Translate this page into:
Exploring tazarotene’s role in dermatology

*Corresponding author: M. Bhagyashree, Department of Dermatology, RVM Institute of Medical Sciences and Research Centre, Hyderabad, Telangana, India. bhagya828@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Bhagyashree M, Bukke P, Matety AR. Exploring tazarotene’s role in dermatology. CosmoDerma. 2025;5:19. doi: 10.25259/CSDM_179_2024
INTRODUCTION
Topical forms of Vitamin A have been widely used since 1971. Topical retinoids are either natural or synthetic. Adapalene, tazarotene, and bexarotene are synthetic retinoids and have different chemical structures, making it more challenging for the metabolic pathway.[1] Tazarotene is an acetylenic synthetic retinoid introduced in 1997 and has a different chemical structure than other retinoids. It does not occur naturally and is approved by the United States of Food and Drug Administration (US FDA) for acne vulgaris, psoriasis, and photodamaged.[1]
STRUCTURE
Tazarotene is a synthetic retinoid and has a different structure when compared to other retinoids. It has a rigid ring-locked structure that gives limited conformational flexibility [Figure 1a].
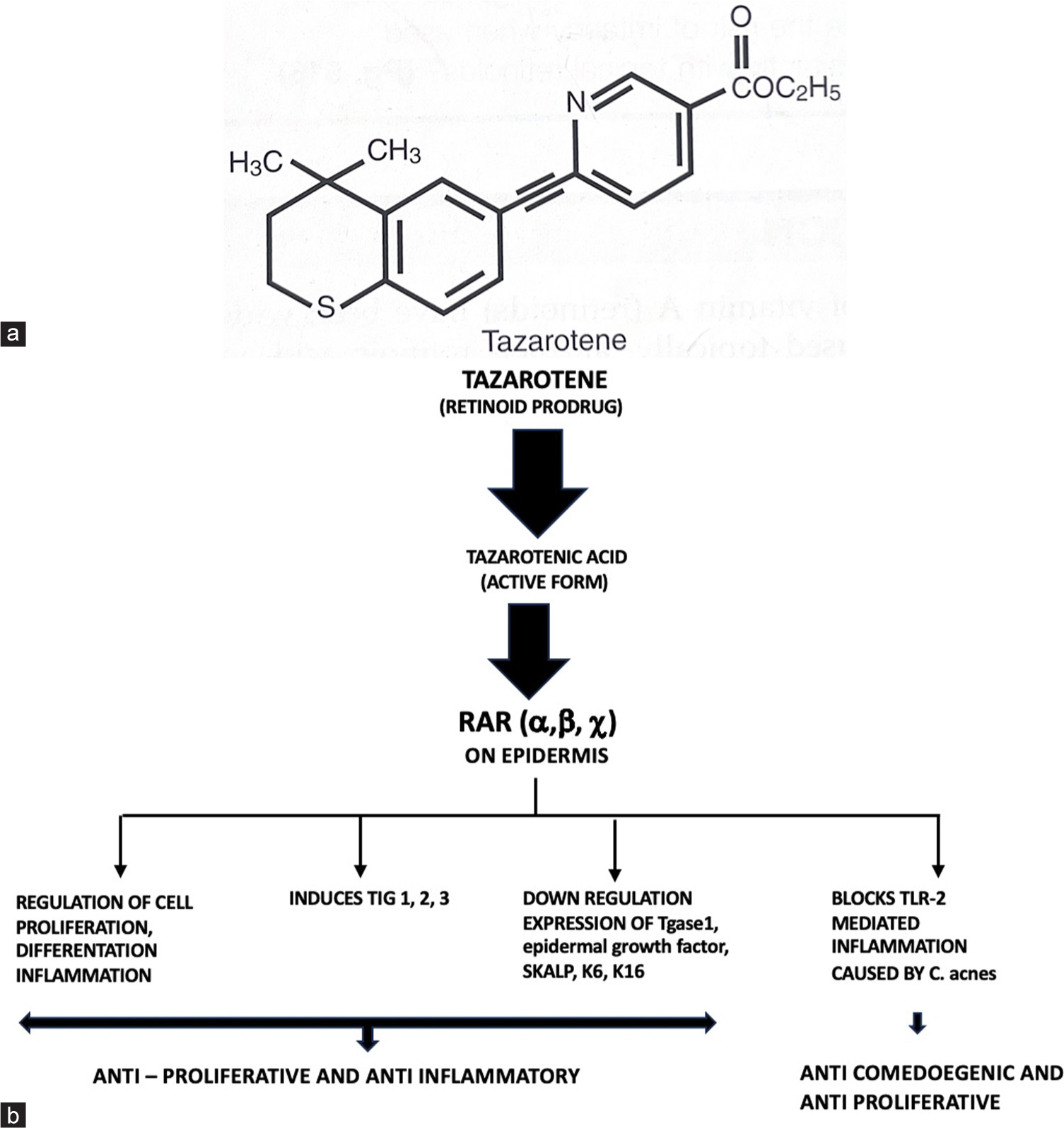
- (a) Structure of tazarotene, (b) Mechanism of action of tazarotene. RAR: Retinoid acid receptor, TIG: Tazarotene inducible genes, TLR: Toll like receptor, Tgase1: Transglutaminase 1, SKALP: Skin derived antileukoproteinase, K6, K16: Hyperproliferative keratins keratin 6, keratin 16.
