibiting the use of allergens in cosmetic products The various studies on the treatment of PCD have been summarised in Table 5.
| Study | Treatment given | Follow up | Results | Complication |
|---|---|---|---|---|
| Cho et al.[48]n = 21 (Koreans, Fitzpatrick skin type III to IV) |
Six sittings of mid fluence (laser intensity of 3.5-5 J/ cm2, 5-mm spot size, and 10 Hz frequency). Q-switched Nd-YAG 1064 nm laser with an interval of 40 days in between two sittings |
34 weeks | Out of 21 patients, two showed >75% improvement, eight and six patients 50-75% and 25-50% improvement, respectively. <25% improvement was seen in two patients While three had no improvement. |
Pruritus and prolonged erythema was seen in one patient each |
| Xu et al.[54] 10 (Chinese, Fitzpatrick skin type III to V) |
Oral tranexamic acid 250 mg BD for 6 months with 150 mg oral glycyrrhizin in the first 3 months |
24 weeks | 50-75% Improvement was seen in seven patients.While two patients had 25-50% improvement, one had <25% Improvement. |
None |
| Kwon et al.[50] eight (Koreans, Fitzpatrick skin type III to V) |
Low-fluence Q-switched 1064 Nd-YAG laser at 3-week intervals. Hydroquinone 4% cream topically every night andoral tranexamic acid 250 mg/day |
54 weeks | Three patients and five patients had >75% improvement and 50-75% improvement respectively. |
None |
| Chung et al.[55] 6 (Koreans, Fitzpatrick III and IV) |
Dual-pulse mode Q-switched Nd-YAG laser with fluence of 2-4 J/cm2, frequency 10 Hz and spot size of 7 mm every 2 weeks for 4 months |
20 weeks | Four patients and two patients had 50-75% improvementand 25-50% Improvement respectively. |
None |
| Wang et al.,[47] three (Chinese, Fitzpatrick III and IV) |
Glycyrrhizin compound (150 mg/d), vitamin C (100 mg/d), and salicylic acid 30% peels every 2 weeks. | 24 weeks | All three patients had significant improvement. | Mild burning |
| Acquired dermal macular hyperpigmentation | ||||
| Bishnoi et al.,[56] 43 (Indian, Fitzpatrick III-V) | Mycophenolate mofetil 2 g/day for 24 weeks | 36 weeks | One patient had >50 % decrease in dermal pigmentation area and severity index. >40-50% in 10 patients, >30-40% in 15 patients. | Leucopenia in one patient, Transaminitis and hyperbilirubinemia in two patients. |
Nd-YAG- Neodymium-doped yttrium aluminum garnet
CONCLUSION
There has been considerable progress in our knowledge about the disease mechanism and possible allergens that cause PCD. Dermoscopy and patch/photo-patch testing can be used for establishing the diagnosis and identifying possible allergens. Treatment is challenging as the pigmentation is recalcitrant or is very slow to resolve. An updated knowledge about the disease can aid dermatologists in efficient management of this enigmatic disease.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Acquired dermal macular hyperpigmentation: An update. Indian Dermatol Online J. 2021;12:663.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented cosmetic dermatitis. Int J Dermatol. 1984;23:299-305.
- [CrossRef] [PubMed] [Google Scholar]
- The association of race/ethnicity and patch test results: North American Contact Dermatitis Group, 1998-2006. Dermatitis. 2016;27:288-92.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile and allergens in pigmented cosmetic dermatitis and allergic contact dermatitis to cosmetics in India. Dermatitis. 2018;29:264-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented contact dermatitis. Indian J Dermatol Venereol Leprol. 2007;73:285-7.
- [CrossRef] [PubMed] [Google Scholar]
- Contact dermatitis due to an optical whitener in washing powders. Br J Dermatol. 1969;81:799-03.
- [CrossRef] [PubMed] [Google Scholar]
- Occupational pigmented contact dermatitis from Naphthol AS. Contact Dermatitis. 1976;2:129-34.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented contact dermatitis from azo dyes: I. Cross-sensitivity in humans. Contact Dermatitis. 1980;6:330-6.
- [CrossRef] [PubMed] [Google Scholar]
- “Brilliant lake red R as a cause of pigmented contact dermatitis,.”. Contact Dermatitis. 1979;5:297-304.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic melanodermatitis due to the rubber peephole of a ship radarscope. Contact Dermatitis.. 1978;4:245-6.
- [CrossRef] [PubMed] [Google Scholar]
- Allergen controlled system (ACS) 1974 Kanehara Shuppan Co.
- Pigmented contact dermatitis due to musk moskene. J Dermatol. 1991;18:420-4.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented cosmetic dermatitis from contact allergy to a toilet soap containing chromium. Contact Dermatitis. 1982;8:29-31.
- [CrossRef] [PubMed] [Google Scholar]
- Prurigo pigmentosa from contact allergy to chrome in detergent. Contact Dermatitis. 2001;44:289-92.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Rep. 2020;6:476-8.
- [CrossRef] [PubMed] [Google Scholar]
- Non-eczematous pigmented interface dermatitis from para-tertiary-butylphenol-formaldehyde resin in a watchstrap adhesive. Contact Dermatitis. 2001;44:45-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented contact dermatitis secondary to benzyl salicylate. Acta Derm Venereol. 2013;93:590.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic contact cheilitis due to isopalmityl diglyceryl sebacate and pentaerythritol rosinate in the lipsticks. Environ Dermatol. 2003;10:70-4.
