Translate this page into:
Overlap syndrome: Juvenile dermatomyositis with systemic lupus erythematosus and lupus hepatitis in a child: An uncommon presentation

*Corresponding author: Archana Singal, Department of Dermatology Dermatology, University College of Medical Sciences, New Delhi, India. archanasingal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pal V, Singal A, Diwaker P. Overlap syndrome: Juvenile dermatomyositis with systemic lupus erythematosus and lupus hepatitis in a child: An uncommon presentation. CosmoDerma. 2024;4:118. doi: 10.25259/CSDM_112_2024
Abstract
Autoimmune connective tissue disease in children carries a unique management challenge to the treating physician due to the potential years of disease burden and resultant complications. Early diagnosis and treatment positively impact disease course. Overlap syndrome in children is a rare entity. We report a case of juvenile dermatomyositis with systemic lupus erythematosus who on follow-up developed lupus hepatitis. Lupus hepatitis in children with overlap syndrome is not a well-known entity and there is paucity of literature describing this association.
Keywords
Juvenile dermatomyositis
Systemic lupus erythematosus
Overlap syndrome and lupus hepatitis
INTRODUCTION
Juvenile dermatomyositis (JDM) is a rare autoimmune disorder and a subtype of inflammatory myopathies.[1] The incidence of JDM is 2–4/million.[2] Childhood systemic lupus erythematosus (SLE) is a rare disease with a prevalence of 3.3−8.8/1,00,000 children.[3] SLE-associated hepatitis, also known as lupus hepatitis, is characterized by subclinical and mild elevation of liver enzymes and affects 3–8% of SLE patients. Liver involvement occurs in 50–60% of SLE patients during their lifetime.[4,5] Lupus hepatitis also indicates SLE activity in most cases.[5] Overlap syndrome with JDM and SLE is rare and the occurrence of lupus hepatitis with overlap syndrome in a child is a unique entity. Herein, we describe a case of JDM with SLE who subsequently developed lupus hepatitis.
CASE REPORT
A 15-year-old boy was admitted to the dermatology inpatients in September 2020 with multiple painful joints of hands, knee, and elbow, diffuse asymptomatic erythema around the eye, chest, and neck, photosensitivity, weakness of proximal muscles of both arms and thighs and multiple painful ulcers on the back, buttocks, and elbow joint. Ulcers over the elbow joint were associated with chalky white discharge. There was no history of oral ulcers, Raynaud’s phenomenon, sclerodactyly, and dryness of mucosae. Family history was not significant.
On physical examination, we observed normal vital parameters, pallor, and myopathic gait. Mucocutaneous examination revealed heliotrope rash involving the periocular area and nasolabial fold [Figure 1a] and multiple erythematous grouped papules over bilateral interphalangeal and metacarpophalangeal joints [Figure 1b]. The patient had multiple ulcers over the trunk with irregular margins, sloping edges, and floor-slowing pale granulation tissue smeared with pus [Figure 1c]. Ulcer over the elbow joint showed chalky white discharge suggestive of calcinosis cutis. Scalp examination revealed diffuse non-cicatricial alopecia involving the occipital and bilateral temporoparietal area [Figure 1d]. Proximal muscles of bilateral upper and lower limbs showed reduced power with medical research counsel grade 3/5 except the hip abductor, which showed power of 2/5. The limb girth of the bilateral arm was reduced to 10 cm. The range of motion of bilateral knee and elbow joints was reduced. Nailfold capillaroscopy showed reduced capillary density and the presence of tortuous and dilated capillaries [Figure 2a]. The rest of the mucosae and systemic examination was within normal limits. Based on history and clinical examination, a provisional diagnosis of JDM was made.