MECHANISM OF ACTION
Tazarotene is a prodrug that is rapidly hydrolyzed in tissue to an active metabolite termed tazarotenic acid. Tazarotenic acid is retinoic acid receptor-specific (RAR-α, β, γ). It has a high selectivity for RAR-γ receptor, RAR-β. The predominant type of RAR expressed in the human epidermis, indicating that it may be an important mediator of retinoid action in the skin. But does not have any affinity for retinoid X receptors (RXRs).[2]
By binding to different RAR, tazarotenic acid modulates the expression of retinoid-responsive genes including:
Regulation of cell proliferation, cell differentiation, and inflammation
It downregulates the abnormal expression of keratinocyte transglutaminase 1 (Tgase 1), epidermal growth factor receptor, involucrin, skin-derived antileukoproteinase (SKALP), small praline-rich protein 2 expression, and hyperproliferative keratins K6 and K16
Tazarotene also induces the tazarotene-inducible gene-1,2,3 (TIG), which reduces keratinocyte proliferation and atypical keratinocytes[3]
Suppression of activation of the activator protein 1, which results in reduced expression of several matrix metalloproteinases from keratinocytes, which are increased in acne vulgaris
Decreased expression of toll-like receptor (TLR) 2 and decrease in ligand binding with Propionibacterium acnes, which results in inhibition of the TLR-2-induced innate response that triggers inflammation in acne[4] [Figure 1b].
PHARMACOKINETICS
Half-life is <20 min
Maximum concentration of Tazarotenic acid is by 9 h
The terminal half-life of tazarotenic acid is approximately 18 h
Total systemic absorption is 5% in normal skin and 15% in psoriatic skin
Degradation by oxidation to inactive sulfoxide and sulfone derivatives that are excreted in the urine, feces, and skin desquamation.[1]
INDICATIONS
FDA approved
Chronic plaque psoriasis
Acne vulgaris
Photodamaged skin.[5]
Off-label indications
Seborrheic keratosis (SKs)
Atrophic scar
Mycosis fungoides
Hyperpigmentation
Genodermatosis (Lamellar Ichthyosis, Erythrokeratoderma variabilis)
Keratosis pilaris.
Contraindications
Pregnancy
Hypersensitivity to tazarotene or the vehicle
Eczematous skin
Sunburn
Exposure to extreme weather
Composition
It is available in
EFFICACY OF TAZAROTENE IN DERMATOLOGY
Role of tazarotene in psoriasis
The study by Weinstein et al. found that tazarotene creams (0.05% and 0.1%), when applied once daily, were significantly more effective than a placebo in reducing the severity of plaque psoriasis. The 0.1% concentration showed the greatest clinical improvement. Both formulations demonstrated sustained therapeutic effects for up to 12 weeks after treatment. The creams were well-tolerated and generally safe, with minimal side effects, supporting their use in psoriasis treatment.[6]
Lebwohl et al. explored the effects of combining tazarotene 0.1% gel with topical corticosteroids for treating psoriasis which resulted in significantly greater reductions in scaling, redness, and overall lesion severity compared to tazarotene with placebo. Additionally, the combination therapy led to fewer local side effects like irritation. Overall, the study concluded that combining tazarotene with corticosteroids enhances effectiveness while reducing adverse reactions.[8]
Combining narrow band ultraviolet B therapy (NBUVB) therapy with topical tazarotene 0.05% gel significantly improves psoriasis treatment by accelerating clearance, reducing the number of treatment sessions, and lowering the overall cumulative NB-UVB dose. This combination therapy is well-tolerated and effective, particularly for patients with treatment-resistant plaque psoriasis.[9]
Role of tazarotene in acne
Only the 0.1% strength is approved by the US FDA for the treatment of acne. Cream 0.1% is indicated for acne vulgaris, and gel 0.1% is indicated for mild-to-moderate acne vulgaris
A study done by Swaroop showed that tazarotene 0.1% gel is a better anti-comedogenic agent with a rapid rate of clinical improvement when compared to adapalene 0.1% gel. The efficacy of both topical agents is similar for inflammatory lesions (papules and pustules).[7]
Tanghetti et al. concluded in their study that a once-daily application of 0.045% tazarotene helped in the reduction of moderate-to-severe acne and decreased patient-perceived oiliness.[10]
In photodamaged skin
Chronic sun exposure can lead to photodamage, which can present as fine and coarse wrinkles, sallowness, laxity, dyspigmentation, and tactile roughness.[11]
This study assessed the effects of tazarotene 0.1% cream on photodamaged skin. Over 24 weeks, tazarotene resulted in significant improvements compared to the vehicle, including reduced cell abnormalities in the epidermis and melanocytes, increased skin thickness and polarity, and a trend toward stratum corneum compaction. In addition, it caused widened intercellular spaces (epidermal edema). Overall, tazarotene effectively improved the structure of photodamaged skin and reduced cellular irregularities.[12]
