Translate this page into:
Linear immunoglobulin A/immunoglobulin G bullous dermatosis with celiac disease in a 26-year-old Filipino male

*Corresponding author: Christine Lyka Raymundo Sayson, Department of Dermatology, Research Institute for Tropical Medicine, Muntinlupa, Philippines. christinelyka.sayson@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dayrit JF, Sayson CR, De Leon RA, Santi ER. Linear immunoglobulin A/immunoglobulin G bullous dermatosis with celiac disease in a 26-year-old Filipino male. CosmoDerma. 2024;4;73. doi: 10.25259/CSDM_67_2024
Abstract
Linear immunoglobulin A/immunoglobulin G bullous dermatosis (LAGBD) is a rare form of autoimmune blistering disease characterized by circulating anti-keratinocyte cell surface antibodies from both the immunoglobulin A and immunoglobulin G. It presents as blisters, erosions, and pustules with erythematous lesions, primarily on the trunk and extremities. A 26-year-old Filipino male presented with a 4-year history of multiple pruritic vesicles on an erythematous base on the mentum spreading on the face, scalp, neck, upper trunk, and upper extremities. Skin biopsy and direct immunofluorescence suggest a diagnosis of LAGBD. The patient underwent esophagogastroduodenoscopy, which revealed duodenal villous atrophy. A small bowel biopsy revealed duodenal villous atrophy with a predominance of lymphocytes. He was treated with dapsone for two years with remarkable improvement. He was also advised to do a strict, lifelong gluten-free diet. After a 5-year follow-up, he only experienced occasional very mild flares treated with topical clobetasol 5% cream. We present a rare case of a patient with LAGBD with celiac disease. Systemic involvement, particularly gastroenteropathy, should also be investigated. Dapsone and a gluten-free diet have been the primary treatment modalities for this case.
Keywords
Linear immunoglobulin A/immunoglobulin G bullous dermatosis
Celiac disease
Dapsone
Gluten-free diet
INTRODUCTION
Autoimmune blistering diseases (AIBDs) result from the immune system’s loss of tolerance to hemidesmosome components that lead to the activation of humoral and cellular responses.[1] The two groups of AIBD are the pemphigus group, which has autoantibodies to keratinocyte cell surfaces, and the pemphigoid group, which has autoantibodies to the epidermal basement membrane zone.[2] Bullous pemphigoid is characterized by the deposition of immunoglobulin G (IgG) and C3 along the basement membrane zone. In contrast, linear immunoglobulin A (IgA) bullous dermatosis (LABD) is defined by the deposition of IgA at the basement membrane.[1] A rare form of AIBD known as linear IgA/IgG bullous dermatosis (LAGBD) is distinguished by circulating anti-keratinocyte cell surface antibodies from both the IgA and IgG classes.[1-3]
Clinically and histologically, LABD can resemble dermatitis herpetiformis (DH) [Table 1].[4,5] Direct immunofluorescence (DIF) distinguishes LABD from the other AIBD. The disease entity of LAGBD has yet to be established since there are few cases reported. Blisters, erosions, and pustules with erythematous lesions, primarily on the trunk and extremities, are among the clinical manifestations of LAGBD. The DIF revealed the presence of both IgA and IgG antibodies in the epidermal basement membrane zone.[2,3] Herein, we present a case of a Filipino male with a recurrent history of pruritic papulovesicular lesions on the face, trunk, and upper extremities. Clinical and DIF findings were consistent with LAGBD. Esophagogastroduodenoscopy revealed duodenal villous atrophy, suggesting celiac disease. The patient was managed with dapsone and a gluten-free diet, and remission was observed. LABD with concomitant celiac disease is rarely reported; it is critical to identify gluten sensitivity in patients who are not responding to treatment.