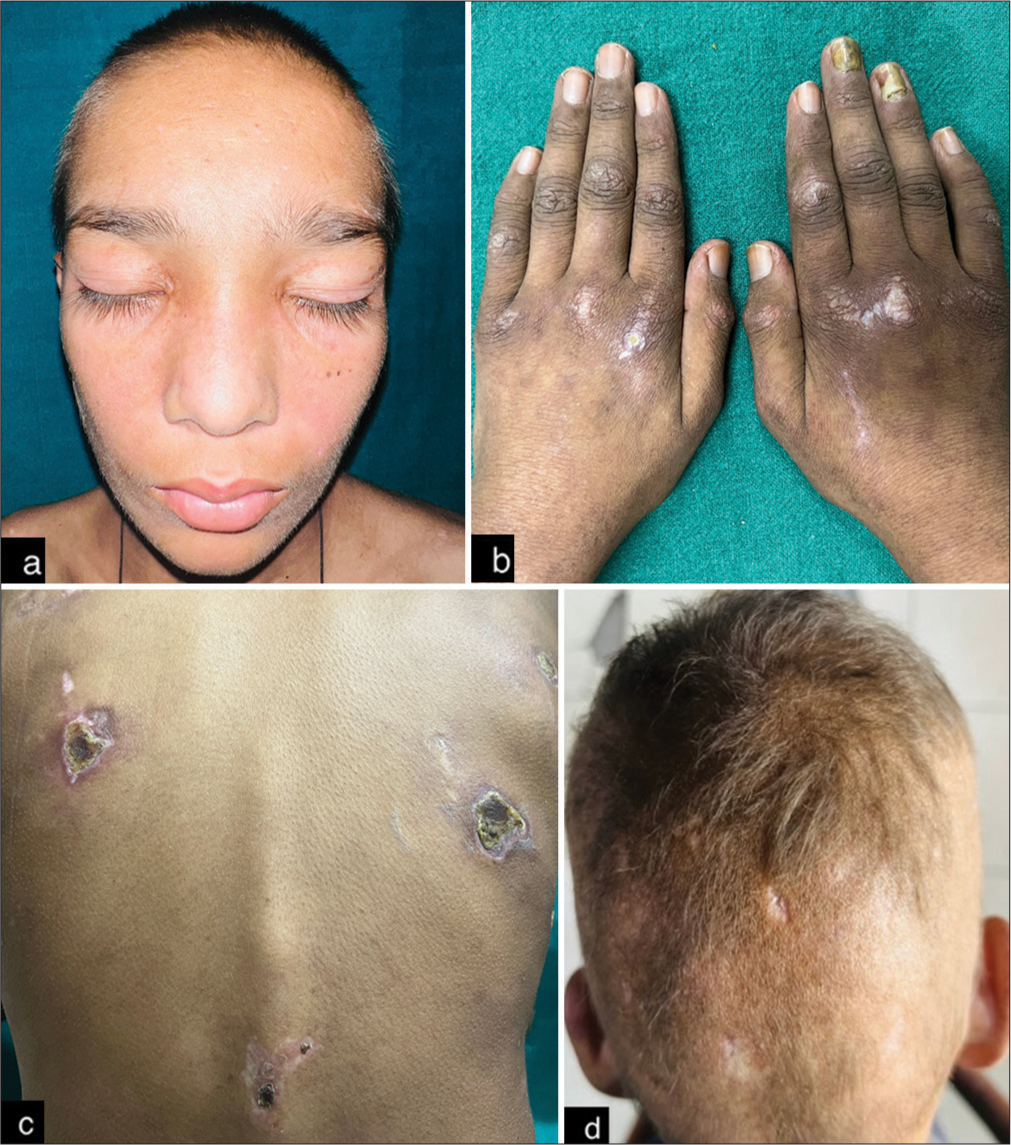
- (a) Diffuse violaceous facial erythema involving periocular area and nasolabial fold, (b) multiple violaceous papules were present over bilateral interphalangeal and metacarpophalangeal joints, (c) multiple ulcer over the trunk with irregular margin, sloping edges and floor slowing pale granulation tissue smeared with pus, and (d) diffuse non-cicatricial alopecia involving occipital and bilateral temporoparietal area.
