Translate this page into:
Ara-C face: A malar rash

*Corresponding author: Kaushal K. Verma, Department of Dermatology and Venereology, All India Institute of Medical Sciences, New Delhi, India. prokverma@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gaurav V, Lal A, Manandhar K, Anand GR, Arava S, Verma KK. Ara-C face: A malar rash. CosmoDerma. 2024;4:58. doi: 10.25259/CSDM_47_2024
Dear Sir,
Cytarabine (cytosine arabinoside; Ara-C), a commonly used chemotherapeutic agent for acute myeloid leukemia (AML), has been linked to various undesirable effects. Cutaneous manifestations have been reported in 20 – 72% of patients manifesting as acral erythema, urticaria, maculopapular eruptions, and rarely neutrophilic eccrine hidradenitis, and eccrine squamous syringometaplasia.[1] We report a case of cytarabine-induced facial erythema in a child with AML.
A 2-year-old girl on induction chemotherapy for AML was referred to the dermatology outpatient for a rash over the face. She had undergone two cycles of induction chemotherapy with cytarabine, daunorubicin, and etoposide. Following the completion of the second cycle, the child began receiving high-dose cytarabine (1000 mg) twice daily for three consecutive days (D1, D3, and D5). On the sixth day following high-dose cytarabine, the pediatric oncology team referred the child to the dermatology department for mildly itchy diffuse erythematous macular lesions over bilateral cheeks. Interestingly, similar but milder bilateral cheek erythema with itching had been observed immediately after completing the second cycle of induction chemotherapy, which resolved spontaneously within two to three weeks. The parents denied any topical application over the face or any history of photosensitivity. There was no personal or family history of atopy. These episodes were not associated with any systemic symptoms.
On cutaneous examination, ill-defined bright erythematous plaque that partially blanched on pressure was noted over the cheeks, while sparing the nasolabial folds [Figure 1]. A punch biopsy was obtained from the lesion, revealing a mild perivascular infiltrate composed of lymphocytes, histiocytes, and occasional plasma cells, along with some pigment incontinence in the upper dermis [Figure 2]. No atypical cell infiltration or eosinophils were observed. The histopathological findings were incompatible with other potential causes such as Sweet syndrome, leukemia cutis, and drug-induced lupus erythematosus. Routine hematological and biochemical investigations were normal except for leukocytosis. The ANA profile, including anti-histone, anti-Ro/La, and dsDNA, was negative. Since daunorubicin and etoposide have not been reported to cause localized erythema of the face and considering the temporal relationship between the clinical features and the administration of cytarabine, a clinical diagnosis of toxic erythema of chemotherapy involving the face, secondary to cytarabine was made.
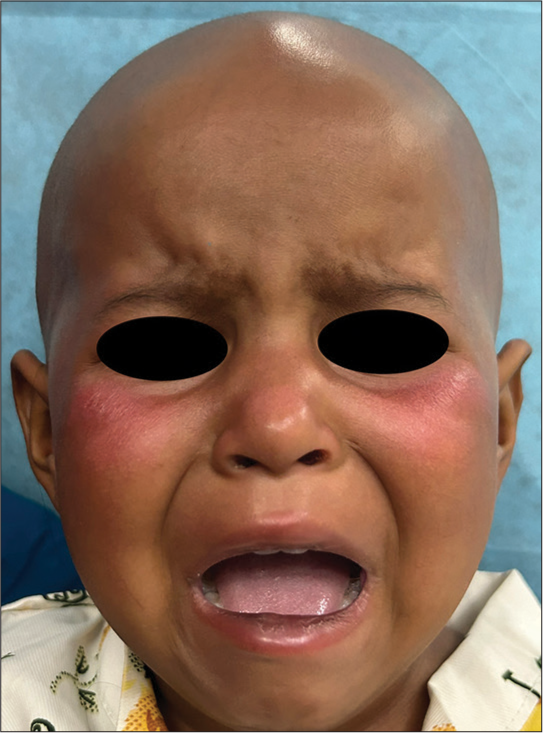
- Ill-defined bright erythematous plaque over the cheeks.
