Translate this page into:
Evaluation of the effectiveness of a moisturizing cream as an adjuvant in the treatment of eczema: A preliminary real-world study report

*Corresponding author: Monil Yogesh Neena Gala, Department of Medical Affairs, Dr. Reddy’s Laboratories Ltd., Hyderabad, Telangana, India. monil.yogesh@drreddys.com
-
Received: ,
Accepted: ,
How to cite this article: Gala MY, Muchhala S, Rathod R, Mane A, Bhagat S, Kotak B. Evaluation of the effectiveness of a moisturizing cream as an adjuvant in the treatment of eczema: A preliminary real-world study report. CosmoDerma 2022;2:97.
Abstract
Objectives:
Eczema is a papulosquamous disease characterized by itchy, dry, rough, flaky, inflamed, and irritated skin on arms, inner elbows, backs of the knees, or head. Numerous skin conditions can be managed with the help of moisturizers. Venusia® Max is a moisturizing cream meant for eczema with a unique combination of four butters – shea, cocoa, mango, and aloe that act as emollients along with glycerin, propylene glycol, emulsifying wax, and cyclomethicone. The study’s objective is to assess Venusia® Max cream’s moisturizing efficacy when used as an adjuvant to the main line of treatment for eczema.
Material and Methods:
This monocentric, real-world setting study evaluates the effectiveness of Venusia® Max as an adjuvant, along with prescribed treatment in patients with eczema, versus prescribed treatment only (without Venusia® Max) as well as baseline. One hundred and twenty subjects enrolled in the study were divided in two groups: 1. Receiving Venusia® Max cream as an adjuvant, along with prescribed treatment and 2. receiving prescribed treatment only. The subjects were assessed for eczema area and severity index (EASI) scores, skin hydration using moisture meter-SC, transepidermal water loss (TEWL) using VapoMeter, and subject self-assessment of itching, cutaneous dryness, and burning sensation.
Results:
In the group with Venusia® Max cream, significant reduction in the EASI score, burning sensation, itching, and TEWL along with significant increase in skin hydration was observed when compared to baseline.
Conclusion:
Thus, Venusia® Max cream offers a novel and effective topical treatment for the dry skin of eczema patients.
Keywords
Eczema
Moisturizer
Steroid
Topical treatment
INTRODUCTION
Eczema (atopic eczema or atopic dermatitis, AD) is a widespread and recurring inflammatory skin disorder. According to Global Burden of Skin Disease research (2010), eczema affects ~230 million people globally.[1] There is a 2.7% overall prevalence of current eczema with 0.3% severe eczema symptoms in Indian children (6–7 years). In addition, 3.6% prevalence of current eczema and 0.4% prevalence of severe eczema are observed in Indian children (13–14 years), according to ISAAC Phase 3 trial.[2,3]
Severe excoriation and pruritus, erythematous, xerotic, lichenified, and fissured skin, with greater risk of skin infection are all characteristics of eczema.[4,5] Eczema results in cutaneous inflammation, immunological dysregulation with a T-helper 2 cell-biased response, and epidermal barrier failure and is caused by hereditary and environmental effects.[6]
Acute lesions are distinguished by ill-defined red scaly patches, frequently accompanied by edema and vesicle development, whereas chronic lesions typically comprise lichenification and pigmentation.[4] The extent and severity of eczema vary greatly and range from mild to severe. Mild eczema features include localized, occasionally dry, and mildly scaly patches. Moderate eczema features are slightly more redness and swelling, but little or no oozing or crusting. Severe eczema includes generalized involvement of entire body, resulting in acute skin failure with widespread, red, oozing, and secondary infected lesions. Both objective and subjective eczema symptoms, such as itching and insomnia, help to determine its clinical severity.[4]
Because AD is a chronic condition, best long-term care of the disease entails controlling cutaneous and systemic inflammation, usually by control of aggravation factors, appropriate general skin care, and topical therapies.[1] The most common intervention utilized as first-line treatment for treating eczema includes topical corticosteroids as they reduce inflammatory immune response, resulting in quick alleviation. The effectiveness of topical steroids is governed by appropriate strength, dose, and administration method. Basic eczema treatment involves moisturizers, which lessen the effectiveness and quantity of pharmacological therapies.[7] They alleviate intensity of AD-related symptoms by a 4-step process: Skin barrier repair, rising the water content, transepidermal water loss (TEWL) depletion, and restoration of ability of lipid barriers to attract, hold, and redistribute water.[1,8,9]
Emollients present in the moisturizers help in smoothening of skin by filling up the spaces between skin flakes with oil droplets.[8] Many trials have proved potent long- and short-term steroid-sparing effect of moisturizers in mild-to-moderate eczema.[10] The present study compared the effectiveness of a moisturizing cream as an adjuvant, along with prescribed treatment (Venusia® Max + prescribed treatment) versus baseline, and versus prescribed treatment only (without moisturizing cream as adjuvant), in patients with eczema.
