Translate this page into:
Evaluation of skin hydration and anti-itch properties after application of a novel urea-based moisturizing cream

*Corresponding author: Biswajit Aich, Medical Affairs, Dr. Reddys Laboratories Ltd, Hyderabad, Telangana, India. biswajitaich@drreddys.com
-
Received: ,
Accepted: ,
How to cite this article: Aich B, Kumbhar P, Sanghavi A, Muchhala S, Katare S, Kotak B. Evaluation of skin hydration and anti-itch properties after application of a novel urea-based moisturizing cream. CosmoDerma. 2025;5:40. doi: 10.25259/CSDM_209_2024
Abstract
Objectives
Moisturizers are crucial in improving skin hydration and reducing transepidermal water loss (TEWL), especially in individuals with dry skin. This study aims to evaluate Venusia Ureka Cream’s efficacy in enhancing skin hydration, reducing TEWL, and providing itch relief in individuals with dry, itchy skin.
Materials and Methods
This single-center, observational study enrolled participants with dry skin. Participants applied Venusia Ureka Cream at four test sites on their forearms, with four control sites left untreated. Skin hydration and TEWL were measured using the MoistureMeter stratum corneum and VapoMeter, respectively, at baseline, 12, 24, and 36 h after application. Pruritus was assessed using a 10-point Visual Analog Scale at baseline, 6, and 12 h.
Results
The study included 32 participants (mean age: 28.66 ± 8.68 years; 75% female). Skin hydration improved significantly at occluded test sites, increasing from baseline (10.71 ± 3.45) to 36 h (37.48 ± 16.33; P < 0.001), reaching normal hydration levels, while unoccluded sites showed modest but significant improvement (10.68 ± 3.36–15.23 ± 8.05; P < 0.001). TEWL increased significantly at 24 h under occluded conditions (P = 0.040), with no significant changes at other time points or under unoccluded conditions (P > 0.05). Pruritus scores decreased by 54.6% at 6 h (P < 0.001) and 94.7% at 12 h (P < 0.001). No adverse reactions were observed.
Conclusion
Venusia Ureka Cream effectively enhances skin hydration, reduces TEWL, and provides rapid relief from pruritus. The product is well-tolerated and offers sustained hydration, making it a promising solution for individuals with dry, itchy skin.
Keywords
Dry skin
Itch relief
Moisturizers
Skin hydration
Transepidermal water loss
INTRODUCTION
The skin serves as the body’s first line of defense against external environmental factors, with the stratum corneum (SC) playing a crucial role in maintaining skin hydration and preventing the penetration of harmful substances.[1] Proper functioning of the SC is vital for healthy skin, as it acts as a barrier that retains moisture and protects against excessive transepidermal water loss (TEWL), microbial invasion, and environmental damage.[2,3] Xeroderma, or dry skin, is a common clinical condition that can lead to significant discomfort and is frequently encountered in dermatological practice. Moisturizers are key to enhancing and maintaining skin hydration, supporting the skin barrier, and preventing the onset of dry skin. Globally, approximately 75% of young individuals use moisturizing creams daily.[4] By reducing TEWL, moisturizers play a pivotal role in preserving skin barrier function and preventing conditions such as pruritus and other barrier-related disorders.[5]
Hyperkeratotic disorders, including psoriasis, chronic hand eczema (CHE), and ichthyosis, affect millions globally, presenting with pruritus, erythema, xerosis, compromised epidermal barrier function, and persistent inflammation due to a skewed immune response. Psoriasis is marked by parakeratosis, psoriasiform hyperplasia, and elevated β-defensin 2 and interleukin-36γ levels, while CHE shows a lack of the granular layer and fewer inflammatory markers.[6] Ichthyosis is characterized by a thickened cornified layer, increased non-polar lipids, and the significant role of interleukin-1 alpha in hyperkeratosis.[7] Treatment typically combines topical therapies and moisturizers to reduce inflammation, restore the skin barrier, and relieve symptoms, with moisturizers being crucial for maintaining hydration and enhancing treatment efficacy. Innovative approaches include Halobacterium halobium lysate with light therapy for psoriasis[8] and selenium-containing keratolytic agents for hyperkeratosis.[9,10]
Venusia Ureka Exfoliating and Anti-itch Moisturizing Cream is a novel formulation designed to address dry, thick, and itchy skin. With its unique combination of Urea, Pramoxine, Allantoin, Phytosqualan, and Shea Butter, this cream helps to offer a triple-action effect: hydration, exfoliation, and itch relief. It is formulated to provide rapid relief from skin irritation and dryness while promoting skin repair and barrier restoration. This study aimed to evaluate the skin hydration and anti-itch properties of Venusia Ureka Cream, assessing its efficacy in improving skin hydration, reducing TEWL, and alleviating itchiness in individuals.
MATERIALS AND METHODS
Setting and design
This single-center, observational study was conducted at C.L.A.I.M.S. Pvt. Ltd. in Mumbai, India. Before the study, the Principal Investigator or Co-investigator explained the study’s objectives, potential risks, and benefits to all participants. All participant queries were addressed, and informed consent was obtained from those willing to participate.