| LABD | Dermatitis Herpetiformis | |
|---|---|---|
| Cutaneous lesions | Childhood-onset LABD or CBDC
LABD
|
Diffuse, symmetrical, grouped erythematous papules, plaques, herpetiform vesicles and blisters with erosions, excoriations, and hyperpigmentation on the face, extensor surfaces of extremities, shoulders, and buttocks |
| Histopathology | Subepidermal blister with superficial infiltrate of neutrophils in the dermis | Subepidermal blisters with neutrophils and few eosinophils at the papillary tips |
| Direct immunofluorescence |
|
Granular IgA at the basement membrane zone |
IgA: immunoglobulin A, IgG: immunoglobulin G, LABD: Linear immunoglobulin A bullous dermatosis, CBDC: Chronic bullous dermatosis of childhood
CASE REPORT
A 26-year-old Filipino male, who works as a seafarer, presented with a 4-year history of multiple pruritic vesicles on an erythematous base on the mentum spreading on the face, scalp, neck, upper trunk, and upper extremities [Figure 1]. The condition was exacerbated by cold weather and alcohol intake. No oral or genital lesions were noted. There are no other associated signs and symptoms, such as fever, myalgia, or weight loss. No diarrhea nor steatorrhea was experienced. The patient was then diagnosed with DH, as confirmed by skin biopsy, and was initially given prednisone 20 mg once a day and clindamycin. Due to the persistence of pruritic vesicular lesions on the face, neck, upper trunk, and upper extremities, treatment was shifted to dapsone 50 mg half tablet once a day and noted improvement of skin lesions characterized by drying and decreased in erythema and pruritus. However, recurrences of lesions prompted treatment adjustments, including dexamethasone lotion, dapsone 50 mg half tablet once a day, and a gluten-free diet. Regular follow-up was done, and dapsone was continued. In the interim, there was a note of intermittent cutaneous eruptions relieved by pharmacologic intervention. Complete blood count and fasting blood sugar were normal. However, the patient still had an intake of gluten-containing products such as bread and had occasional alcohol intake.

- Multiple erythematous papulovesicular lesions on the (a and b) face, (c) trunk, and (d) upper extremity. (e) Vesicular lesions noted on close-up.
Due to the subsequent recurrences of lesions, repeat skin biopsy and DIF studies were done. Hematoxylin and eosin-stained skin biopsy sections revealed spongiosis of the epidermis and a subepidermal blister with neutrophils [Figure 2a]. The DIF showed deposits of IgA and IgG in a linear pattern [Figures 2b and c]. The patient also underwent esophagogastroduodenoscopy, revealing duodenal villous atrophy [Figure 3]. A biopsy was done, which showed a chronic inflammatory pattern with a predominance of lymphocytes.
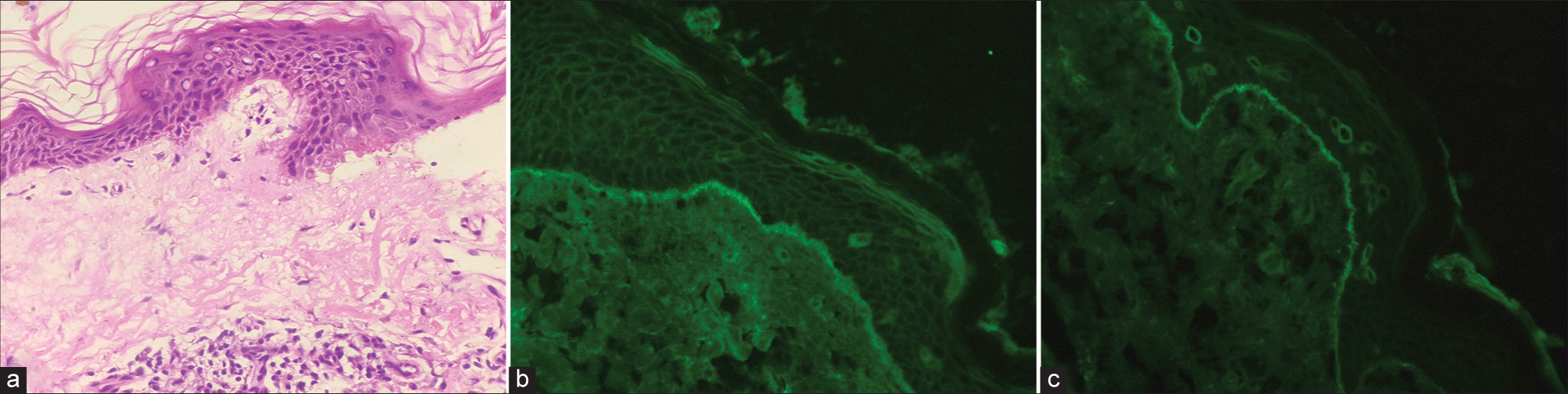
- (a) Spongiosis of the epidermis and a subepidermal blister with neutrophils (H&E x100). (b) Linear deposits of immunoglobulin G on direct immunofluorescence (DIF). (c) Linear deposits of immunoglobulin A on DIF.