MATERIAL AND METHODS
Study design
This was a real-world, two-group, randomized, and monocentric study conducted in a clinic to evaluate effectiveness of Venusia® Max cream as an adjuvant, when used with the main line of treatment for eczema.
Ethical approval
The study was approved by the Institutional Ethics Committee and was conducted in accordance with the protocol (Protocol No.: CL/095/0121/STU; Version No.: 1; dated January 15, 2021), Declaration of Helsinki and its amendments in conformity with the Good Clinical Practices principles, New Drug and Clinical Trial rules 2019, and Indian Council of Medical Research guidelines concerning medical research in humans. This trial was registered with Clinical Trial Registry of India (CTRI Registration No: CTRI/2021/06/034406).
Study participants
One hundred and twenty adult male and female subjects between 18 and 55 years of age, with AD, eruptive eczema, chronic lichenified eczema, lichen simplex chronicus, discoid eczema, allergic eczema, with EASI scores 8–21, and history of allergic dermatitis or contact allergy to cosmetics were included in the study.
Pregnant women, lactating mothers, those with dry skin, chronic illness which may influence the study results, those with any clinically significant systemic disease, with hypersensitivity to any cosmetic product or raw material, any cutaneous conditions on test site (scars, moles, papules, etc.), and localized psoriasis patients were excluded from the study.
The study details, including potential risks and benefits, were explained to the participants by the principal investigator/ coinvestigator before screening for the study. The queries of all volunteers were cleared by the principal investigator/ coinvestigator and willing participants were consented for the study.
Study settings
The study was conducted at Skin Clinic, Nashik Road, Maharashtra, India.
Sample size and study treatment
One hundred and twenty participants were randomized into two groups:
Receiving Venusia® Max cream as an adjuvant, in addition to prescribed treatment and
Receiving only prescribed treatment.
Venusia® Max is a plant-based moisturizer which is a product of Dr. Reddy’s Laboratories Ltd., Hyderabad, India, that improves skin hydration, beneficial in eczema. It contains a unique combination of four butters – shea, cocoa, mango, and aloe that act as emollients along with glycerin, propylene glycol, emulsifying wax, and cyclomethicone. Prescribed treatment included application of topical steroid (clobetasone [0.05% w/w] cream, twice daily).
Randomization
The randomization of the participants was based on their EASI scores such that the groups were matched equally with no significant difference in baseline scores. One group received prescribed treatment with Venusia® Max cream and other group received only prescribed treatment without any Venusia® Max cream.
Outcomes
Patients were evaluated on the basis of clinical evaluation, instrumental evaluation, and self-assessment. Clinical evaluation was done by assessing EASI scored in terms of percentage or numbers. To determine moisture level of skin surface, TEWL and skin hydration measurements (instrumental evaluation) were performed. Results were used to provide information with regard to status of permeability barrier in the two groups. Condition of all subjects was first stabilized for 15–20 min, in a climate- and humidity-controlled room. TEWL value was measured using VapoMeter and skin hydration was measured using Moisture Meter-SC (MMSC). Self-assessment was done by assessing cutaneous dryness, itching severity, and burning sensation. 5-D itch scale is a reliable, multidimensional scale used to evaluate itching that has been validated in individuals suffering from chronic pruritus and has been found to identify “change over time.” The scale comprises five domains, namely, duration, degree, direction, disability, and distribution measured on a 5-point Likert scale. Investigator administered self-assessment questionnaire to evaluate cutaneous dryness and burning sensation. Participants were called for follow-up visits at week 2 and week 4 wherein same parameters were measured.
Statistical analysis
Statistical analysis was carried out using 10.0 version of statistical software SPSS. Student’s t-test was used to test for statistically significant differences (P < 0.05) between time points in skin hydration using MMSC and TEWL using VapoMeter. Mann–Whitney U-test was used to test for statistically significant differences (P < 0.05) between the groups in EASI score, 5-D pruritus score, and score of burning sensation. Fisher’s exact test was used to test for statistical difference in subject self-assessment of cutaneous dryness.