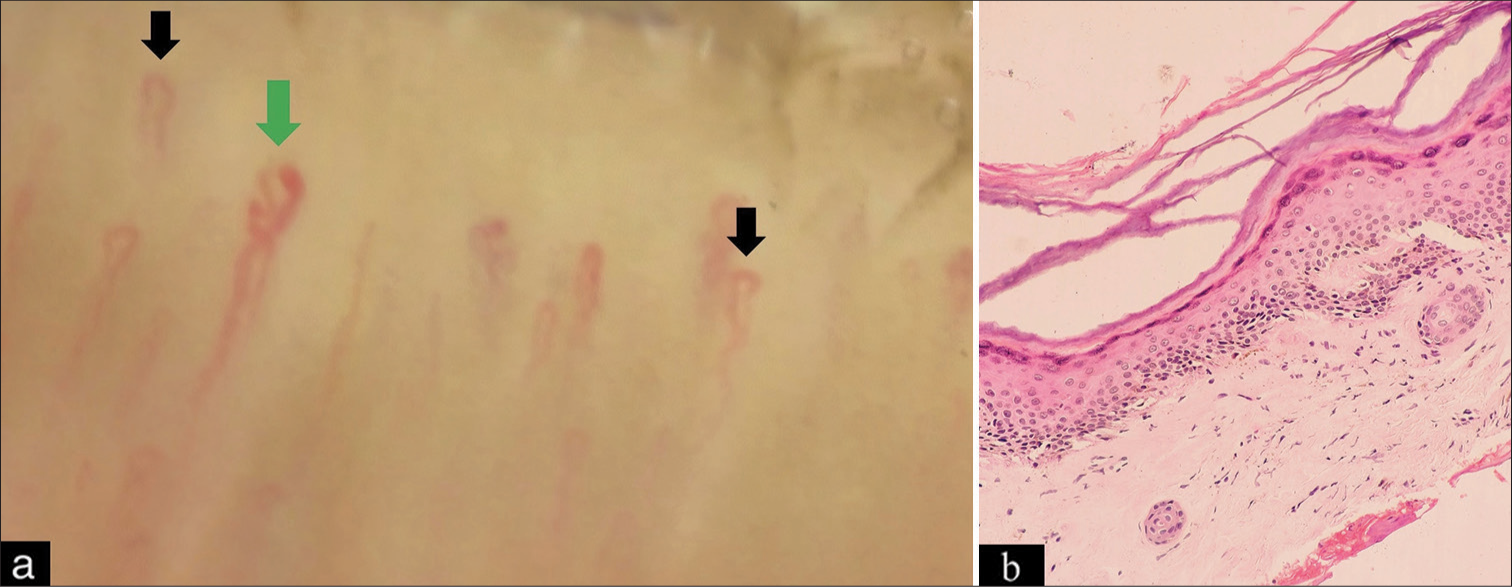
- (a) Nailfold capillaroscopy showing tortuous (black arrow) and dilated capillary (green arrow) and (b) hyperkeratotic mildly acanthotic epidermis with focal pigment incontinence in the upper dermis (Hematoxylin and eosin, magnification ×400).
Laboratory investigations are shown in Table 1. Total and differential leucocyte counts and kidney function tests were within normal limits. Biopsy from the papule present over the interphalangeal joint showed hyperkeratotic, mildly acanthotic epidermis with focal pigment incontinence in the upper dermis [Figure 2b]. Myositis-specific antibody profile, electromyography, and direct immunofluorescence could not be done due to feasibility constraints.
| Investigations | Result | Normal range |
|---|---|---|
| Hemoglobin (g/dL) | 8.4 with dimorphic anemia | 11–16 |
| ESR (mm/h) | 31 | <20 |
| Total protein (g/dL) | 8.1 | 6.4–8.2 |
| Albumin (g/dL) and globulin (g/dL) | 3.5/5.1 | 3.5–5.0 |
| Total bilirubin (mg/dL) | 0.7 | 0.1–1.2 |
| ALP (U/L) | 286 | 44–147 |
| AST (U/L) | 36 | 15–37 |
| ALT (U/L) | 41 | 16–63 |
| LDH (U/L) | 524 | 120–246 U/L |
| Creatinine kinase NAC IU/l | 109 | 55–170 |
| Creatinine kinase MB IU/L | 11 | 0–25 |
| HIV, HBsAg, Anti-HCV | Non-reactive | |
| ANA | 1:160 | <1:40 |
| Anti-DsDNA | Negative | |
| Anti-U1RNP | 2+ | |
| c-ANCA/p-ANCA | Negative | |
| Rheumatoid factor (IU/mL) | 11.87 | <18 |
| 24 hr urinary protein | 194 mg | <150 mg/24 h |
| 2D electrocardiogram | Ejection fraction 45–50% with mild left ventricular dysfunction and global hypokinesia | |
| X-ray right elbow joint | Radiopaque foci suggestive of calcification | |
| MRI of proximal muscle | Inflammation and edema suggestive of dermatomyositis | |
| Biopsy-papule over dorsum of the hand | Hyperkeratotic mildly acanthotic epidermis with focal pigment incontinence in the upper dermis. | |
| Biopsy-ulcer | Epidermal acanthosis and dermis showed vascular proliferation along with moderate mixed inflammatory infiltrate. |
ALP: Alkaline phosphatase, AST: Aspartate transaminase, ALT: Alanine transaminase, LDH: Lactate dehydrogenase, BUN: Blood urea nitrogen, ESR: Erythrocyte sedimentation rate, ANA: Anti-nuclear antibody, dsDNA: double-stranded deoxyribonucleic acid antibody, Anti-U1RNP: Anti-U1 ribonucleoprotein, p- and c- ANCA: Perinuclear- and cytoplasmic-anti-neutrophil cytoplasmic antibodies
The diagnosis of overlap syndrome
The JDM (heliotrope rash, Gottron sign, proximal muscle weakness, and raised muscle enzyme) with SLE (anti-nuclear antibody, anti-U1 ribonucleoprotien [U1-RNP] antibody, non-scarring alopecia, fever, and arthritis) was thus made.
