Translate this page into:
Large ulcerated hemangioma treated with topical timolol drops

-
Received: ,
Accepted: ,
How to cite this article: Sethi JK, Syiemlieh A. Large ulcerated hemangioma treated with topical timolol drops. CosmoDerma. 2024;4:21. doi: 10.25259/CSDM_257_2023
Abstract
Infantile hemangiomas (IH) are the most common benign tumors of infancy and undergo spontaneous remission by about four to five years of age. Usually, only superficial and uncomplicated hemangiomas are treated by topical timolol while larger and complicated hemangiomas require oral propranolol or corticosteroids. Uncommonly reported in the literature is that even larger complicated IHs on low-risk sites can be treated with timolol drops locally. Our case is unique, as we treated a large ulcerated IH with oral and topical antibiotics and only topical timolol drops with rapid and complete resolution of symptoms.
Keywords
Infantile hemangioma
Large hemangioma
Ulcerated hemangioma
Timolol drops
Crusted hemangioma
INTRODUCTION
Hemangiomas are broadly classified as congenital hemangiomas (CH) and infantile hemangiomas (IH). The CH is clinically apparent from birth while IH is evident a few weeks to months after birth. The IH is the most common benign tumor of infancy affecting about 5% of babies.[1] Although spontaneous resolution is the norm in IH, some are complicated by ulceration, which can lead to scarring or even gross disfigurement.
CASE REPORT
We present a case of a 4-month-old male baby, who presented with a single reddish lesion on the back of the left thigh seen within a few days after birth. It was gradually increasing in size. The parents did not complain of evidence of pain associated with the lesion. There was history of raw area over the lesion one month back with thick brown deposit on the surface after application of local remedies. The mother was 36 years old at the time of pregnancy, and her obstetric history was uneventful, and the patient was a single baby born at term by normal vaginal delivery with 2.8 kg of birth weight. On examination, the pulse, blood pressure, and heart sounds were normal. On cutaneous examination, there was a single lobulated erythematous plaque measuring 4 × 4 cm over the middle third of the posterior aspect of the left thigh with thick black-brown crust on the surface [Figure 1]. There was no increased temperature locally or no bruit on auscultation. The differential diagnosis we considered was congenital hemangioma, but our patient presented after birth, which gradually increased in size. Kaposiform hemangioendothelioma and Kasabach-Merritt Syndrome were also considered, but the clinically well-defined and lobulated surface, superficial location of the lesion, and the gradual increase in size along with the normal hemoglobin and platelet counts helped us rule out this fatal syndrome.
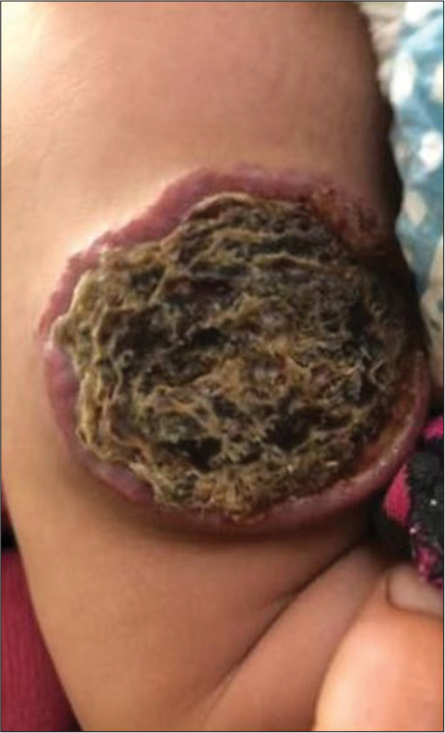
- A 4-month-old male baby presented with a single 4 × 4 cm reddish plaque with lobulated surface and a large central crust over the posterior aspect of the left thigh consistent with a crusted infantile hemangioma.