Participants
The study included 32 participants; women aged 18–55 with dry skin (baseline MoistureMeter SC [MMSC] readings below 20). Eligibility criteria required participants to have healthy skin on the forearms, free from scars, moles, or tattoos, and to provide informed consent. Exclusion criteria included menopausal, pregnant, or lactating women; those with known allergies to any test product ingredient; individuals on systemic or topical medical treatment within the past month; participants with chronic illnesses or skin conditions (such as atopic dermatitis, psoriasis, eczema, and hypothyroidism) that could interfere with the study outcomes; and those involved in any other clinical investigations within the preceding month.
Procedures
Following informed consent, participants were screened and enrolled based on inclusion and exclusion criteria. The forearms of each participant were cleaned, and they were acclimatized for 45 min in a controlled environment (humidity 40–60%, temperature 20–22°C). Eight 2 × 2 cm test sites were marked on each participant’s volar forearm, with four sites designated for test product application and four for control (no product application). Baseline measurements of skin hydration (MMSC) and TEWL (using VapoMeter) were taken for all eight sites.
Approximately 0.030 g of Venusia Ureka Cream (Batch No. BZL4004) was applied to four test sites (sites 1, 3, 5, and 7) by massaging for 30 s. Control sites (sites 2, 4, 6, and 8) did not receive any product application. After application, sites 1, 2, 3, 4, 5, and 6 were occluded, while sites 7 and 8 were left unoccluded for the entire study duration. At 12 h, occlusion was removed from sites 1 (test) and 2 (control) to take MMSC and VM measurements. This process was repeated at 24 and 36 h for sites 3 and 4, and sites 5 and 6, respectively. Extended occlusion enhances drug absorption and therapeutic outcomes by maintaining prolonged contact with the skin, benefiting conditions, such as psoriasis or chronic wounds. All procedures were carried out by qualified study personnel, and observations were documented in the case report form.
Outcome measures
The primary outcomes assessed were skin hydration and TEWL to evaluate skin barrier function. The MMSC measured the skin hydration level, with electrical capacitance readings that correlate to moisture content. MMSC values below 20 indicate dry skin, values between 20 and 40 indicate normal hydration and values above 40 indicate well-hydrated skin.[11]
TEWL was measured using the VapoMeter, which recorded changes in relative humidity and temperature in a closed chamber upon skin contact. TEWL values were calculated based on relative humidity increases, with reductions in TEWL readings indicating less moisture loss from the skin and an improvement in skin barrier function.
Safety assessments
Throughout the study, safety assessments were conducted to monitor any adverse reactions or intolerances to the test product. Participants were observed by qualified personnel for any signs of skin irritation or discomfort following product application. In addition, participants were instructed to report any adverse skin reactions immediately. All reported reactions or events, if any, were documented in the case report form to ensure a thorough safety evaluation. The safety of the test product was a primary consideration, and no participants experienced adverse effects during the study period.
Statistical analysis
An independent statistician conducted the statistical analysis using Statistical Package for the Social Sciences version 10.0. Data from continuous variables were summarized by treatment group, with descriptive statistics including the number of observations, mean, standard deviation (SD), or median values, and minimum and maximum ranges. Student’s t-test and analysis of variance with repeated measures were used to compare mean differences in MMSC and VM readings. Bonferroni correction was applied for post hoc analysis, with a P < 0.05 considered statistically significant.
RESULTS
Demographics
The study included 32 participants with a mean age of 28.66 years (SD = 8.68), ranging from 18 to 47 years [Table 1]. Of the participants, 25% were male (8) and 75% were female (24) [Figure 1].
| Parameter | Value |
|---|---|
| Number of participants | 32 |
| Age (years) | |
| Mean | 28.66 |
| SD | 08.68 |
| Range | 18.00–47.00 years |
SD: Standard deviation
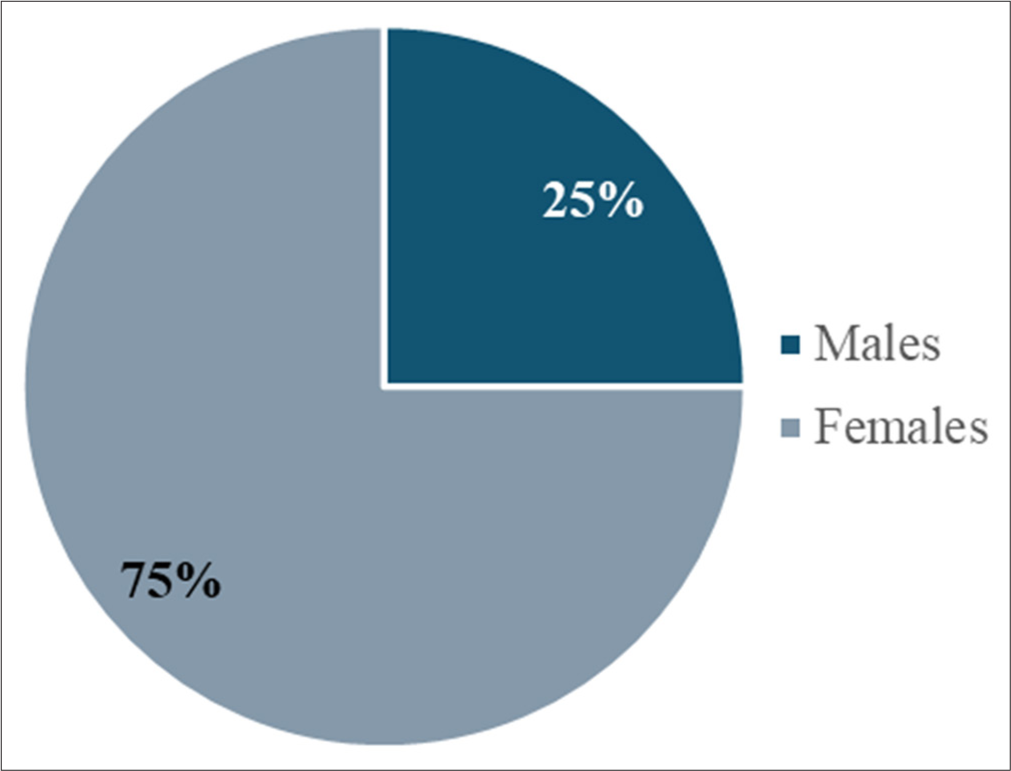
- Gender-wise distribution of the participants.
