Translate this page into:
Clinico-mycological profile of dermatophytosis in a tertiary care hospital in North-Eastern India

*Corresponding author: Anita Marak, Department of Dermatology and STD, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India. anita.marak@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Paul D, Marak A, Thappa DM, Verma S, Lamba R, Chhangte MZ, et al. Clinico-mycological profile of dermatophytosis in a tertiary care hospital in North-Eastern India. CosmoDerma. 2023;3:190. doi: 10.25259/CSDM_215_2023
Abstract
Objectives:
While studies from different parts of India do show an increasing trend and change in the pattern of dermatophytosis, the same cannot be said about Northeast India. This study was therefore undertaken to identify the clinical and mycological profile of dermatophytosis in a tertiary care hospital in North-Eastern India. Furthermore, this study will help to identify the various risk factors and study the impact of comorbidities on the disease pattern.
Material and Methods:
All diagnosed cases of dermatophytosis (either KOH mount or culture positive or both) were included in the study. Data collection was done in the preformed pro forma, which included demographic and clinical variables, presence of risk factors, and comorbidities.
Results:
A total of 111 diagnosed cases of dermatophytosis were included in our study with ages ranging from 3 to 73 years and a mean age of 32.8 years. In adults, the majority belonged to 21–30 years (34.2%) while the pediatric population comprised 8.1%. KOH mount positivity was seen in 90.1%, and culture positivity was seen in 87.4%. History of topical steroid use was present in 69.4% of patients while 62.2% had unhygienic practices, and 9% had immune-compromised status. Combined infection (27.9%) was the most common clinical type followed by tinea cruris (24.3%), tinea corporis (22.5%), and onychomycosis (14.4%). Trichophyton rubrum was found to be the most common isolate.
Conclusion:
Our study revealed that combined infection (27.9%), tinea cruris (24.3%), and tinea corporis (22.5%) continue to be the three most common presentations clinically while onychomycosis (14.4%) has become less frequent of a presentation. Interestingly, our study revealed that it was the most frequent manifestation in patients with comorbidities (38.46%). T. rubrum and Trichophyton mentagrophytes were the predominant species that were isolated.
Keywords
Dermatophytosis
Tinea
Trichophyton
KOH mount
Steroid
North-Eastern
INTRODUCTION
Dermatophytosis is predominantly a tropical disease, and recently there has been a dramatic change in the trend of this disease. Not only there has been an increase in its incidence at an alarming rate in our country[1] but also the clinical presentation in terms of disease severity, treatment response, relapse rate,[2] and changing mycological profile in different parts of the world have all led to its increased morbidity impacting on the quality of life[3] and posing a greater therapeutic challenge.
There are studies supporting these changes from different parts of the country; however, studies pertaining to this part of India where climatic conditions favor the growth of the fungi are almost negligible. Hence, we undertook this study to have a better understanding of the clinical presentation, the demographic profile, associated risk factors, and the current mycological profile prevalent in this part of India and compare its trends with other parts of the country.
MATERIAL AND METHODS
An observational cross-sectional study was conducted in a tertiary care hospital in North-Eastern India from March 2020 to June 2021 after obtaining ethical approval from the Institute Ethics Committee.
All diagnosed cases of dermatophytosis (either KOH mount or culture positive or both) were included in the study. Written informed consent was obtained from all the study subjects/ guardians after explaining the nature and purpose of the study. A detailed history was obtained from patients in a pre-designed pro forma including age, sex, occupation, state of residence, socioeconomic status, duration of disease, history of steroid use, history of antifungal use, immunocompromised status, hygienic practices, and comorbidities.
Statistical analysis
Descriptive statistics were used for data analysis. The continuous variables are presented as mean ± standard deviation (± SD) or median (range) if the data are skewed. Categorical variables are presented as absolute numbers and percentages.
RESULTS
A total of 111 diagnosed cases of dermatophytosis were included in our study with a prevalence of 6.32 among our outpatient department (OPD) patients. The mean age of the study population was 32.8 years with ages ranging from 3 to 73 years.