The hemogram was normal, and local ultrasonography showed a superficial localized well-defined hyperechoic lesion with no deep extension. A diagnosis of superficial IH was made, and the patient was treated with oral amoxicillinclavulanate for five days, topical mupirocin ointment, and 0.5% timolol drops topically 3 drops twice daily for three months with reduction of crusting within just 10 days and with clearance of the crust and almost flattening of the plaque by one month [Figures 2 and 3]. One year and four months later, a hypertrophic scar can be seen over the site of the hemangioma [Figure 4]. Two years after the treatment, the parents do not report any recurrence of the lesion.

- A direct view of the hemangioma on posterior aspect of the left thigh with bent popliteal fossa showing the sequence of resolution of the crusting from (a) a well-formed plaque on the first day, to (b) a reduction in size can be seen by day 10 and (c) a shrunken plaque with a thin central crust seen one month later.
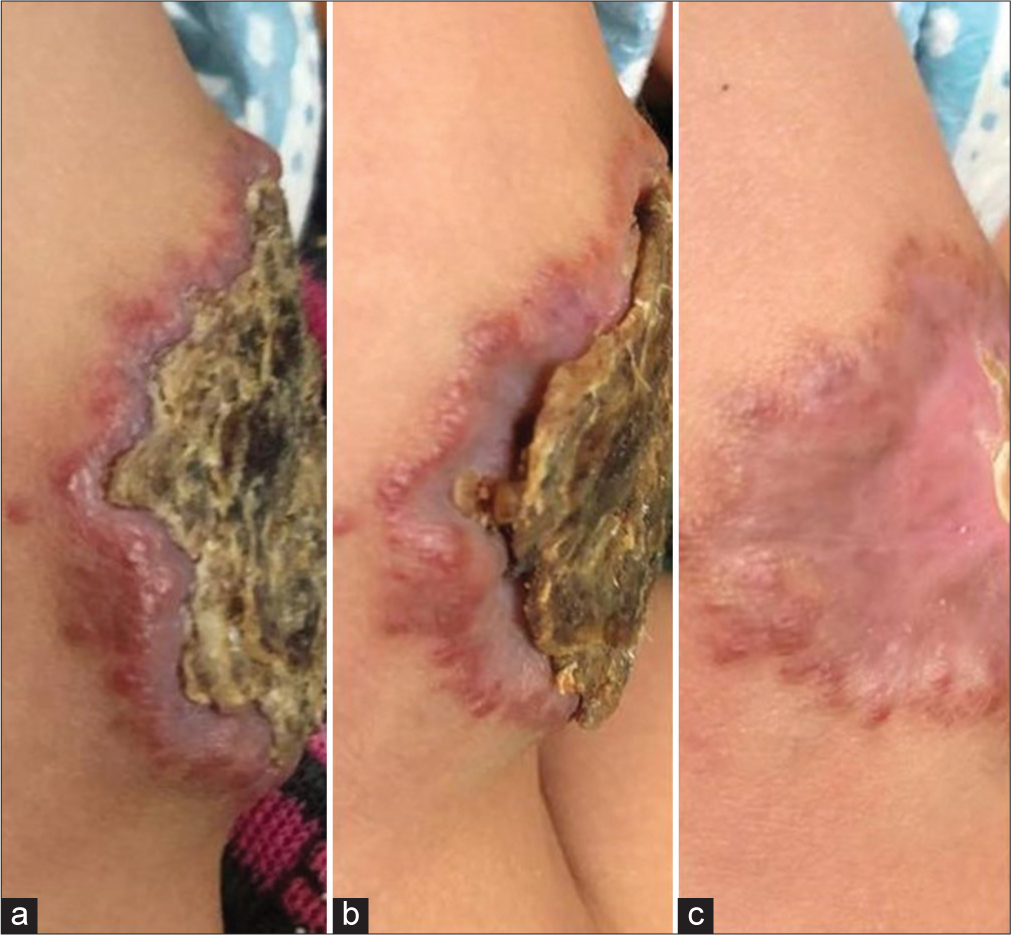
- A lateral profile of the plaque showing (a) the edematous rim with well-formed crust on day of presentation, (b) reduction of edema and shrinkage of the crust by day 10 and (c) clear involution with almost flattening of the lesion by one month.