The patient was treated with tablet prednisolone 20 mg/day, hydroxychloroquine (HCQ) 150 mg/day, mycophenolate mofetil (MMF) 500 mg BD, aspirin 75 mg daily, and iron-folic acid-Vitamin B12 supplements along with intralesional triamcinolone acetonide in calcinosis cutis lesions. At one month, improvement in anemia, muscle weakness, and muscle power was noted and ulcers healed with an atrophic scar. Subsequently, prednisolone was tapered gradually and stopped at seven months while the patient continued HCQ, MMF, and low-dose aspirin. Three months later, the patient presented with acute exacerbation of skin lesions with increased fatigue and muscle weakness. The patient was re-admitted and started on 20 mg daily oral prednisolone. In the 2nd week, raised aspartate aminotransferase (AST): 191 U/L (normal - 15–37 U/L) and alanine aminotransferase (ALT): 227 U/L (normal-16–63) were found on routine hematological screening viral serology for hepatitis A, B, C, and E which was negative. We stopped potential hepatotoxic drugs (HCQ and MMF). However, liver enzymes continued to rise further (AST/ALT: 250 U/L/390 U/L at 2 weeks).
In view of non-reactive viral serology, withdrawal of hepatotoxic drug, non-feasibility of autoimmune hepatitis (AIH)-related antibody profile, and parent’s refusal for liver biopsy, a diagnosis of lupus hepatitis was considered and prednisolone was increased to 30 mg (1 mg/kg). This led to a progressive fall in the liver enzyme (AST-118 U/L/ALT-155 U/L) within 2 weeks. The patient was discharged but was lost to follow-up.
DISCUSSION
Overlap syndrome is characterized by fulfillment of classification criteria for two or more autoimmune connective tissue diseases.[6] Overlap myositis, defined as the presence of idiopathic inflammatory myositis (Polymyositis, dermatomyositis, etc.) with other connective tissue diseases, is a rare disease and its prevalence in patients with SLE is low (4–16%).[7] Our patients also had positive U1-RNP antibodies but did not fulfill the criteria for mixed connective tissue disease. To the best of our knowledge, there has been only one case reported in the literature of JDM and SLE with hepatitis, though JDM with SLE has been reported.[8,9] The previously reported cases of overlap syndrome are detailed in Table 2. Approximately 25–59% of the SLE patients develop transaminitis during their lifetime. The most commonly identified causes are drugs and viral hepatitis. However, in 28–42% of patients, in the absence of no obvious cause, it is attributed to SLE disease itself.[4] Lupus hepatitis has a benign course, is associated with a good prognosis, and responds well to corticosteroid therapy with no long-term complications as compared to AIH which has a poor prognosis, and higher rates of progression to liver cirrhosis, hepatocellular carcinoma, and death.[4,5]
| S. No. | Age/Sex | Clinical feature | Laboratory investigation | Antibody profile (positive) | Treatment |
|---|---|---|---|---|---|
| Macêdo et al., 2010 SLE, JDM, Urticarial vasculitis |
12/M | Proximal muscle weakness Heliotrope rash Gottron signs Fever Malar rash Psychosis Weight loss |
Anemia Leukopenia Thrombocytopenia Elevated muscle enzymes |
ANA Anti-dsDNA Low C3 and C4 C1q deficiency |
methylprednisolone pulse, deflazacort, azathioprine 150 mg/day HCQ 200 mg OD |