Demographic profile of the study population
Tables 1 and 2 show the demographic profile of our study population.
| Age in group | Number of patients | Percentage |
|---|---|---|
| ≤20 | 18 | 16.2 |
| 21–30 | 38 | 34.2 |
| 31–40 | 30 | 27.0 |
| 41–50 | 12 | 10.8 |
| 51–60 | 10 | 9.0 |
| >60 | 3 | 2.7 |
| Total | 111 | 100.0 |
| Sex | Number of patients | Percentage |
| Male | 85 | 76.6 |
| Female | 26 | 23.4 |
| Total | 111 | 100 |
| Socioeconomic status | Number of patients | Percentage |
| Lower middle | 57 | 51.4 |
| Upper middle | 27 | 24.3 |
| Upper lower | 24 | 21.6 |
| Upper | 3 | 2.7 |
| Total | 111 | 100 |
| Number | Mean | SD | Minimum | Maximum | Median | |
|---|---|---|---|---|---|---|
| Age (in years) | 111 | 32.8 | 13.0 | 3.0 | 73.0 | 30.0 |
| Disease duration (in months) | 111 | 15.8 | 18.4 | 0.5 | 96.0 | 6.0 |
SD: Standard deviation
Age group distribution
In our study, the majority of cases were seen in the age group 21–30 years (34.2%) followed by 31–40 years (27%). Nine patients (8.1%) belonged to the pediatric age group. The mean age (mean ± SD) of the study population was 32.8 ± 13.0. The youngest patient was three years old while the oldest patient was 73 years old. The median age of patients was 30 years.
Sex distribution
The majority of patients were male (76.6%) with a male-to-female ratio of 3.26.
Socioeconomic status
The majority of patients 57 (51.4%) belonged to lower middle socioeconomic status followed by upper middle (24.3%) and upper lower (21.6%). Only three patients (2.7%) were from the upper class.
Duration of disease
The mean duration of the disease was 15.8 months, and the median disease duration was six months while the maximum disease duration noted was 96 months (8 years).
Risk factors and comorbidities
Table 3 shows various risk factors and comorbidities associated with dermatophytosis in our study.
| Personal hygiene | Number of patients | Percentage |
|---|---|---|
| Unhygienic | 69 | 62.2 |
| Hygienic | 42 | 37.8 |
| History of steroid use | Number of patients | Percentage |
| Yes | 77 | 69.4 |
| No | 34 | 30.6 |
| History of anti-fungal use | Yes | No |
| Yes | 33 | 29.7 |
| No | 78 | 70.3 |
| Immunocompromised status | Number of patients | Percentage |
| Yes | 10 | 9.0 |
| No | 101 | 91.0 |
| Total | 111 | 100 |
| History of other comorbidities | Number of patients | Percentage |
| No comorbidities | 98 | 88.3 |
| DM | 7 | 6.3 |
| Hypothyroidism | 2 | 1.8 |
| Alopecia areata | 1 | 0.9 |
| Leprosy | 1 | 0.9 |
| Psoriasis | 1 | 0.9 |
| Hypertension+DM | 1 | 0.9 |
| Total | 111 | 100.0 |
DM: Diabetes mellitus
Hygienic versus unhygienic practices
Unhygienic practices of sharing towels and clothes among family members and not taking baths daily were seen in a significant proportion (62.2%) of patients in this study.
History of steroid and antifungal use
History of topical steroid formulation was seen in 77 (69.4%) patients, and 33 (29.7%) patients had a history of antifungal use. Combined usage of topical steroids and antifungal agents was noted in 12.6% of patients.
Immunocompromised status
Among study participants, 10 (9.0%) patients had immunocompromised status. The most common clinical presentation was tinea corporis and onychomycosis in this group (30% each). Trichophyton growth was detected in 80% of patients.
History of comorbidities
A total of 11.7% of patients had the presence of some comorbidities of which diabetes mellitus (DM) (6.3%) was the most common comorbidity noted followed by hypothyroidism (1.8%). Among other comorbidities, alopecia areata, leprosy, psoriasis, and both hypertension and DM were distributed equally in the study (0.9% each). Table 3 shows the associated risk factors and comorbidities in our study population.
Clinical profile of patients in our study
Combined infection (27.9%) was the most common clinical type followed by tinea cruris (24.3%), tinea corporis (22.5%), and onychomycosis (14.4%). Combined infection of tinea cruris and tinea corporis [Figure 1], which comprised 22.5% of total cases, was the most common followed by tinea cruris + tinea corporis + tinea faciei [Figure 2], which is 1.8% of total cases. Tinea faciei [Figure 3] comprised 6.3% of cases while tinea pedis was 2.7% of total cases in the study.

- Combined infection – tinea corporis with cruris.

- Tinea corporis with faciei.

- Tinea faciei involving right side of face.
In male patients, 29.4% of the patients had combined infection of which tinea corporis + tinea cruris was most common (24.7%) followed by tinea cruris [Figure 4] (24.7%) and tinea corporis (21.1%). Among female patients, tinea corporis (26.9%) and tinea cruris (23.1%) were the most common clinical type, and combined infection comprised 19.2% of total female participants.