Tazarotene in SKs
SKs are benign epidermal neoplasms presenting as waxy, brown-to-black papules and plaques. Patients often seek removal for cosmetic reasons or irritation. Twice daily application of 0.1% tazarotene cream caused remarkable clinical and histopathological correlation.[13]
Tazarotene in mycosis fungoides
Mycosis fungoides is an uncommon skin lymphoma with tumor node metastasis, early-stage shown to have a better prognosis. Topical treatments are first-line treatments in patch/plaque disease (Stage IA, IB, II A).[14]
This open-label study by Besner et al., evaluated topical tazarotene as monotherapy for early-stage cutaneous T-cell lymphoma, specifically stages IA to IIA, in 10 patients. After 6 months of treatment, 60% of patients achieved a complete response (CR), with a mean time to CR of 3.8 months. Reductions in erythema, scaling, and lesion size were observed, and 83% of responders maintained CR for at least 6 months. The treatment was well-tolerated, with no disease progression in non-responders.[15]
Tazarotene in hyperpigmentation
Tazarotene also led to improvements in hyperpigmentation, an inflammation-associated sequela of acne. Tazarotene 0.045% lotion was safe and well tolerated, with no application-site irritation or dermatitis after 12 weeks of once-daily treatment.[16]
Effects of tazarotene in genodermatoses
Type I lamellar ichthyosis improved by tazarotene 0.1% gel in a 30-year-old woman with type I lamellar ichthyosis is reported. The drug was applied to 15% of the total body surface area as follows: once daily for 2 weeks, 3 times a week for a further 2 weeks, followed by a once-weekly maintenance application. During the 1st week of treatment, a reduction of scaling on treated areas was observed. After 4 months of maintenance application, there was a marked overall improvement in the treated areas.[17]
Application of tazarotene 0.1% cream for a short contact period in case of linear Dariers disease showed improvement with minimal side effects within 4 weeks.[18]
Erythrokeratoderma variabilis, also known as Mendes da Costa syndrome, is a genodermatosis characterized by well demarcated, variable, transient, figurate patches of erythema, and localized or generalized hyperkeratotic plaques. In a case report, a child with erythrokeratoderma variabilis with no family history of this entity was successfully treated with topical tazarotene after no improvement with conventional treatment.[19]
USES OF TAZAROTENE IN OTHER CONDITIONS
Confluent and reticulated papillomatosis of Gougerot and Carteaud is an uncommon dermatosis of unclear cause that can be recalcitrant to therapy. A case report of an 11-year-old girl with an eruption that was completely cleared using tazarotene gel which was well tolerated.[20]
Keratoderma blennorrhagicum, a manifestation of Reiter’s disease which responded well to tazarotene 0.1% gel after being obstinate to other treatments.[21]
A randomized, placebo-controlled study showed that tazarotene 0.05% cream significantly improved pruritus, erythema, and roughness in keratosis pilaris on the posterior arms, proving it effective and well tolerated.[22]
ADVERSE EFFECTS
Mild-to-moderate local irritation
Erythema as seen with the “retinization period”
Peeling.[23]
SAFETY PROFILE
First, percutaneous penetration is limited, with <6% of the applied drug being absorbed into the bloodstream
Second, tazarotene and its metabolites are not lipophilic, hence, minimal systemic absorption. Hence, it has more of cutaneous effects
They are rapidly eliminated from the blood in the urine and feces
The risk of teratogenic effects is minimal
Overall, tazarotene has a good safety profile and is not associated with contact sensitization, phototoxicity, photoallergic reactions, mutagenicity, or carcinogenicity.[24]
CONCLUSION
Tazarotene topical is a type of retinoid derived from vitamin A that is available as a cream, gel, lotion, or foam. It is used in the treatment of inflammatory dermatoses or to improve the appearance and texture of skin. Combining it with other therapies could boost effectiveness and reduce side effects. Advances in delivery systems could reduce irritation, and its potential in anti-aging, systemic inflammatory diseases, and even cancer therapies warrant further exploration. Overall, while tazarotene may not be the first-line option for every dermatological condition, it can be a valuable treatment option in certain cases, particularly when other treatments have been ineffective or when used off-label for specific indications.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required, as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Topical retinoids In: Wolverton SE, ed. Comprehensive dermatologic drug therapy (3rd ed). Netherlands: Elsevier; 2103. p. :505-17.