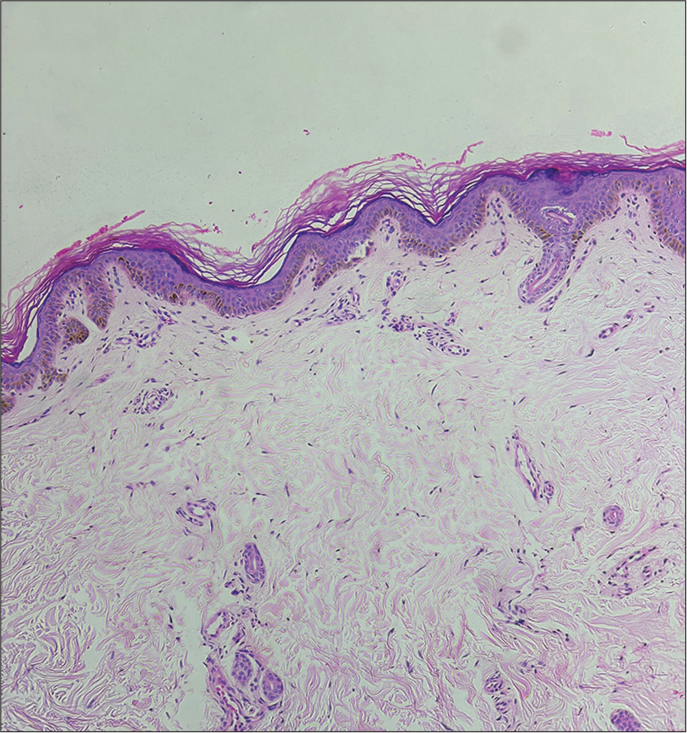
- Mild-to-moderate perivascular lymphohistiocytic infiltrate and pigment incontinence in the upper dermis (H&E; ×40).
The child was treated symptomatically with antihistamines, emollients, and cold compressions resulting in a significant reduction in erythema and pruritus by day 21 of starting high-dose cytarabine chemotherapy, allowing continuation of the treatment regimen. Thereafter, the child was lost to follow up.
Cytarabine is a chemotherapeutic agent that inhibits cell division by inhibiting deoxyribonucleic acid synthesis during the S-phase of the cell cycle. It has been effectively used in the treatment of hematological malignancies, especially AML. It has been linked to cardiovascular, dermatologic, gastrointestinal, hepatic, hematological, and immunologic side effects. Acute generalized exanthematous pustulosis, alopecia, cutaneous ulcers, ephelids, pruritus, skin rash, and urticaria are the most common skin reactions.[2] Clinical trials with cytarabine have shown that high doses (1000 – 3000 mg/m2) are more effective than standard (100 mg/m2) or intermediate doses (500 – 1000 mg/m2) as they have been associated with improved outcomes and long-term disease-free survival. Though, the aforementioned adverse effects are particularly seen with high doses of cytarabine.[3] However, 18% of cutaneous reactions can occur with low-dose cytarabine as well, and there is no consensus currently on the relationship between dosage and cutaneous reactions to cytarabine.[4]
Cutaneous adverse reactions caused by cytarabine are frequent and typically appear as morbilliform rashes primarily on the acral sites and skin folds, with occasional involvement of the elbows, knees, neck, and ears. Specifically, ear involvement is termed “Ara-C ears,” representing a less common form of acral erythema.[5] It can also cause generalized papular purpuric eruption or violaceous erythema.[3] The exact mechanism of cytarabine-associated cutaneous toxicity is unknown. However, a delayed-type hypersensitivity has been suggested. Cytarabine-induced photosensitivity could be another possible mechanism. Predominant involvement of hands and feet has been noted. In the present patient, there was predominant involvement of the face involving the malar area, a site of involvement seen in other conditions as well. Hence, obtaining a definitive diagnosis is crucial to avoid unnecessary discontinuation of chemotherapy. Clinical trials have shown that the most effective treatment for cutaneous adverse reactions to cytarabine is the suspension of therapy. However, since cutaneous reactions are typically self-limiting, the decision to stop the chemotherapy should not rely solely on dermatological outcomes. To alleviate cutaneous symptoms, topical steroids, cold compresses, antihistamines, and analgesics may be utilized. Moreover, complications are infrequently reported, and re-challenging with cytarabine is generally considered safe.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- High-dose cytosine arabinoside-induced cutaneous reactions. J Eur Acad Dermatol Venereol. 2002;16:481-5.
- [CrossRef] [PubMed] [Google Scholar]
- Isolated bilateral ear and scalp rash after cytarabine therapy for acute myelogenous leukemia: A report and literature review. Am J Ther. 2019;26:e653-5.
- [CrossRef] [PubMed] [Google Scholar]
- Generalized benign cutaneous reaction to cytarabine. J Am Acad Dermatol. 2015;73:821-8.
- [CrossRef] [PubMed] [Google Scholar]
- Ara C face: Cytarabine related dermatological toxicity. Asian J Med Sci. 2022;13:169-70.
- [CrossRef] [Google Scholar]
- Bilateral ear swelling and erythema after chemotherapy: A case of ara-C ears. J Clin Oncol. 2012;30:e146.
- [CrossRef] [PubMed] [Google Scholar]