Skin hydration (MMSC)
Skin hydration was assessed at baseline and 12, 24, and 36 h after application using the MMSC. For occluded test sites, a statistically significant increase in hydration was observed at all-time points (P < 0.001). At baseline, MMSC readings indicated dry skin (mean = 10.71 ± 3.45). After applying Venusia Ureka Cream, hydration improved markedly, reaching the normal skin category within 12 h (mean = 33.25 ± 15.79) [Figure 2]. This effect was sustained for 36 h (mean = 37.48 ± 16.33), consistently outperforming control sites (baseline to 36-h mean difference = 26.46 ± 15.42, P < 0.001) [Table 2 and Figure 3]. For unoccluded sites, hydration also improved significantly (P < 0.001) from baseline (mean = 10.68 ± 3.36) to 36 h (mean = 15.23 ± 8.05), though hydration levels remained in the dry skin category (MMSC <20) throughout the study [Figure 4]. The improvement was less pronounced than at the occluded sites Table 3 and Figure 5.
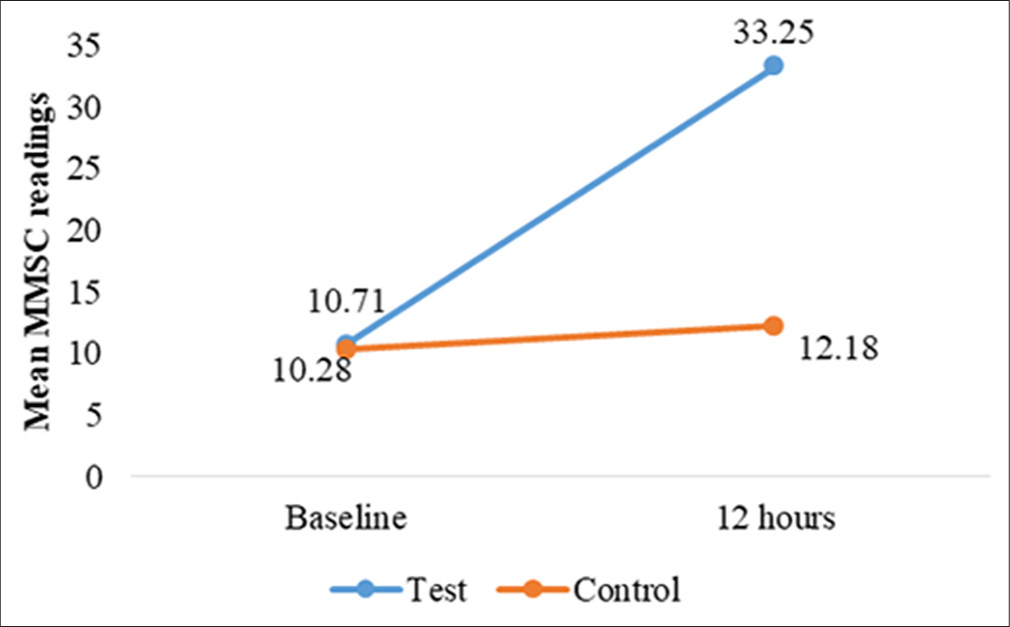
- Changes in mean MoistureMeter stratum corneum (MMSC) readings, for test and control site at 12 h.