RESULTS
Between July 8, 2021, and December 15, 2021, 120 patients were recruited, wherein seven subjects dropped out. Out of 113 patients, 56 subjects in the group with Venusia® Max and 57 subjects in the group without Venusia® Max completed the study [Figure 1].

- Flowchart of study participants.
Subject demographics
A total of 67 males and 46 females were included in the study. The average age of participants was found to be 38.88 years in Venusia® Max plus prescribed treatment group which was comparable to 37.98 years in prescribed treatment only group. Demographic details of all the study participants are summarized in [Table 1]. Subject demographics were comparable between the two groups.
| Parameters | Venusia® Max+Prescribed treatment (n=56) | Prescribed treatment only (n=57) | P-value |
|---|---|---|---|
| Age (Mean±SD) | 38.88±11.19 | 37.98±10.59 | 0.661# |
| Gender | |||
| Male, n(%) | 31 (55.4) | 36 (63.2) | 0.398@ |
| Female, n(%) | 25 (44.6) | 21 (36.8) |
Clinical evaluation
EASI score
Both Venusia® Max plus prescribed treatment (P = 0.001) and prescribed treatment only (P = 0.001) significantly decreased EASI score after 4 weeks compared to baseline [Figure 2]. Mean EASI score showed a significant reduction of 19.4% and 21.6% from baseline in Venusia® Max + prescribed treatment group at the end of weeks 2 and 4, respectively. There was a 22.6% and 35.0% reduction observed in prescribed treatment only group at weeks 2 and 4. At week 2, there was no significant difference in the EASI scores observed between the groups. However, there was a significant decrease (P = 0.003) in the EASI score at week 4 between the two groups.

- Changes in EASI score in the two groups over 4 weeks study duration.
Instrumental evaluation
Skin hydration using MMSC
At baseline, mean MMSC readings were 8.59 in Venusia® Max plus prescribed treatment group and 9.41 in prescribed treatment only group. At baseline, there was no statistical difference between MMSC readings for the two groups [Figure 3]. After application of Venusia® Max along with prescribed treatment, there was a significant 1.87-fold and 2.19-fold increase at the end of 2 weeks and 4 weeks when compared with baseline MMSC readings. In the prescribed treatment only group, there were 1.44-fold rise at the end of 2 weeks and 1.73-fold rise at the end of 4 weeks. At week 2 and week 4, mean skin hydration was significantly higher (P = 0.001) in Venusia® Max plus prescribed treatment group as compared to prescribed treatment only group.

- Skin hydration using Moisture Meter-SC in the two groups over 4 weeks study duration.
TEWL using VapoMeter
At baseline, there was no statistical difference in VapoMeter readings observed in the two groups. Similar to skin hydration, there was a significant decrease in TEWL compared to baseline after application of Venusia® Max (with prescribed treatment) for 2 weeks (36.92 vs. 31.75; P = 0.001) and 4 weeks (36.92 vs. 29.35; P = 0.001), as shown by decrease in mean VapoMeter readings. There were 1.16-fold and 1.26-fold reduction in Venusia® Max + prescribed treatment group at 2 and 4 weeks, respectively. A 1.12-fold and 1.19-fold reduction in prescribed treatment only group were observed at weeks 2 and 4 [Figure 4]. There were no significant differences observed in mean VapoMeter readings between both the groups.
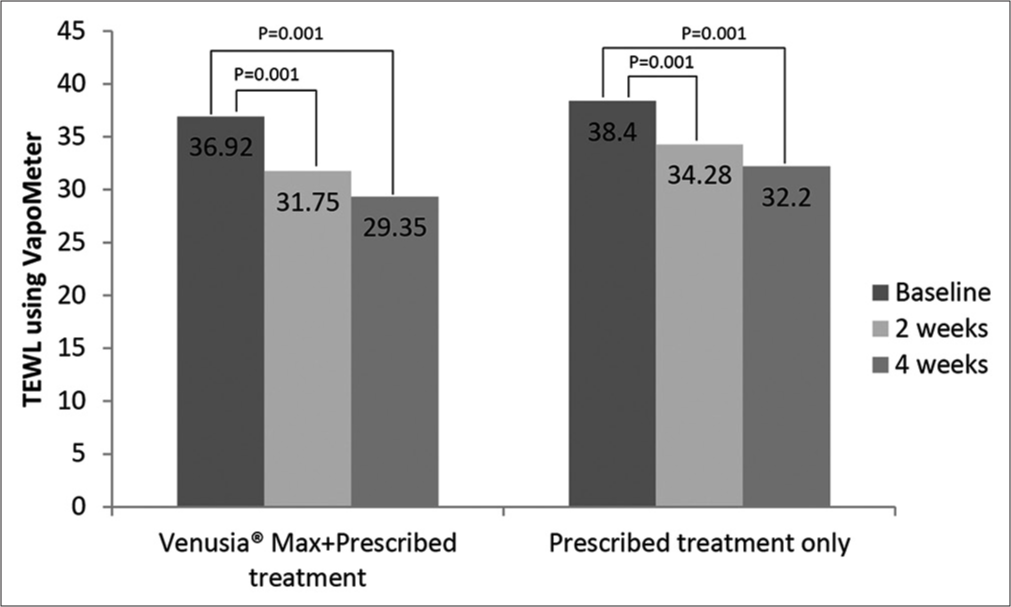
- Transepidermal water loss using VapoMeter in the two groups over 4 weeks study duration.