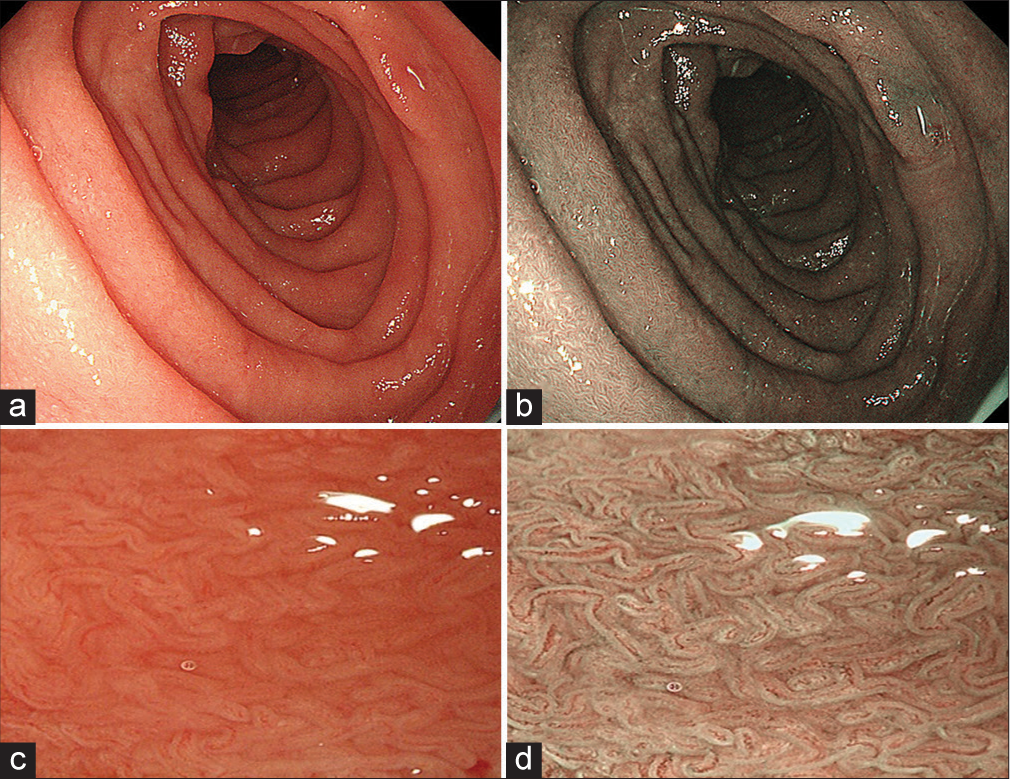
- (a) Conventional esophagogastroduodenoscopy showing continuous and regular duodenal mucosa and (c) flattening of villi. (b and d) Narrow band imaging showing irregular arrangement and flattening of villi on duodenal mucosa suggestive of villous atrophy.
The lesions were noted to have subsided after treatment with dapsone [Figure 4]. Dapsone was continued for two years with remarkable improvement. In addition, a strict, lifelong gluten-free diet was recommended. After a 5-year follow-up, the patient only experienced occasional mild flares managed with topical clobetasol 5% cream.