- Extensive tinea cruris.
Among the pediatric age group, tinea corporis (44.44%) was the most common type followed by combined infection (22.22%). Tinea capitis, tinea faciei, and onychomycosis were the other clinical presentations in this age group (11.1% each).
Table 4 represents the clinical profile of patients in our study.
| Clinical type | Number of patients | Percentage |
|---|---|---|
| Combined infection | 31 | 27.9 |
| A. Tinea cruris+Tinea corporis | 25 | 22.5 |
| B. Tinea cruris+Tinea corporis+Tinea faciei | 2 | 1.8 |
| C. Tinea cruris+Tinea faciei | 1 | 0.9 |
| D. Tinea cruris+Tinea pedis | 1 | 0.9 |
| E. Tinea corporis+Tinea pedis | 1 | 0.9 |
| F. Tinea corporis+Tinea faciei | 1 | 0.9 |
| Tinea cruris | 27 | 24.3 |
| Tinea corporis | 25 | 22.5 |
| Onychomycosis | 16 | 14.4 |
| Tinea faciei | 7 | 6.3 |
| Tinea pedis | 3 | 2.7 |
| Tinea capitis | 1 | 0.9 |
| Tinea manuum [Figure 5] | 1 | 0.9 |
| Total | 111 | 100.0 |
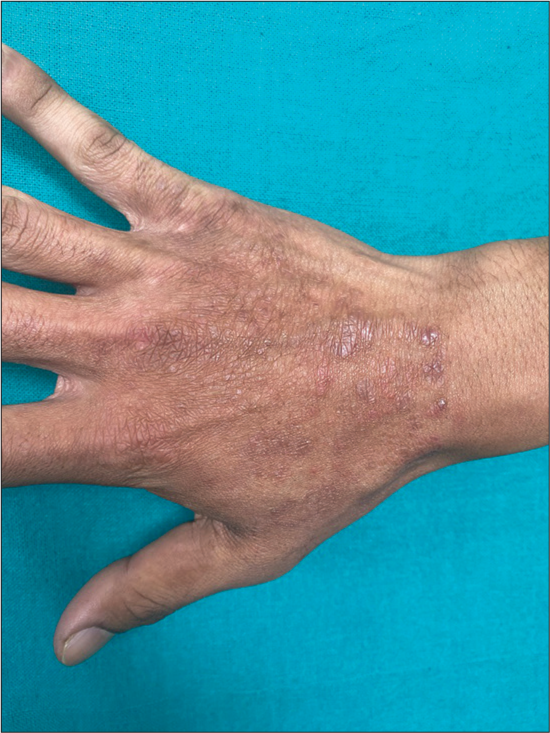
- Tinea manuum extending to involve the dorsum of hand.
Cultural characteristics of our study
Species grown in culture
Trichophyton rubrum was the most common species grown (26.8%) followed by Trichophyton mentagrophytes (17.5%) and Trichophyton tonsurans (15.4%). In another 23 patients, there was growth of Trichophyton, but species identification could not be done. Mixed growth was seen in 11.3% of patients among which 6.18% of patients showed mixed growth of T. mentagrophytes and T. tonsurans. Trichophyton verrucosum was isolated in 3.09% of patients while Trichophyton interdigitale and Trichophyton ajelloi were grown in 1.03% each. T. mentagrophytes (22.2%) was the most common isolate among the pediatric age group. Pie Diagram 1 shows the distribution of species grown in culture.