- A hypertrophic scar is seen 1 year and 4 months after the start of therapy over the site of the ulcerated hemangioma on the posterior left thigh.
DISCUSSION
The IHs are seen in more than half the cases in the head and neck region followed by the trunk and lastly the extremities, seen only in about 15% of patients.[2] They may be single, multiple or segmental and are also classified as superficial, deep, and mixed as discussed in Table 1.[3]
| Superficial | Deep | Mixed | |
|---|---|---|---|
| Site in the skin | In the superficial dermis | Reticular dermis and subcutaneous fat | Both |
| Morphology | Usually well-defined, bright red, lobulated | Ill-defined and bluish | Has features of both |
| Age at presentation | Usually present by 4 weeks of age | May present 1–2 months later than the superficial IH | |
| Proliferative stage | Rapid in first 3 months, then slower up to months of age | Up to 1 year of age | |
| Plateau stage | Stableup to 1 year of age | ||
| Involution stage | Begins by 1 year and is usually complete by 4 years of age. | Up to 8 years of age |
The etiopathogenesis involves a hypoxic state that is commonly seen in multiple pregnancies, low birth weight, premature babies, elderly mothers, and procedures such as amniocentesis or chorionic villous sampling.[2] In our case, the older age of the mother is likely what the precipitating factor for the hemangioma. This hypoxia in turn upregulates glucose transporter 1 and vascular endothelial growth factor resulting in angiogenesis.
The diagnosis is done clinically and rarely doubtful cases may require a biopsy. An ultrasonography, magnetic resonance imaging (MRI), and computed tomography can be done for deep hemangioma. An MRI may be required in cases involving the head and neck region and the sacrocaudal region or near vital organs to look for invasion. Echocardiography may be required in large IH or multiple IH to look for cardiac insufficiency.
Previously, oral glucocorticoids were the standard of therapy. Now with the advent of beta-blockers such as propranolol, the first line of treatment has shifted.
Oral propranolol in doses starting with 0.6 mg/kg increasing to 2–3 mg/kg/day every week if required is reserved for resistant IH. There is a need for caution due to the risk of bradycardia, hypotension, hypoglycemia, and bronchospasm. Propranolol being lipophilic can cross the blood-brain barrier and result in reduce sleep and irritability. Atenolol is a selective beta-1 blocker and has fewer side effects in comparison. Timolol is a common topical beta-blocker and has been found to not have significant difference in effect compared to oral propranolol in superficial cases with almost no side effects.[4]
Oral prednisolone (2–4 mg/kg/day) is usually tapered over weeks to months but is associated with side effects such as irritability, hypertension, bone demineralization, growth retardation, and cardiomyopathy. Intralesional and topical corticosteroids can be used for smaller focal lesions.
Other alternative and adjuvant therapy are lasers. The most commonly used laser for IH is the pulsed dye laser, which emits light at wavelengths of 585 and 595 nm, at longer pulse durations of about 10 ms, having a penetration of 1.5 mm and is thus used for superficial IHs. Lasers such as the 1064 nm wavelength Q-Switched Neodymium-Doped Yttrium Aluminium Garnet laser, which penetrates up to 6 mm are commonly used for deep and mixed IH. More invasive procedures such as sclerotherapy are performed for larger or complicated IH. Surgery is usually done for IHs that can cause obstruction or even heart failure.