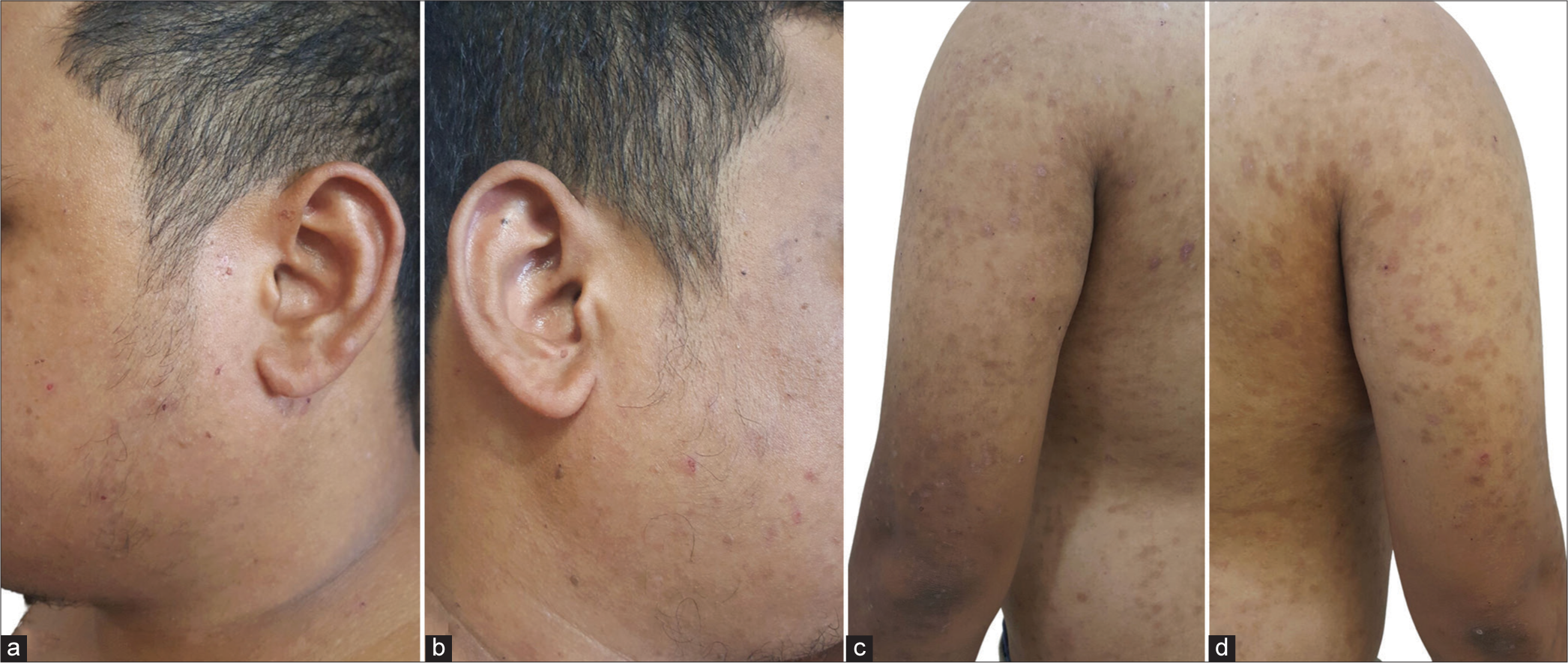
- Drying and healing of skin lesions on the (a and b) face and (c and d) trunk on follow-up.
DISCUSSION
The LAGBD is a rare type of AIBD characterized by the presence of both IgA and IgG antibodies in the epidermal basement membrane zone.[1-3] The incidence of LABD is 0.3–2.3/million. However, the incidence of LAGDB is unknown due to the small number of reported cases.[1] The majority (51%) affect those over 60, while 42.3% affect those between the ages of 15 and 60. Gastrointestinal diseases and malignancy are associated with LAGBD. Most frequently, IgA and IgG antibodies target BP180, and less often, they target laminin-322 and BP230.[1] The LAGBD patients had prominent reactivity to laminin-322, an epidermal basement membrane zone-specific laminin.[3]
The LAGBD presents as multiple pruritic vesicles, bullae, and pustules with erosions on an erythematous base, usually seen on the trunk and extremities in an annular pattern. Involvement of the oral and ocular mucosa may also develop.[1,2] Histopathologic findings reveal subepidermal blister and inflammatory infiltrates, with a predominance of neutrophils arranged in a band pattern along the dermoepidermal junction.[6] The DIF typically exhibits linear and homogenous deposition of IgA, IgG (up to 25% of cases), and C3 along the basal membrane.[3,6,7] Indirect immunofluorescence also shows positive IgA and IgG anti-keratinocyte cell surface antibodies.[2,3]
Initially, the patient was diagnosed as a case of DH, which is another type of AIBD. A case report also identified a possible overlap of DH with LAGBD.[8] The DH presents with erythematous pruritic vesicles on the elbows, knees, and buttocks. Histologically, DH has subepidermal blisters with neutrophils, while on DIF, it presents with granular IgA deposition.[8] Both DH and LAGBD were compatible with the clinical features in this case. With the DIF showing both linear deposition of IgA and IgG along the basal membrane, the patient can be classified as a case of LAGBD.