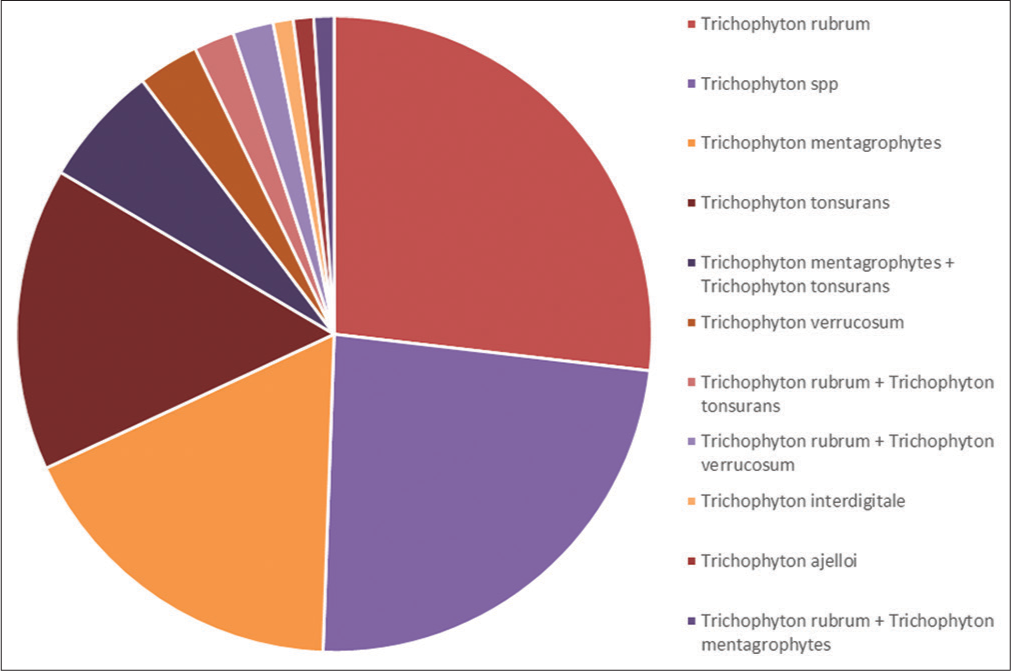
- Species grown in culture.
Species variability and laboratory characteristics according to clinical type and gender
In combined infection, T. mentagrophytes and T. tonsurans were the most common growth comprising 22.58% each followed by T. rubrum (19.35%). Mixed growth of T. mentagrophytes and T. rubrum was seen in 9.67%. The KOH mount positivity rate was 100% among these patients.
Among tinea cruris patients, T. rubrum (22.22%) was the most common growth followed by T. tonsurans (18.51%). Mixed growth was seen in 14.8% of patients. The KOH positivity rate was 81.48%.
In tinea corporis patients, T. rubrum (28%) and T. mentagrophytes (16%) were the most common isolates, and mixed growth was seen in 12% of patients. The KOH positivity rate in this category was found to be 92%.
In onychomycosis, Trichophyton growth was seen in 87.5% while species identification could not be done in a significant proportion (62.5%). The KOH positivity rate was lower (75%) in comparison to other clinical variants in this study.
Among male patients, T. rubrum (25.88%) was the most common species isolated followed by T. tonsurans (15.2%). In another 21.17% of patients, Trichophyton growth was detected, but further species identification was not possible. Mixed growth was seen in 8.23%, and growth could not be detected in 10.58%. On the other hand in females, T. mentagrophytes (19.23%) was the most common species followed by T. rubrum (15.38%). Mixed growth was detected in 15.38% while 19.23% of patients showed no growth in culture. The KOH mount positivity rate was higher in males than in females (91.76% vs. 84.61%).
The overall KOH mount positivity in this study found 90.1% and 87.4% of patients had positive culture growth. Table 5 shows the species variability and KOH mount positivity among different clinical types and genders in our study.
| Clinical type | Most common species grown | KOH positivity rate |
|---|---|---|
| Combined infection | T. mentagrophytes | 100% |
| Tinea cruris | T. rubrum | 81.48% |
| Tinea corporis | T. rubrum | 92% |
| Onychomycosis | Trichophyton spp. | 75% |
| Gender | Most common species grown | KOH positivity |
| Male | T. rubrum | 91.76% |
| Female | T. mentagrophytes | 84.61% |
| KOH mount | Number of patients | Percentage |
| Fungal hyphae present | 100 | 90.1% |
| Fungal hyphae absent | 11 | 9.9% |
| Culture report | Number of patients | Percentage |
| Growth present | 97 | 87.4% |
| No growth | 14 | 12.6% |
T. mentagrophytes: Trichophyton mentagrophytes, T. rubrum: Trichophyton rubrum
Adults versus pediatric characteristics
The M:F ratio was 3.5 in the pediatric age group compared to 3.26 in adults. Among the pediatric age group, tinea corporis (44.44%) was the most common type followed by combined infection (22.22%) in comparison to adults where combined infection (27.9%) was the most common clinical type followed by tinea cruris (24.3%) and tinea corporis (22.5%). The culture positivity rate was lower than the adult age group (55.55% v/s 87.4%). T. mentagrophytes (22.22%) was the most common species isolated, while in adults the most common isolate was that of T. rubrum (26.8%). There were no immunocompromised patients in the pediatric age group in comparison to adults (9%). Unhygienic practices and a history of steroid use before seeking treatment were present in 66.67%, respectively, which was very similar to the adult age group (Unhygienic – 62.2% and history of steroid use – 69.4%).