Self-assessment
Cutaneous dryness
Subjects’ self-assessment of cutaneous dryness indicated no improvement in cutaneous dryness in both the groups at 2 weeks and 4 weeks compared with baseline. There was no significant change in cutaneous dryness perception by patients probably because the eczematous skin remained dry and eczema did not cleared completely even after 4 weeks of treatment in both the groups.
Itching
At baseline, mean 5-D pruritus score was 15.96 in the Venusia® Max + prescribed treatment group and 15.58 in prescribed treatment only group. Mean total 5-D pruritus score showed a significant reduction of 17.0% and 24.4% in Venusia® Max + prescribed treatment group at weeks 2 and 4, respectively. On the contrary, 9.1% reduction at week 2 and 15.2% at week 4 were observed in prescribed treatment only group. At week 2 and week 4, the reduction in 5-D pruritus score was significantly more in Venusia® Max + prescribed treatment group as compared to prescribed treatment only group [Figure 5].

- 5-D pruritus score in the two groups over 4 weeks study duration.
Burning sensation
Mean score of burning sensation reported at baseline was 3.54 in Venusia® Max + prescribed treatment group which was comparable to 3.21 in prescribed treatment only group. At the end of week 2, there was a significant reduction in burning sensation in Venusia® Max + prescribed treatment and in prescribed treatment only group, as shown by decrease in the mean score of burning sensation from baseline. The mean score of burning sensation was 2.67 and 2.76 in Venusia® Max + prescribed treatment group and 2.52 and 2.01 in prescribed treatment only group, at week 2 and week 4, respectively. However, intragroup comparison at week 2 and week 4 demonstrated no significant difference in mean scores of burning sensation.
Safety assessment
During the course of the study, no participant reported any adverse events or serious adverse events.
DISCUSSION
Eczema is a chronic skin condition with symptoms such as dry skin and severe itching due to faulty skin barrier function and has a detrimental effect on patients’ life. Choice of therapy, therefore, makes a positive impact on patients’ social and personal life.[11] According to global and Indian guidelines, moisturizers are considered as basic treatment for eczema to be applied after proper hydration to improve barrier function in both adults and children.[1,7,12-18] According to Indian Dermatology Expert Board, moisturizers are cornerstone of therapy and should be used throughout all therapy lines and in maintenance phase.[1] The American Academy of Dermatology recommends that patients with AD should use moisturizers as an integral part of their care.[12,19] Emollient use is regarded as standard therapy, steroid free, and helpful for treating eczema.[20] NICE guidelines have recommended use of emollients for managing eczema in children under 12 years.[21]
Venusia® Max moisturizer tested in the study is paraben, phthalate, alcohol, mineral oil, animal origin, sulfate free (PAMAS free) and dye free and is comprised of various ingredients combined in a balanced ratio to provide deep and long-lasting moisturizing effect and, therefore, provide benefits to patients with eczema.[22,23] For people with Fitzpatrick skin phototypes III–V, Venusia® Max cream is a well-tolerated and non-irritant moisturizer.[23]
Although steroids are the therapy of choice, they have several negative effects such as atrophic skin changes including steroid atrophy, telangiectasia, striae, purpura, easy bruising, and ulceration. Other possible adverse effects include infections, acneiform eruption, tachyphylaxis, hirsutism, hyperpigmentation, hypopigmentation, and, rarely, allergic contact dermatitis.[24] Findings of the present study demonstrated various benefits of Venusia® Max when used as an adjuvant to prescribed treatment (i.e., topical steroid) such as reducing eczema severity, pruritus, burning sensation, and TEWL along with increasing skin hydration.