Dapsone is the most often used medication for the treatment of linear LABD. It is also considered an effective therapy in LAGBD. It prevents neutrophil adhesion and the release of proteases and tissue-damaging oxidants. Adverse effects of dapsone include hemolysis, methemoglobinemia, neutropenia, neuropathy, and hepatitis.[1] Dapsone dosage is usually started at low doses and gradually increased to a maintenance dose of 100–200 mg/day in adults.[9] Due to its adverse effects, other alternative treatments may be used. Rituximab and intravenous immunoglobulin (IVIg) are used for severe and resistant cases. Systemic corticosteroids are used in combination with dapsone or IVIg. Systemic and topical corticosteroids or nicotinamide are utilized in the pediatric population.[10]
Intermolecular epitope spreading, defined as autoreactive B- or T-cell response from the endogenous epitope to a secondary epitope over time, is frequently associated with IgA antibodies.[11] Epitope spreading is believed to be the pathogenic process responsible for relapse and refractoriness in AIBD.[12] Treatment for refractory LAGBD has been reported to be effective with omalizumab, a monoclonal immunoglobulin E antibody. The patient’s lesions may clear after three months with omalizumab.[13]
The patient was managed with dapsone, but recurrences continued, necessitating further investigations. Esophagogastroduodenoscopy revealed villous atrophy indicative of celiac disease. LABD has a 0–24% incidence of gluten-sensitive enteropathy or celiac disease. A small bowel intestinal biopsy reveals villous blunting with increased intraepithelial lymphocytes. A case of LABD with celiac disease was successfully managed with a gluten-free diet combined with 25 mg of dapsone daily. Skin lesions cleared, and a repeat biopsy of the small intestine showed normal villous architecture.[14] In this case, the patient significantly improved when dapsone and a gluten-free diet were combined.
CONCLUSION
We report a case of a 26-year-old Filipino male with LAGBD with celiac disease who has been maintained on dapsone but still had episodes of cutaneous exacerbations. Such a condition is not commonly encountered in the Philippines. Skin lesions of LAGBD have similarities with other AIBD, which clinically present as pruritic bullous lesions. The DIF is an essential tool to make the appropriate diagnosis. Systemic involvement, particularly gastroenteropathy, should also be investigated. Dapsone and a gluten-free diet have been the primary treatment modalities for this case. Additional studies, locally, would help investigate features of LAGBD and its association with other conditions.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Immunologic overlap in a case of linear IgG/IgA bullous to dermatosis responsive rituximab. JAAD Case Rep. 2021;9:57-60.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and immunological study of 30 cases with both IgG and IgA Anti-Keratinocyte cell surface autoantibodies toward the definition of intercellular IGG/IGA dermatosis. Front Immunol. 2018;9:994.
- [CrossRef] [PubMed] [Google Scholar]
- Three cases of linear IGA/IGG bullous dermatosis showing IgA and IgG reactivity with multiple antigens, particularly laminin-332. JAMA Dermatol. 2013;149:1308.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-8.
- [CrossRef] [PubMed] [Google Scholar]
- Linear IgA and IgG bullous dermatosis. An Bras Dermatol. 2016;91(5 Suppl 1):32-4.
- [CrossRef] [PubMed] [Google Scholar]
- Immunoelectronmicroscopic differentiation of linear IgA bullous dermatosis of adults with coexistence of IgA and IgG deposition from bullous pemphigoid. J Am Acad Dermatol. 1992;27:394-9.
- [CrossRef] [PubMed] [Google Scholar]
- A case of possible concurrence of dermatitis herpetiformis and linear immunoglobulin A/immunoglobulin G bullous dermatosis. Acta Dermatovenerol Croat. 2021;29:116-7.
- [Google Scholar]
- Dapsone in the management of autoimmune bullous diseases. Dermatol Clin. 2011;29:561-4.
- [CrossRef] [PubMed] [Google Scholar]
- Updates in the diagnosis and management of linear IGA disease: A systematic review. Medicina. 2021;57:818.
- [CrossRef] [PubMed] [Google Scholar]
- Humoral epitope spreading in autoimmune bullous diseases. Front Immunol. 2018;9:779.
- [CrossRef] [PubMed] [Google Scholar]
- IgA autoantibody may be the foremost pathogenic in three cases of linear IgA/IgG bullous dermatosis. Australas J Dermatol. 2023;64:e224-8.
- [CrossRef] [PubMed] [Google Scholar]
- Linear IgA/IgG bullous dermatosis successfully treated with omalizumab: A case report. Clin Case Rep. 2022;10:e05368.
- [CrossRef] [PubMed] [Google Scholar]
- Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-9.
- [CrossRef] [PubMed] [Google Scholar]