Pattern of seeking consultation among patients
In our study, 14 (12.6%) patients sought consultation from a dermatologist, 18 (16.2%) patients visited a general physician, one (0.9%) patient visited both general physician and dermatologist, 58 (52.3%) patients purchased medicines over the counter, and five (4.5%) patients consulted a dermatologist and also took medication over the counter. Pie Diagram 2 shows the pattern of consultation seeking among patients.
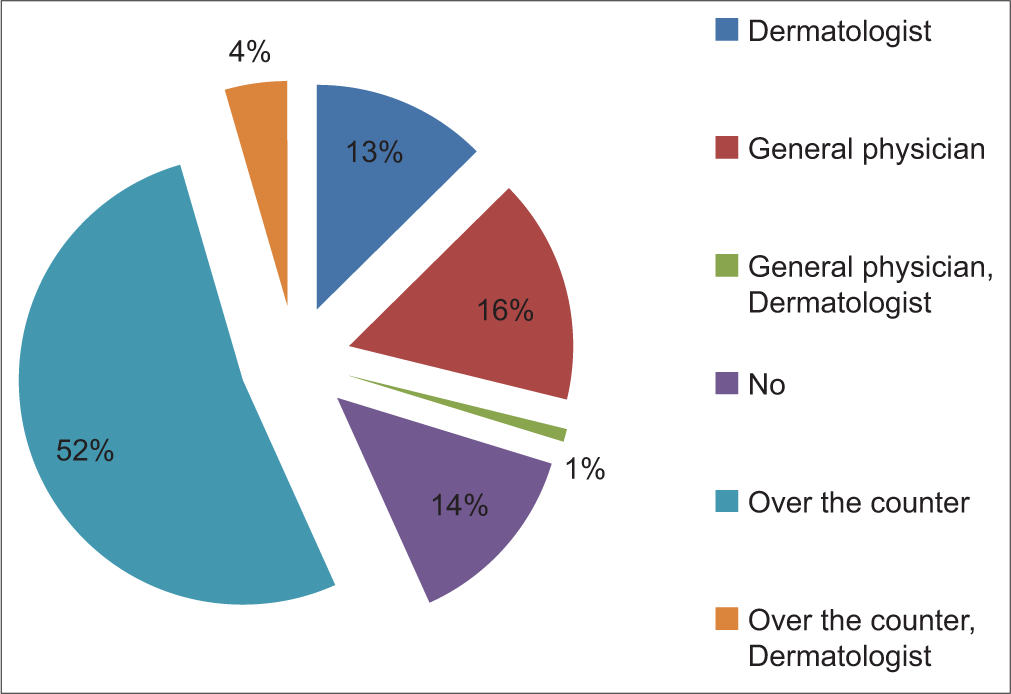
- Pie chart showing pattern of seeking consultation among patients.
DISCUSSION
This study intends to determine the recent trend and clinical-with-mycological pattern in dermatophytosis in Northeast India and also analyze if these changes reflect or differ from other parts of India.
Demographic profile
In our study, the majority of cases belong to the age group of 21–30 years, which is similar to the finding of Noronha et al.[4] in North Karnataka and Agarwal et al.[5] in North India, but unlike Vineetha et al.[6] where second decade means 11-20 years was the most common age group affected in the study. The increased incidence in this age group can be attributed to increased outdoor activities and physical exertion leading to increased sweating and maceration of the skin. The next common age group affected was 31–40 years. Due to the COVID pandemic, the number of young population visiting hospitals reduced drastically could be the reason for less number of patients belonging to the 11–20 years age group in our study.
The majority of the patients were male in this study, and the percentage of male patients was 76.6%. This finding was similar to other similar studies conducted by Sahai and Mishra,[7] Lakshmanan et al.,[8] and Kaur.[9] The increased male preponderance is explained by increased outdoor activity and involvement in activities that lead to increased perspiration such as farming, labor activities, and hot and humid conditions. On the other hand, females may be reluctant to seek medical attention due to the involvement of body areas that are difficult to expose such as axillae and groins and less involvement in outdoor activities that can be the factor for lesser incidence.
When comparing the socioeconomic status, 51.4% of patients belong to lower middle socioeconomic status, which is similar to the finding of Agarwal et al.[5] Noronha et al.[4] and Poluri et al.[10] In their respective studies, they have stated lower socioeconomic class as the most commonly affected category. During the COVID pandemic, people belonging to lower socioeconomic class faced difficulty due to the lack of availability of public transportation and other restrictions, which may have led to less participation of lower socioeconomic class patients in this study.