| Belgaumkar et al., 2023 JDM-SLE |
16/F | Proximal muscle weakness Heliotropic rash Gottron papule Fever Joint pain Alopecia Photosensitivity RP Psychiatric symptoms |
Anemia Elevated muscle enzymes |
ANA anti-dsDNA anti-Ro/SSA Low C3, C4 |
Prednisolone (1 mg/kg/day) HCQ 200 mg BD aspirin 75 mg OD Nifedipine 20 mg OD Cilastazole 50 mg BD Olanzapine 5 mg OD |
| Sulaiman et al. 2021 JDM, SLE, Lupus cerebritis and hepatitis |
17/F | Proximal muscle weakness, heliotrope rash, discoid rash fever, shortness of breath, bilateral scleritis, oral ulcers | Elevated muscle enzymes Proteinuria |
ANA ds-DNA borderline positive anti-M2 alpha anti-M2 beta |
prednisolone 60 mg daily (1 mg/kg/day) HCQ 200 mg OD MMF 500 mg BD cyclosporine 50 mg BD |
| Our case | 15/M | Proximal muscle weakness Heliotrope rash Gottron papule Fever Joint pain Alopecia |
Anemia Elevated muscle enzymes |
ANA U1-RNP c-ANCA |
Prednisolone 20 mg OD HCQ 200 mg/100 mg alternate day MMF 500 mg BD Aspirin 75 mg HS |
JDM: Juvenile dermatomyositis, SLE: Systemic lupus erythematosus, ANA: Anti-nuclear antibody, dsDNA: double-stranded deoxyribonucleic acid antibody, HCQ: hydroxychloroquine, U1RNP: U1 ribonucleoprotein, c-ANCA: Cytoplasmic- anti-neutrophil cytoplasmic antibodies, MMF: Mycophenolate mofetil, RP: Raynaud’s phenomenon, SSA: Sjogren’s-syndrome-related antigen A.
CONCLUSION
With this case, we would like to draw attention toward the significance of regular and long-term follow-up in the early recognition of the complications and prompt initiation of treatment as it responds well to systemic immunosuppressants.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- New myositis classification criteria-what we have learned since Bohan and Peter. Curr Rheumatol Rep. 2018;20:18.
- [CrossRef] [Google Scholar]
- Juvenile dermatomyositis-clinical phenotypes. Curr Rheumatol Rep. 2019;21:74.
- [CrossRef] [Google Scholar]
- Overlap syndrome of juvenile dermatomyositis with systemic lupus erythematosus: A rare case report of diagnostic perplex. Dermatol Rev. 2023;4:223-7.
- [CrossRef] [Google Scholar]
- Lupus hepatitis and autoimmune hepatitis (lupoid hepatitis) Am J Med Sci. 2017;353:329-35.
- [CrossRef] [Google Scholar]
- Lupus hepatitis, more than just elevated liver enzymes. Scand J Rheumatol. 2020;49:427-33.
- [CrossRef] [Google Scholar]
- Systemic lupus erythematosus and overlap: A clinician perspective. Clin Dermatol Rev. 2019;3:12-7.
- [CrossRef] [Google Scholar]
- The clinico-serological spectrum of overlap myositis. Curr Opin Rheumatol. 2018;30:637-43.
- [CrossRef] [Google Scholar]
- Juvenile systemic lupus erythematosus and dermatomyositis associated with urticarial vasculitis syndrome: A unique presentation. Rheumatol Int. 2012;32:3643-6.
- [CrossRef] [Google Scholar]
- Overlap syndrome: Dermatomyositis and systemic lupus erythematosus with cerebral lupus and autoimmune hepatitis: A case report. Ann Arthritis Clin Rheumatol. 2021;4:1023.
- [Google Scholar]