- [Google Scholar]
- Pigmented contact cheilitis from ricinoleic acid in lipsticks. Contact Dermatitis. 2003;49:48-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented contact cheilitis from dipentaerythritol fatty acid ester. Contact Dermatitis. 2008;59:117-8.
- [CrossRef] [PubMed] [Google Scholar]
- Kumkum-induced dermatitis: An analysis of 46 cases. Clin Exp Dermatol. 2007;32:385-7.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatoses due to Indian cultural practices. Indian J Dermatol. 2015;60:3-12.
- [CrossRef] [PubMed] [Google Scholar]
- The role of patch testing with Indian cosmetic series in patients with facial pigmented contact dermatitis in India. Indian J Dermatol. 2021;66:81-6.
- [CrossRef] [PubMed] [Google Scholar]
- Acquired diffuse slate-grey facial dyspigmentation due to henna: An unrecognized cause of pigment contact dermatitis in Korean patients. Eur J Dermatol. 2018;28:644-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathologic features of skin reactions to temporary tattoos and analysis of possible causes. Arch Dermatol. 2002;138:88-92.
- [CrossRef] [PubMed] [Google Scholar]
- Contact dermatitis due to henna, solvent red 1 and solvent red 3. A case report. Contact Dermatitis. 1992;27:346-7.
- [CrossRef] [PubMed] [Google Scholar]
- Contact sensitization to hair colours in acquired dermal macular hyperpigmentation: Results from a patch and photo-patch test study of 108 patients. J Eur Acad Dermatol Venereol. 2019;33:1349-57.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented contact dermatitis due to therapeutic sensitizer as complication of contact immunotherapy in alopecia areata. J Dermatol. 2010;37:888-93.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented contact dermatitis due to kojic acid. A paradoxical side effect of a skin lightener. Contact Dermatitis. 2010;62:63-4.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented contact dermatitis from topical minoxidil 5%. Contact Dermatitis. 2002;46:246.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented contact cheilitis: From green tea? Contact Dermatitis. 2010;62:60-1.
- [CrossRef] [PubMed] [Google Scholar]
- Paracrine roles of hormone receptors in Riehl’s melanosis: A quantitative analysis of oestrogen and progesterone receptor expression patterns. Exp Dermatol. 2021;30:396-01.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatoscopic evaluation and histopathological correlation of acquired dermal macular hyperpigmentation. Int J Dermatol. 2017;56:1395-9.
- [CrossRef] [PubMed] [Google Scholar]
- Four views of Riehl’s melanosis: Clinical appearance, dermoscopy, confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2014;28:1199-06.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented facial contact dermatitis to benzyl salicylate: A comparative histopathological and immunohistochemical study of the involved skin and the positive patch test site. Am J Dermatopathol. 2019;41:443-7.
- [CrossRef] [PubMed] [Google Scholar]
- A novel scale for measurement of acquired dermal macular hyperpigmentation severity. J Eur Acad Dermatol Venereol. 2018;32:e251-3.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of riehl’s melanosis on quality of life in Korean patients: A cross-sectional comparative study. J Dermatol. 2020;47:893-7.
- [CrossRef] [PubMed] [Google Scholar]
- Psychological disturbances in patients with pigmentary disorders: A cross-sectional study. J Eur Acad Dermatol Venereol. 2020;34:392-9.
- [CrossRef] [PubMed] [Google Scholar]
- Combination therapy with salicylic acid chemical peels, glycyrrhizin compound, and vitamin C for Riehl’s melanosis. J Cosmet Dermatol. 2020;19:1377-80.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of Riehl’s melanosis with mid-fluence Q-switched Nd: YAG 1064-nm laser. Lasers Surg Med. 2020;52:753-60.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Q-switched Nd: YAG laser on the clinical, pigmentary, and immunological markers in patients with lichen planus pigmentosus: A pilot study. Dermatol Ther. 2020;33:e13208.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study for triple combination therapy with a low-fluence 1064 nm Q-switched Nd: YAG laser, hydroquinone cream and oral tranexamic acid for recalcitrant riehl’s melanosis. J Dermatol Treat. 2017;28:155-9.
- [CrossRef] [PubMed] [Google Scholar]
- Screening patch tests for pigmented contact dermatitis in Israel. Contact Dermatitis. 1999;40:155-7.
- [CrossRef] [PubMed] [Google Scholar]
- Patch testing and histopathology in thai patients with hyperpigmentation due to erythema dyschromicum perstans, lichen planus pigmentosus, and pigmented contact dermatitis. Asian Pac J Allergy Immunol. 2014;32:185-92.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy and patch testing in patients with lichen planus pigmentosus on face: A cross-sectional observational study in fifty Indian patients. Indian J Dermatol Venereol Leprol. 2017;83:656-62.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study of oral tranexamic acid and glycyrrhizin compound in the treatment of recalcitrant riehl’s melanosis. J Cosmet Dermatol. 2019;18:286-92.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study of a novel dual-pulsed 1064 nm Q-switched Nd: YAG laser to treat Riehl’s melanosis. J Cosmet Laser Ther. 2014;16:290-2.
- [CrossRef] [PubMed] [Google Scholar]
- Oral mycophenolate mofetil in the treatment of acquired dermal macular hyperpigmentation: An open-label pilot study. Australas J Dermatol. 2021;62:278-85.
- [CrossRef] [PubMed] [Google Scholar]