Risk factors associated with and pattern of seeking consultation
Risk factors associated showed an interesting finding that 69.4% of participants in our study gave a history of use of topical steroid formulation, which was reported much lower in studies conducted by Vineetha et al.[6] and Singh et al.[11] (42% and 21.7%). Furthermore, 29.7% of patients used antifungal agents in either topical or systemic form while Singh et al.[11] reported topical antifungal use in 23.1% of patients.
The majority of patients (52.3%) were taking medications over the counter without consultation from a dermatologist or general physician. Only a very small number, i.e., 12.6% of study participants consulted a dermatologist, and 16.2% consulted a general physician for their problem. Lack of patient education and awareness along with a lack of availability of specialists in rural areas and easy availability of topical steroid preparation over the counter may lead to a higher rate of topical steroid abuse among people of this region. Using topical antifungal preparations in inadequate dosage and for insufficient periods without proper prescription may lead to the rise of chronic and recalcitrant cases as seen in our study. The pattern of seeking consultation among patients has been studied for the first time in this region.
Another interesting finding in our study was a much lower rate of chronic infection among treatment naive patients in comparison to those who either used topical steroid formulations or antifungal agents or both.
The second most common risk factor seen was unhygienic practices of not taking baths regularly and sharing personal clothes including towels with family members and with roommates in 62.2% of patients. Vineetha et al.[6] reported sharing of towels (22%) in their study in chronic cases. Unhygienic practices lead to the spread of infection, which can be taken care of by proper counseling.
Comorbidities
The percentage of immunocompromised patients in our study was found to be 9% while Noronha et al.[4] found 19.6% of the study participants to be immunocompromised. As the immunocompromised patients during the COVID pandemic strictly avoided going outdoors, could be the reason for the lower proportion found in our study.
In our study, DM (7.2%) was the most common comorbidity followed by hypothyroidism (1.8%). Noronha et al.[4] also reported DM in 23.5% of patients as the most common comorbidity, and Vineetha et al.[6] reported diabetes followed by thyroid disorders as the common morbidities in their study. Hypertension (0.9%), leprosy (0.9%), psoriasis (0.9%), and alopecia areata (0.9%) were the other comorbidities noted in our study.
The association of dermatophytosis with the presence of comorbidities is essential, especially in current times where there is a rampant increase in its prevalence. Many such comorbidities are known to either affect or alter the natural course of skin diseases like dermatophytosis potentially giving rise to chronic and recalcitrant cases increasing the number of combined infections or involvement of greater body areas than isolated involvement and also impacting the choices of available options for treatment of the disease, as it can lead to various drug interactions.
Clinical profile
When comparing the differences in various clinical types of dermatophytosis, we found that the most common presentation was the combined infection comprising 27.9% followed by tinea cruris (24.3%), tinea corporis (22.5%), and onychomycosis (14.4%) while other studies conducted by Lakshmanan et al.,[8] Bhagra et al.,[12] and Poluri et al.[10] reported tinea corporis as the most common clinical manifestation. This difference can either be attributed to different geographical variations or can be a reflection of a change in the trend of the disease in the last few years where there is an increased occurrence of combined infection or simultaneous involvement of multiple body areas as compared to isolated body involvement. Only one study conducted by Singh et al.[11] also resonated with a similar finding to our study where the combined infection was found to be the most common clinical manifestation. However, they reported a much higher percentage of 77%. Another study conducted by Lyngdoh et al.[13] reported tinea pedis as the most common clinical type in Meghalaya as compared to this study, thereby showing a change in the trend of the disease.
We also noted that there was some difference when comparing the clinical presentations between the two genders. The combined infection was the most common clinical type in males accounting for 29.4% while in females tinea corporis (26.9%) was the most common, and combined infection (19.2%) was the third most common presentation in females. This difference can either be due to a smaller sample size or the fact that females sought treatment earlier than males thereby reducing the spread of infection to other body parts.
Among the pediatric age group, tinea corporis (44.44%) was the most common type followed by combined infection (22.22%) in comparison to adults where combined infection (27.9%) was the most common clinical type followed by tinea cruris (24.3%) and tinea corporis (22.5%). An interesting finding in our study is that patients with comorbidities presented with onychomycosis (38.46%) as the most common clinical type of dermatophytosis, which was otherwise found only in 11.22% of patients without comorbidities, and it represented the fourth most common clinical presentation in them. Based on this, it can be hypothesized that the presence of onychomycosis can be considered as an indicator or a surrogate marker of comorbidities and alert clinicians to either ask or look for their presence in patients. The previous studies did not compare the clinical profile based on gender, pediatric age group, and patients with comorbidities as we did in this study.