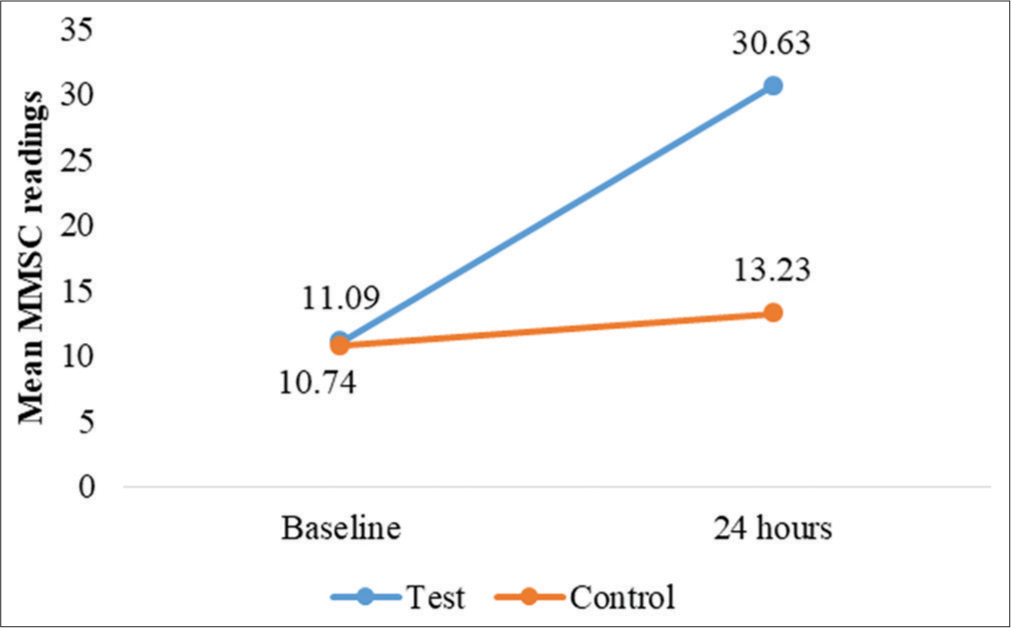
- Changes in mean MoistureMeter stratum corneum (MMSC) readings, for test and control site at 24 h.
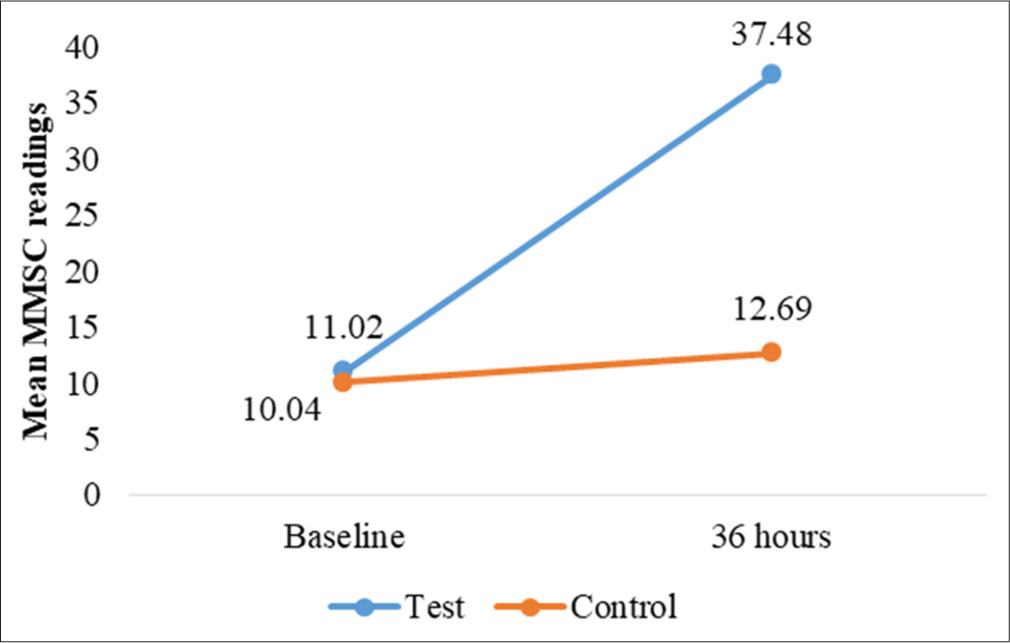
- Changes in mean MoistureMeter stratum corneum (MMSC) readings, for test and control site at 36 h.

- Changes in mean MoistureMeter stratum corneum (MMSC) readings for test and control site at 12, 24, and 36 h.
| Time point (hours) | Mean skin hydration (test site) | Mean skin hydration (control site) | P-value | Mean diff (P-value) |
|---|---|---|---|---|
| 12 | ||||
| Baseline | 10.71±03.45 | 10.28±3.01 | 0.597 (NS) | *0.001 |
| At 12 h | 33.25±15.79 | 12.18±3.42 | - | |
| 24 | ||||
| Baseline | 11.09±02.78 | 10.74±2.97 | 0.628 (NS) | *0.001 |
| At 24 h | 30.63±12.87 | 13.23±4.26 | - | |
| 36 | ||||
| Baseline | 11.02±03.85 | 12.69±3.49 | 0.259 (NS) | *0.001 |
| At 36 h | 37.48±16.33 | 8.76±1.53 | - | |
| Time point (hours) | Mean skin hydration (test site) | Mean skin hydration (control site) | P-value | Mean diff (P-value) |
|---|---|---|---|---|
| 0 | 10.68±3.36 | 10.29±3.37 | 0.644 (NS) | |
| 12 | 16.14±5.45 | 11.22±3.50 | - | *0.001 |
| 24 | 15.30±5.89 | 11.84±3.78 | - | *0.001 |
| 36 | 15.23±8.05 | 11.70±3.62 | - | *0.035 |
TEWL, VapoMeter
TEWL measurements provided insights into changes in skin barrier function and moisture retention. At the occluded test sites, TEWL increased slightly over time for both sites, with a statistically significant mean difference at 24 h (P = 0.040). However, no other time points showed significant changes [Table 4]. For unoccluded test and control sites, while TEWL readings increased slightly from baseline, no significant differences were observed between test and control sites (P > 0.05) [Table 5 and Figures 6-9].