EASI (objective measure of severity of eczema) helped compare the effectiveness of Venusia® Max cream in relation to the signs of eczema in the present study. A meta-analysis of 77 randomized clinical trials revealed that moisturizers achieve a significant reduction in investigator-assessed disease severity scores and fewer flares in people with eczema, especially when paired with corticosteroids.[25,26] Another study demonstrated treatment success in 87.5% after 28 days of study period and a significant drop in the EASI score by day 28 after using antioxidant rich topical cream on patients twice a day.[27] The present study is in accordance with Guanti et al., 2022, and showed combination of Venusia® Max cream and prescribed treatment was effective in reducing the EASI scores in comparison to the baseline EASI scores. The study, thus, demonstrated a substantial reduction in severity of eczema by Venusia® Max cream.
Maintenance of healthy skin barrier function is important to attenuate susceptibility of the skin to various irritants, allergens, and microbes. The previous qualitative researches have demonstrated the role of emollients in improving skin hydration. A study showed increase in skin hydration and decreased TEWL facilitating restoration of xerotic skin by over-the-counter moisturizers.[9] A randomized, evaluator-blinded, and comparative study revealed that Venusia® Max moisturizing cream increased MMSC values by 92.7% indicating a long-lasting skin hydration along with reducing TEWL readings by 17.1%, 15.7%, 10.3%, and 5.6% at 4, 10, 24, and 36 h, respectively, compared to baseline. However, TEWL readings increased at 24 h (compared to 10 h readings) after application that was maintained till 36 h.[28] The present study demonstrated that Venusia® Max results in intense hydration level of the skin surface as shown by significant increase in MMSC readings in the group receiving Venusia® Max along with prescribed treatment versus baseline as well as group receiving prescribed treatment only. Similarly, a significant decrease in TEWL was observed after application of test product indicating reduced water loss and better epidermal barrier repair by Venusia® Max. Using moisturizer product, Venusia® Max, therefore, will be desirable in terms of restoring the skin barrier by increasing the cutaneous hydration observed as a result of reduction in TEWL.
The manual procedures for diagnosing skin diseases take a long time, demand expertise and excellent visual perception, and are error prone.[29] In the present study, clinical dryness self-assessment score has been obtained using a scale that had a binary response option (yes vs. no). The subject self-assessment evaluation of cutaneous dryness at 2 and 4 weeks showed that there was no change in the dryness perception by the participants after the treatment in both the groups. This finding was in contrast to the study done by Lee et al., 2016, where the usage of eczema cream containing emollients improved the cutaneous hydration score.[30] As skin dryness is prone to subjective analysis, the current binary dependent scale does not allow the clinical data to be represented accurately. Furthermore, burning sensation and itching are also subjective and difficult to measure. Another self-assessment of pruritus was done using the 5-D itch score that is a brief yet multidimensional questionnaire to assess change in itching over time and its effect on the quality of life. The present evaluation using 5-D itch score showed a significant decrease in itching after using Venusia® Max cream when compared to the baseline. Furthermore, there was a significant reduction in burning sensation reported by the subjects who used Venusia® Max as an adjunctive therapy. Positive results obtained in the objective parameters such as EASI score, skin hydration using MMSC, and TEWL using VapoMeter are, therefore, supported by the improvement in clinical dryness and mitigation of itching and burning sensation reported by the subjects. This can be attributed to the reason that Venusia® Max can help in reduction of inflammatory mediators associated with skin inflammation and in direct release of antioxidants, ultimately reducing the itching and burning sensation in eczematous skin. No adverse effects were reported due to Venusia® Max cream.
There were some limitations in this study. The study was done in a single center; hence, multicenter studies are required to reconfirm the efficacy of the test product. Furthermore, the study was conducted for a short duration of 4 weeks for a chronic condition such as eczema. Participants were not followed up on after the trial period to see if the skin barrier improved considerably. A decrease in EASI may have been noticed if the research time had been extended. Similar trials with a follow-up length of up to 8 weeks may be beneficial in patients with eczema. Furthermore, patients were not evaluated for other confounding factors (smoking and alcohol) which may aggravate the dryness of the skin.
CONCLUSION
Venusia® Max offers a novel and effective topical treatment for the dry skin of eczema patients by alleviating the severity of eczema symptoms, dryness, itching, and burning sensation along with increasing hydration and skin barrier function without any untoward side effects. In addition, this formulation could give support to reducing the amount and side effects of topical corticosteroids needed for eczema.