Laboratory characteristics
In this study, 100 out of 111 total participants (90.1%) showed fungal hyphae under direct microscopic examination using a KOH mount [Figure 6]. This finding was similar to Sahai and Mishra[7] (89.6%), Singh et al.[11] (97.7%), and Agarwal et al.[5] (84.67%). On the other hand, Lakshmanan et al.[8] and Munuswamy and Subramanian[14] reported lower KOH mount positivity rates (50.5% and 43%). The KOH mount positivity rate was 100% among patients with combined infection while among onychomycosis patients, only 75% showed KOH mount positivity.
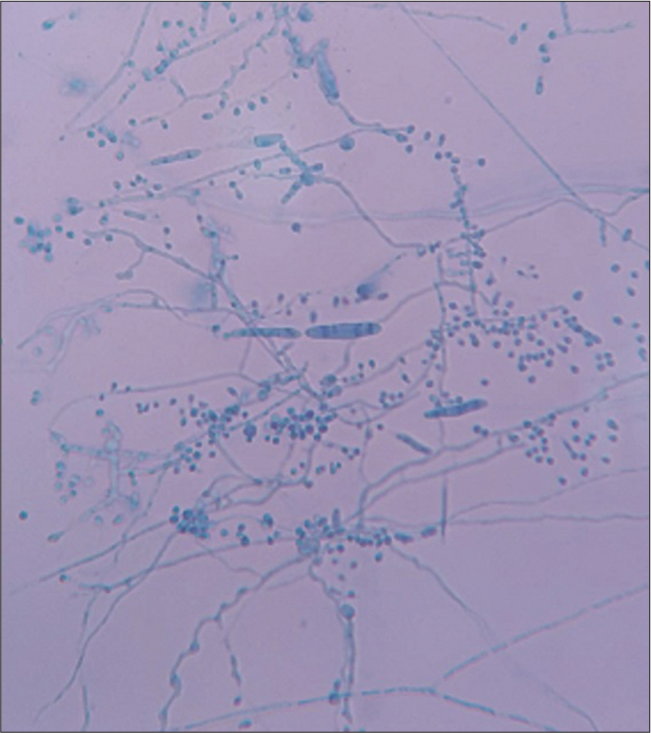
- Lactophenol cotton blue mount showing macroconidia and microconidia of dermatophytes.
The culture positivity rate in our study was 87.4%, which is consistent with Narasimhalu et al.[15] and Agarwal et al.[5] who reported 88.2% and 80%, respectively. On the other hand, Garg et al.[16] and Noronha et al.[4] reported a very low rate of culture positivity of 23.8% and 40%, respectively.
The most common isolate was T. rubrum (26.8%) followed by T. mentagrophytes (17.5%) and T. tonsurans (15.4%). Bhagra et al.[12] also reported T. rubrum (66.17%) as the most common isolate followed by T. mentagrophytes (19.11%). Kaur,[9] Poluri et al.,[10] and Dhayagude et al.[17] all reported T. rubrum as the most common isolate in their respective studies. Agarwal et al.[5] and Singh et al.[11] reported T. mentagrophytes as the most common dermatophyte grown in culture. In this study, 11.34% of patients showed mixed growth in culture out of which T. mentagrophytes and T. tonsurans were the commonest. In another 23.71% of patients, Trichophyton species were grown, but species identification could not be done. Other Trichophyton species that were grown in small numbers were T. verrucosum (2.7%), Trichophyton interdigitale (0.9%), and Trichophyton ajelloi (0.9%). No Microsporum or Epidermophyton growth was seen in our study.
We also found out the common species isolated according to the clinical types, gender, and pediatric age group. In combined infection, T. mentagrophytes and T. tonsurans were the most common growth comprising 22.58%, each followed by T. rubrum (19.35%). Among tinea cruris patients, T. rubrum (22.22%) was the most common growth followed by T. tonsurans (18.51%). Mixed growth was seen in 14.8% of patients. In tinea corporis patients, T. rubrum (28%) and T. mentagrophytes (16%) were the most common isolates, and mixed growth was seen in 12% of patients. In onychomycosis, Trichophyton growth was seen in 87.5% while species identification could not be done in a significant proportion (62.5%). Among male patients, T. rubrum (25.88%) was the most common species isolated followed by T. tonsurans (15.2%) while in females T. mentagrophytes (19.23%) was the most common species followed by T. rubrum (15.38%). T. mentagrophytes (22.22%) was the most common species isolated in the pediatric population.