| Time point (hours) | Mean TEWL (test site) | Mean TEWL (control site) | P-value | Mean diff (Baseline -12 h) (P-value) |
|---|---|---|---|---|
| 12 | ||||
| Baseline | 08.51±2.01 | 8.33±2.27 | 0.738 (NS) | 0.826 (NS) |
| At 12 h | 10.01±2.45 | 9.72±1.90 | - | |
| 24 | ||||
| Baseline | 7.77±1.72 | 7.84±1.98 | 0.881 (NS) | *0.040 |
| At 24 h | 9.75±2.39 | 8.91±1.35 | - | |
| 36 | ||||
| Baseline | 8.00±1.64 | 7.72±1.74 | 0.510 (NS) | 0.441 (NS) |
| At 36 h | 9.44±2.33 | 8.76±1.53 | - | |
| Time point (hours) | Mean TEWL (test site) | Mean TEWL (control site) | P-value | Mean diff (P-value) |
|---|---|---|---|---|
| 0 | 7.88±1.94 | 7.73±1.74 | 0.745 (NS) | |
| 12 | 8.97±2.33 | 8.38±1.48 | - | 0.310 (NS) |
| 24 | 8.76±1.86 | 8.03±1.49 | - | 0.179 (NS) |
| 36 | 8.48±1.71 | 8.06±1.33 | - | 0.528 (NS) |
NS: Not significant, TEWL: Transepidermal water loss
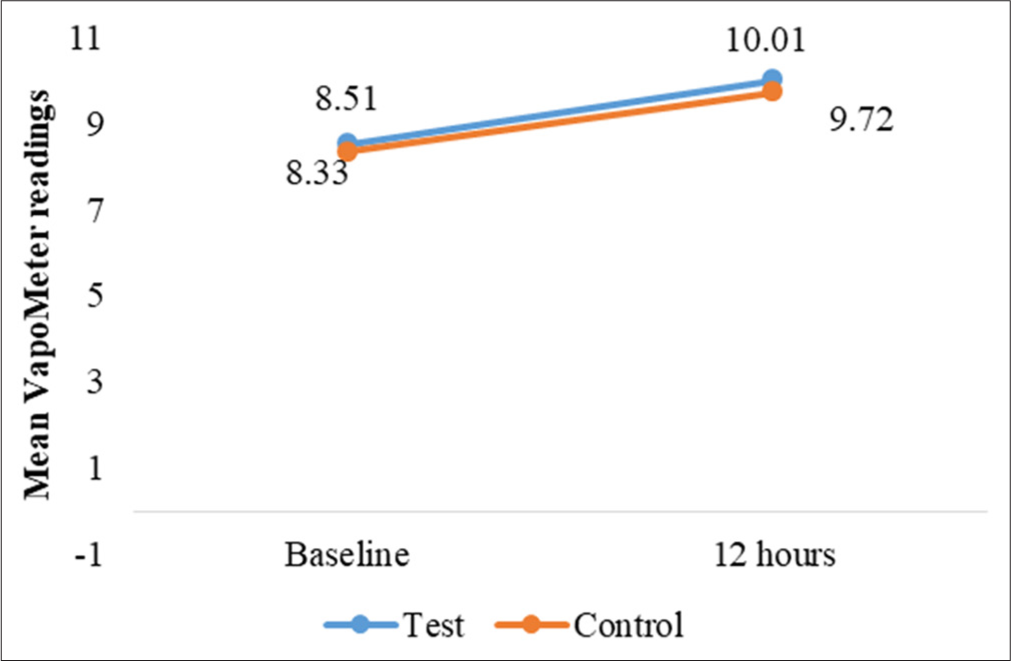
- Changes in mean transepidermal water loss readings, for test and control site at 12 h.
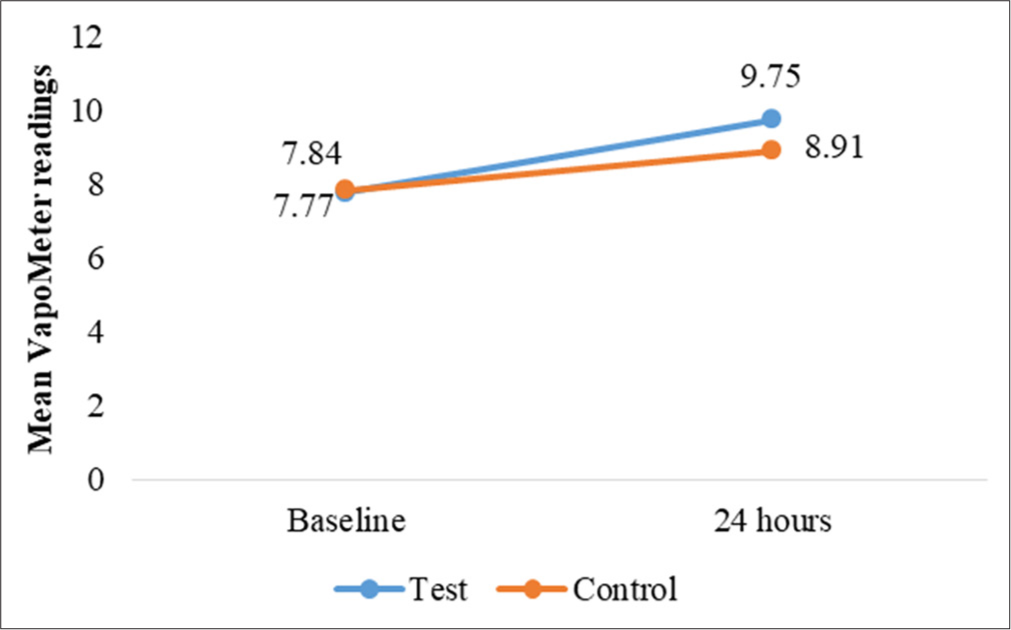
- Changes in mean transepidermal water loss readings, for test and control site at 24 h.