- [CrossRef] [Google Scholar]
- Effects of narrow-band ultraviolet B and tazarotene therapy on keratinocyte proliferation and TIG3 expression. J Dermatol. 2008;35:651-7.
- [CrossRef] [PubMed] [Google Scholar]
- Use of tazarotene foam for the treatment of acne vulgaris. Clin Cosmet Investig Dermatol. 2014;7:165-70.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis. In: Pharmacology and therapeutics. Philadelphia, PA: W B Saunders; 2009. p. :983-1005. Ch. 71
- [CrossRef] [Google Scholar]
- Tazarotene cream in the treatment of psoriasis: Two multicentre, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-7.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of efficacy of once daily 0.1% tazarotene and adapalene gel for the treatment of facial acne vulgaris. Indian J Clin Exp Dermatol. 2015;1:4-8.
- [Google Scholar]
- Tazarotene 0.1% gel plus corticosteroid cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;39:590-6.
- [CrossRef] [PubMed] [Google Scholar]
- Tazarotene gel with narrow-band UVB phototherapy: A synergistic combination in psoriasis. An Bras Dermatol. 2018;93:385-90.
- [CrossRef] [PubMed] [Google Scholar]
- Improvements in acne and skin oiliness with tazarotene 0.045% lotion in patients with oily skin. J Dermatolog Treat. 2023;34:2147391.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous solar ultraviolet exposure and clinical aspects of photodamage. Indian J Dermatol Venereol Leprol. 2012;78(Suppl 1):S9-14.
- [CrossRef] [PubMed] [Google Scholar]
- Histological effects of tazarotene 0.1% cream vs. vehicle on photodamaged skin: A 6-month, multicentre, double-blind, randomized, vehicle-controlled study in patients with photodamaged facial skin. Br J Dermatol. 2004;151:1245-52.
- [CrossRef] [PubMed] [Google Scholar]
- Seborrheic keratoses: A study comparing the standard cryosurgery with topical calcipotriene, topical tazarotene, and topical imiquimod. Int J Dermatol. 2004;43:300-2.
- [CrossRef] [PubMed] [Google Scholar]
- Suggested guidelines for the treatment of mycosis fungoides in countries with limited resources. Dermatol Res Pract. 2023;2023:1360740.
- [CrossRef] [PubMed] [Google Scholar]
- Tazarotene 0.1% cream as monotherapy for early-stage cutaneous T-cell lymphoma. J Cutan Med Surg. 2016;20:244-8.
- [CrossRef] [PubMed] [Google Scholar]
- Safety of tazarotene 0.045% lotion and hyperpigmentation improvements in black participants with moderate-to-severe acne. Skin J Cutan Med. 2023;7:221.
- [CrossRef] [Google Scholar]
- Type I lamellar ichthyosis improved by tazarotene 0.1% gel. Clin Exp Dermatol. 2003;28:391-3.
- [CrossRef] [PubMed] [Google Scholar]
- Short-contact therapy with topical tazarotene in Darier disease. Actas Dermo-Sifiliogr. Actas Dermosifil. 2012;103:255-6.
- [CrossRef] [Google Scholar]
- Erythrokeratoderma variabilis successfully treated with topical tazarotene. Pediatr Dermatol. 2006;23:382-5.
- [CrossRef] [PubMed] [Google Scholar]
- Confluent and reticulated papillomatosis: Response to tazarotene. J Am Acad Dermatol. 2003;48:80-1.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of keratoderma blennorrhagicum with tazarotene gel 0.1%. J Am Acad Dermatol. 2000;43:400-2.
- [CrossRef] [PubMed] [Google Scholar]
- Tazarotene 0.05% cream for the treatment of keratosis pilaris. J Am Acad Dermatol. 2004;50:39.
- [CrossRef] [Google Scholar]
- Efficacy and safety of topical tazarotene: A review. Expert Opin Drug Metab Toxicol. 2009;5:195-210.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43:S31-5.
- [CrossRef] [PubMed] [Google Scholar]