Acknowledgment
The authors thank the study participants, principal investigators, and site staff. The authors also thank WorkSure®, India, for providing medical writing assistance for this manuscript.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
The study was funded by Dr. Reddy’s Laboratories Ltd., Hyderabad, India.
Conflicts of interest
There are no conflicts of interest.
Contribution details
All the authors have been involved in study conceptualization, literature search, and manuscript review.
References
- Guidelines on management of atopic dermatitis in India: An evidence-based review and an expert consensus. Indian J Dermatol. 2019;64:166-81.
- [CrossRef] [PubMed] [Google Scholar]
- The burden of allergic diseases in the Indian subcontinent: Barriers and challenges. Lancet Glob Health. 2020;8:e478-9.
- [CrossRef] [Google Scholar]
- Global variations in prevalence of eczema symptoms in children from ISAAC phase three. J Allergy Clin Immunol. 2009;124:1251-8.e23.
- [CrossRef] [PubMed] [Google Scholar]
- Topical treatments for eczema: A network meta-analysis. Cochrane Database Syst Rev. 2018;2018:CD013205.
- [CrossRef] [Google Scholar]
- Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214-9.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol. 2014;134:345-50.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis: Skin-directed management. Pediatrics. 2014;134:e1735-44.
- [CrossRef] [PubMed] [Google Scholar]
- Skin hydration is significantly increased by a cream formulated to mimic the skin's own natural moisturizing systems. Clin Cosmet Investig Dermatol. 2018;11:491-7.
- [CrossRef] [PubMed] [Google Scholar]
- The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: A randomized controlled study. Dermatology. 2007;214:61-7.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-8.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-32.
- [CrossRef] [PubMed] [Google Scholar]
- Japanese guidelines for atopic dermatitis 2017. Allergol Int. 2017;66:230-47.
- [CrossRef] [PubMed] [Google Scholar]
- Management of pruritus in Indian settings: An expert opinion. Am J Dermatol Venereol. 2021;10:31-43.
- [Google Scholar]
- ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34:2717-44.
- [CrossRef] [PubMed] [Google Scholar]
- A position paper on the management of itch and pain in atopic dermatitis from the international society of atopic dermatitis (ISAD)/oriented patient-education network in dermatology (OPENED) task force. J Eur Acad Dermatol Venereol. 2021;35:787-96.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic dermatitis: A practice parameter update 2012. J Allergy Clin Immunol. 2013;131:295-9.e1-27.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of chronic pruritus: An expert consensus review. Indian J Dermatol. 2017;62:7-17.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-49.
- [CrossRef] [PubMed] [Google Scholar]
- Erratum: Guidelines of care for atopic dermatitis (journal of the american academy of dermatology (March 2004) 50 (391-404)) J Am Acad Dermatol. 2005;52:156.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic Eczema in Under 12s: Diagnosis and Management (NICE Clinical Guidelines, No. 57 London: National Institute for Health and Care Excellence; 2021.
- [Google Scholar]
- Evaluation of comedogenic potential of a paraben-free plant-based butter moisturizing cream: A double-blind, comparative study. Cosmoderma. 2021;1:52.
- [CrossRef] [Google Scholar]
- Plant-based paraben-free moisturizer, Venusia Max cream, is a nonirritant. Cosmoderma. 2022;2:25.
- [CrossRef] [Google Scholar]
- Clinical profile of cutaneous adverse effects induced by topical corticosteroids and their source of information. Int J Res. 2021;7:522.
- [CrossRef] [Google Scholar]
- From the Cochrane Library: Emollients and Moisturizers for Eczema. Dermatology. 2022;238:594-6.
- [CrossRef] [PubMed] [Google Scholar]
- Emollients and moisturisers for eczema. Cochrane Database Syst Rev. 2017;2:CD012119.
- [CrossRef] [Google Scholar]
- Efficacy and safety of an antioxidant-enriched medical device for topical use in adults with eczematous dermatitis. Dermatol Ther (Heidelb). 2022;12:1015-25.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of plant-based paraben free venusia max cream on skin hydration in healthy individuals with dry skin. J Clin Investigat Dermatol. 2021;9:4.
- [CrossRef] [Google Scholar]
- Automatic skin disease diagnosis using deep learning from clinical image and patient information. Skin Health Dis. 2022;2:e81.
- [CrossRef] [PubMed] [Google Scholar]
- A comprehensive review of the treatment of atopic eczema. Allergy Asthma Immunol Res. 2016;8:181-90.
- [CrossRef] [PubMed] [Google Scholar]