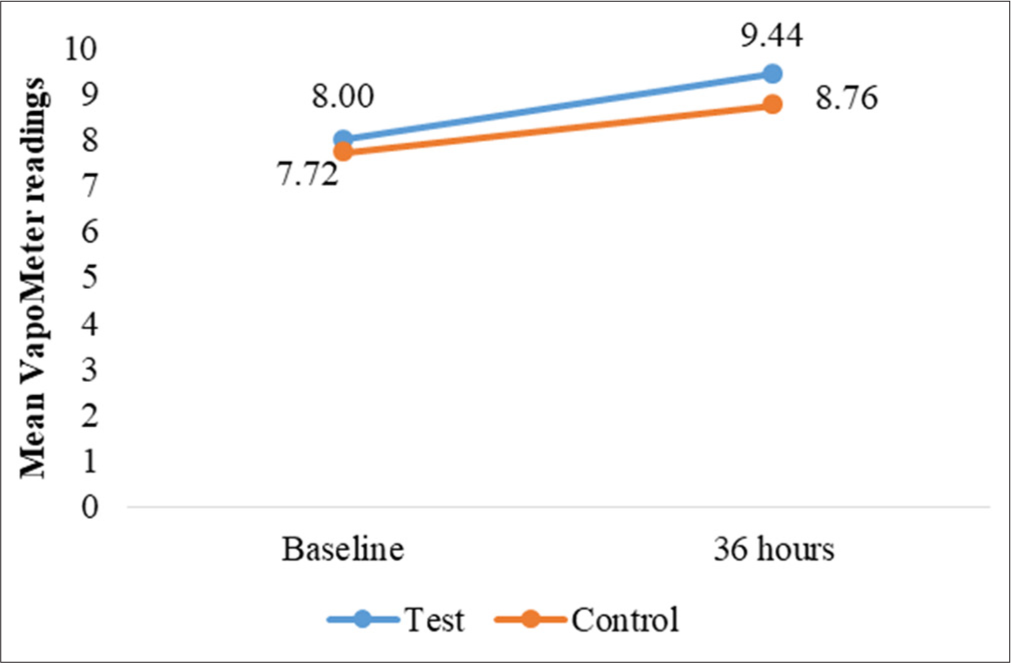
- Changes in mean transepidermal water loss readings, for test and control site at 36 h.

- Changes in mean transepidermal water loss readings, versus control site at 12, 24, and 36 h.
Anti-itch efficacy (Visual Analog Scale [VAS] for pruritus)
Pruritus levels, measured using a 10-point VAS, showed a rapid and substantial reduction. Among 17 participants reporting baseline pruritus (mean score = 5.71 ± 0.85), scores decreased significantly by 54.6% at 6 h (mean = 2.59 ± 1.28, P < 0.001) and by 94.7% at 12 h (mean = 0.29 ± 0.59, P < 0.001) [Table 6]. Our findings showed a significant reduction in pruritus scores at both 6 and 12 h after the application of the test product, with reductions of 54.6% and 94.7%, respectively, for both occluded and unoccluded sites [Table 7 and Figure 10].
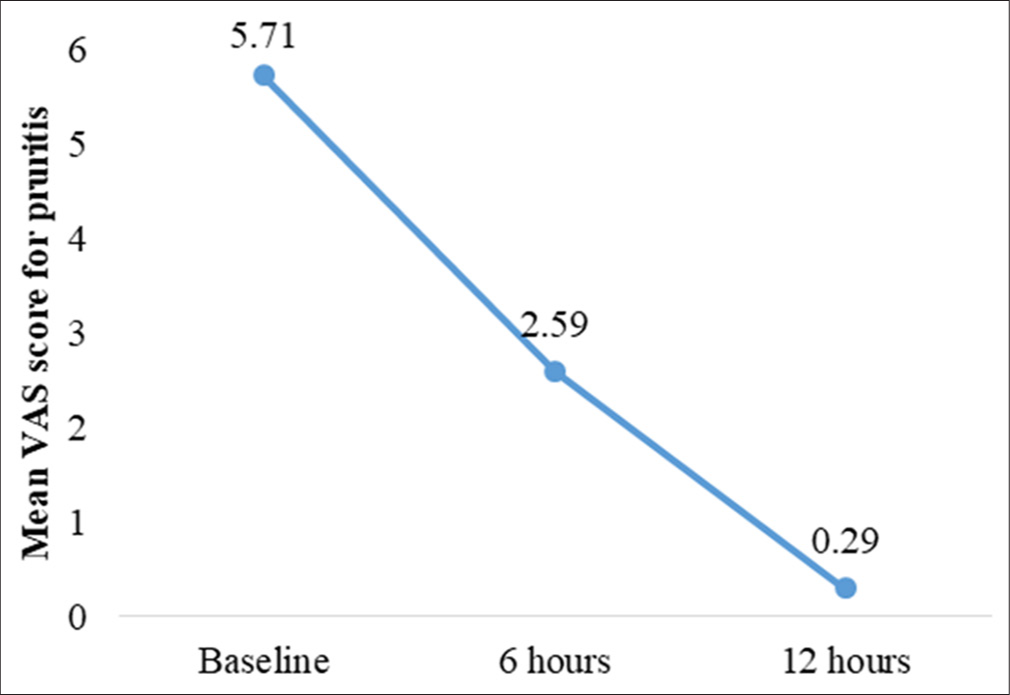
- Changes in mean visual analog scale (VAS) score for pruritis as scored by participants.