The complications of hemangiomas are listed as follows:
Ulceration is the most common complication seen in 25% of patients and is seen with larger and segmental IH.[5] It can result in infection, pain, non-healing, and scarring. This complication is often treated with oral beta-blockers or steroids, which are often complicated by adverse effects and surprisingly recurrence after stopping.[6] A few case reports had treated large ulcerated IH with topical timolol drops with complete resolution by one month and no recurrences at 18 months of follow-up.[7] Chen et al.[8] found in his study that there was no statistical difference between using timolol drops and oral propranolol in ulcerated hemangiomas. The risk factors for ulceration are intertriginous location of the hemangiomas such as the neck-fold, axillae and anogenital region, female gender, prematurity, low birth weight, and friction. It is heralded by black spots or white discoloration.[7]
Disfigurement appearing after resolution are seen as anetoderma, scarring or residual fibrofatty tissue, and telangiectasia.[9] The risk sites for disfigurement are the periorificial and nose areas.
Obstruction and functional impairment especially in the head and neck region, anogenital sites.[5] Facial involvement may lead to astigmatism, amblyopia, difficulty feeding or breathing.
The PHACE(S) is characterized by posterior fossa vascular malformation, segmental hemangiomas of the face, arterial anomalies, coarctation of the aorta, cardiac anomalies, eye abnormalities and in some cases, sternal defects or supraumbilical raphe defects. Any segmental IH of 5 or more cm on the head requires MRI of the head and neck region along with echocardiography and ophthalmology assessment. Those with IH in the sacral area need MRI of the pelvic and lower spine. The LUMBAR syndrome is usually characterized by lower-body hemangioma, urogenital abnormalities, ulceration, myelopathy, bony deformities, and anorectal malformations.
The presence of five or more IH is indicative of internal organ involvement such as the liver. Ultrasonographic evaluation becomes important in such cases.
High output cardiac failure is a complication associated with large or multiple IHs in the liver.
The type of treatment required vary with the depth and location in the body.[10]
Observation can be recommended for small, localized hemangiomas.
Topical therapy is used in superficial, small, or even large hemangiomas having low to no risk of disfigurement.
Systemic therapy is necessary for deep, mixed, segmental or high risk hemangiomas.
Treatment for complicated IH is usually a combination of topical and oral medications with surgery or laser.
CONCLUSION
Our patient had the characteristic, well-defined, lobulated bright red appearance with no evidence of deeper involvement and was thus classified as a superficial IH. The causative factor was likely the maternal age in our case. In addition, considering the risks and even recurrences associated with propranolol and the literature regarding topical therapy even for larger, ulcerated IH at low-risk sites, we were encouraged to treat our patient topically. Other than a few case reports, there have been no large randomized controlled trials to study the same.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Prospective study of infantile hemangiomas: Demographic, prenatal, and perinatal characteristics. J Pediatr. 2007;150:291-4.
- [CrossRef] [PubMed] [Google Scholar]
- Topical timolol vs. oral propranolol for the treatment of superficial infantile hemangiomas. Front Oncol. 2018;8:605.
- [CrossRef] [PubMed] [Google Scholar]
- Identification of putative biomarkers for infantile hemangiomas and propranolol treatment via data integration. Sci Rep. 2020;10:3261.
- [CrossRef] [PubMed] [Google Scholar]
- Propranolol for treatment of ulcerated infantile hemangiomas. J Am Acad Dermatol. 2011;64:827-32.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacious healing of ulcerated infantile hemangiomas using topical timolol. Plast Reconstr Surg Glob Open. 2016;4:e621.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the efficacy between topical timolol and pulsed dye laser in the treatment of ulcerated infantile haemangiomas: A randomized controlled study. J Eur Acad Dermatol Venereol. 2021;35:e303-5.
- [CrossRef] [Google Scholar]
- Infantile hemangioma. Part 2: Management. J Am Acad Dermatol. 2021;85:1395-404.
- [CrossRef] [PubMed] [Google Scholar]
- Infantile hemangiomas: An update on pathogenesis and treatment. J Clin Med. 2021;10:4631.
- [CrossRef] [PubMed] [Google Scholar]