Table 6 shows a comparison of various studies from different parts of India with the present study.
| Author, year (Ref) | M:F | Most common age group (years) | KOH positivity (%) | Culture positivity (%) | Most common clinical type | Most common species grown |
|---|---|---|---|---|---|---|
| Bhagra et al., 2014[12] | 4:1 | 21–30 | 80 | 68 | Tinea corporis | T. rubrum |
| Poluri et al., 2015[10] | 2:1 | 21–30 | 58.1 | 56.3 | Tinea corporis | T. rubrum |
| Noronha et al., 2016[4] | 1.6:1 | 21–30 | 60 | 40 | Tinea corporis | Trichophyton mentagrophytes |
| Ray Ghosh et al., 2018[3] | 3:2 | 21–30 | 72 | 51 | Tinea corporis | T. rubrum |
| Dhayagude et al., 2019[17] | 2.5:1 | 21–40 | 91.4 | 29.4 | Mixed infection (Tinea cruris+Tinea corporis) |
T. rubrum |
| Present study | 3.2:1 | 21–30 | 90.1 | 87.4 | Tinea cruris | T. rubrum |
Limitations of study
The number of patients attending OPD during the COVID pandemic reduced drastically. As a result, the sample size was smaller than the expected one.
CONCLUSION
Our study highlights the importance of understanding the current trend and changing clinical and mycological pattern in Northeast India. Furthermore, it iterates on the association of various risk factors that can lead not only to chronicity and recurrences of the disease but inadequate treatment and thereby guide and equip, especially the medical practitioners from other fields, who are more easily accessible than the limited specialists catering to only a fraction of population here in Northeast India in an attempt to control its rampant increase.
Ethical approval
Approved by the Institutional Ethics Committee at North Eastern Indira Gandhi Regional Institute Of Health & Medical Science, Shillong, number NEIGR/IEC/M10/ T7/2020 dated 14th May 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Financial support and sponsorship
Nil.
References
- The menace of dermatophytosis in India: The evidence that we need. Indian J Dermatol Venereol Leprol. 2017;83:281-4.
- [CrossRef] [PubMed] [Google Scholar]
- Rook's textbook of dermatology (9th ed). Chichester, West Sussex, Hoboken, NJ: John Wiley and Sons Inc; 2016. p. :1.
- [CrossRef] [Google Scholar]
- Changing trend in clinico-mycological profile of dermatophytosis of skin in Eastern India. IP Int J Med Microbiol Trop Dis. 2020;4:159-65.
- [CrossRef] [Google Scholar]
- Clinico-microbiological study of dermatophytosis in a tertiary-care hospital in North Karnataka. Indian Dermatol Online J. 2016;7:264-71.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-mycological study of dermatophytes in a tertiary care centre in Northwest India. Indian J Dermatol Venereol Leprol. 2014;80:194.
- [CrossRef] [PubMed] [Google Scholar]
- Profile of dermatophytosis in a tertiary care center in Kerala, India. Indian J Dermatol. 2019;64:266-71.
- [CrossRef] [PubMed] [Google Scholar]
- Change in spectrum of dermatophytes isolated from superficial mycoses cases: First report from central India. Indian J Dermatol Venereol Leprol. 2011;77:335-6.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological and clinical pattern of dermatomycoses in rural India. Indian J Med Microbiol. 2015;33(Suppl):134-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-mycological pattern of hair and skin infection in New Delhi. Intensive Crit Care. 2017;3:2.
- [CrossRef] [Google Scholar]
- Clinicomycological study of dermatophytosis in South India. J Lab Physicians. 2015;7:84-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicomycological study of dermatophytosis in a tertiary care hospital in Eastern India: A cross-sectional study. Indian Dermatol Online J. 2019;11:46-50.
- [CrossRef] [PubMed] [Google Scholar]
- Mycological pattern of dermatophytosis in and around Shimla hills. Indian J Dermatol. 2014;59:268-70.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-mycological profile of dermatophytosis in Meghalaya. Int J Med Public Health. 2013;3:254-6.
- [CrossRef] [Google Scholar]
- A clinical and mycological study of dermatophytosis in a tertiary care hospital. Int J Res Dermatol. 2021;7:429.
- [CrossRef] [Google Scholar]
- Cross-sectional, clinico-mycological research study of prevalence, aetiology, speciation and sensitivity of superficial fungal infection in Indian patients. Clin Exp Dermatol Res. 2015;7:324.
- [CrossRef] [Google Scholar]
- Rapid detection of dermatophytes from skin and hair. BMC Res Notes. 2009;2:60.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicomycological study of tinea infections in and around Pune. Int J Res Dermatol. 2019;5:598-602.
- [CrossRef] [Google Scholar]