| Time point (hours) | Mean VAS score (pruritus) | Mean difference from baseline | P-value |
|---|---|---|---|
| Baseline | 5.71±0.85 | - | - |
| 6 | 2.59±1.28 | −3.12±1.32 | 0.001 |
| 12 | 0.29±0.59 | −5.41±1.06 | 0.001 |
VAS: Visual Analog Scale
| Time (hours) | Mean pruritus score (VAS) -occluded | Reduction (%) -occluded | Mean pruritus score (VAS) - unoccluded | Reduction (%) - unoccluded |
|---|---|---|---|---|
| Baseline | 5.71±0.85 | 0 | 5.71±0.85 | 0 |
| 6 h | 2.59±1.28 | 54.6 | 2.59±1.28 | 54.6 |
| 12 h | 0.29±0.59 | 94.7 | 0.29±0.59 | 94.7 |
VAS: Visual Analog Scale
Safety evaluation
No adverse skin reactions or intolerances were observed at any test sites during the study, and the participants reported no adverse events.
DISCUSSION
Venusia Ureka Cream significantly improved skin hydration and reduced itchiness within 6 h, with effects lasting up to 12 h. Hydration remained stable at occluded sites for 36 h, while TEWL readings supported enhanced moisture retention and skin barrier function.
Moisturizers are essential for improving skin hydration, especially for individuals with dry skin. They work by increasing water content in the SC, and binding corneocytes together to enhance moisture retention.[3] Studies demonstrate the efficacy of moisturizers, such as Venusia Max Lotion, in sustaining hydration for up to 36 h compared to untreated skin.[11] Devices, such as the MMSC and Corneometer, which employ capacitance-based methods, consistently validate these hydration benefits. Similarly, in studies involving conditions, such as allergic contact dermatitis, single applications of ceramide-based, and other moisturizers have shown lasting hydration effects in both patients and healthy individuals.[12] Our study supports these findings, with Venusia Ureka Cream significantly enhancing hydration at occluded and unoccluded test sites. Occlusion amplified the hydration effect, with MMSC values indicating a shift from dry to normal skin levels within 12 h, and hydration levels remained elevated at 36 h. A study involving 30 healthy women with dry skin reported that after applying Venusia Max Cream, MMSC values increased by 92% at 10 h, 48% at 24 h, and 34% at 36 h compared to baseline. In addition, TEWL values decreased by 17.1% at 4 h, 15.7% at 10 h, 10.3% at 24 h, and 5.6% at 36 h post-application.[4] Urea, a key ingredient in Venusia Ureka Cream, is well-recognized for its moisturizing properties, as shown in formulations containing 3% and 10% urea, which improve skin hydration and reduce TEWL.[13,14] A study on chronic eczematous dermatitis patients further demonstrated the long-term benefits of urea-based products, with significant hydration gains over 4 weeks.[15]
In our study, Venusia Ureka Cream significantly improved skin hydration at both occluded and unoccluded sites, with hydration reaching the normal skin category within 12 h for occluded sites (mean = 33.25 ± 15.79) and sustained for 36 h (mean = 37.48 ± 16.33) (P < 0.001). While hydration also improved for unoccluded sites (baseline mean = 10.68 ± 3.36 to 36-h mean = 15.23 ± 8.05), it remained in the dry skin category throughout the study, with a less pronounced effect compared to occluded sites.
Our study showed that pruritus scores decreased by 54.6% within 6 h and by 94.7% after 12 h, highlighting the cream’s rapid and substantial anti-itch efficacy. Urea-based formulations are known for their effectiveness in reducing itch, particularly when combined with agents, such as polidocanol, which provides fast relief for conditions such as atopic eczema.[16] Clinical studies consistently report reduced itch and improved self-assessed itch scores with urea-based creams, underscoring their strong anti-pruritic potential.[15,17] However, individual response to treatment can vary, and some patients may benefit from additional therapies for optimal relief.
Occlusion with Venusia Ureka Cream notably improves skin hydration and reinforces the skin’s barrier function, as evidenced by reduced TEWL levels at the occluded sites compared to the control group in our study. While some increase in TEWL was observed, the occlusion helped retain moisture without compromising the barrier, whereas unoccluded sites showed milder hydration benefits. TEWL is a key measure of skin barrier health, and research highlights those certain ingredients, such as petrolatum, strengthen the barrier, while others, such as some emulsifiers, may weaken it. Formulation differences, such as the presence of niacinamide, also impact barrier effects, as seen in Crowther et al.’s study, where one moisturizer notably improved SC thickness and function.[18] Venusia Ureka Cream, being free from parabens, alcohol, and mineral oil, minimizes irritation and pore congestion, emphasizing the importance of ingredients that support both hydration and skin barrier integrity.
Clinical studies affirm the safety and tolerability of Venusia Ureka Cream for sensitive skin, with no adverse reactions or intolerances reported. Similarly, a micro peeling cream study demonstrated good tolerability on sensitive skin, enhancing texture without inflammation.[19] Although these studies support the cream’s suitability for sensitive skin, individual variability in skin reactions should be considered.
Strengths and limitations
This study demonstrates several strengths, including the use of validated instruments (MMSC and VapoMeter) for objective assessments, a comprehensive evaluation of hydration, TEWL, and pruritus under both occluded and unoccluded conditions, and rapid as well as sustained results up to 36 h. In addition, the study ensured safety, with no adverse reactions observed, and its focus on individuals with clinically dry skin enhances real-world relevance. However, limitations include small sample size, short duration (36 h), single-center design, exclusion of participants with chronic skin conditions, and the absence of a direct comparator, which limits generalizability and comparative insights.
CONCLUSION
This study demonstrated that Venusia Ureka Cream significantly improves skin hydration, reduces TEWL, and alleviates pruritus in individuals with dry, itchy skin. The cream’s hydrating and itch relief was rapid, with notable improvement in skin hydration within 12 h and a marked reduction in itchiness by 94.7% at 12 h. Occlusion enhanced the cream’s efficacy, contributing to sustained hydration and improved skin barrier function over 36 h. The product was well-tolerated with no adverse reactions reported. These findings support the use of Venusia Ureka Cream as an effective treatment for dry and itchy skin, enhancing both hydration and comfort for users.
Ethical approval
The research/study was approved by the Institutional Review Board at Claims Independent Ethics Committee, number CL/038/0624/STU, dated 22nd July 2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
All the authors are employees of Dr. Reddy’s Laboratories Ltd.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22-42.
- [Google Scholar]
- A look at epidermal barrier function in atopic dermatitis: Physiologic lipid replacement and the role of ceramides. Skin Ther Lett. 2012;17:6-9.
- [Google Scholar]
- Moisturizers: The slippery road. Indian J Dermatol. 2016;61:279-87.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of plant-based paraben free Venusia Max cream on skin hydration in healthy individuals with dry skin. J Clin Invest Dermatol. 2021;9:4.
- [CrossRef] [Google Scholar]
- Over-the-counter topical skin products-a common component of skin disease management. Cutis. 2004;74:55.
- [Google Scholar]
- Differential histopathological and immunohistochemical findings between palmar psoriasis and chronic hand eczema. Eur J Dermatol. 2020;30:710-5.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-1 alpha blockade prevents hyperkeratosis in an in vitro model of lamellar ichthyosis. Hum Mol Genet. 2010;19:2594-605.
- [CrossRef] [PubMed] [Google Scholar]
- Method for treating hyperkeratotic disease with a Halobacterium halobium lysate. US Patent 6238663B1. [Last accessed on 2025 Mar 26]
- [Google Scholar]
- Compositions for the treatment of hyperkeratosis disorders. WO2017182885A2. [Last accessed on 2025 Mar 26]
- [Google Scholar]
- Effects of venusia max lotion on skin hydration and skin barrier in patients with dry skin. Cosmoderma. 2021;1:41.
- [CrossRef] [Google Scholar]
- Skin hydration assessment through modern non-invasive bioengineering technologies. Maedica. 2014;9:33.
- [Google Scholar]
- A double-blind comparison of two creams containing urea as the active ingredient. Assessment of efficacy and side-effects by non-invasive techniques and a clinical scoring scheme. Acta Derm Venereol Suppl (Stockh). 1992;177:34-43.
- [CrossRef] [Google Scholar]
- Improvement of skin moisture and skin texture with urea therapy. Hautarzt. 1989;40:67-70.
- [CrossRef] [Google Scholar]
- Efficacy and tolerability of a repairing moisturizing cream containing amino-inositole and urea 10% in adults with chronic eczematous dermatitis of the hands. Ital J Dermatol Venereol. 2023;158:42-8.
- [CrossRef] [PubMed] [Google Scholar]
- Large-area treatment of itchy, sebostatic dermatoses with a polidocanol-urea combination. Curr Dermatol. 2003;29:77-81.
- [CrossRef] [Google Scholar]
- An evaluation of the moisturizing and anti-itch effects of a lactic acid and pramoxine hydrochloride cream. Cutis. 2004;73:135-9.
- [Google Scholar]
- Measuring the effects of topical moisturizers on changes in stratum corneum thickness, water gradients and hydration in vivo. Br J Dermatol. 2008;159:567-77.
- [CrossRef] [PubMed] [Google Scholar]
- In-vitro efficacy investigation and an open-label, single-arm clinical study of a gentle micropeeling cream for sensitive and non-sensitive skin. Cosmetics. 2022;9:138.
- [CrossRef] [Google Scholar]






