Translate this page into:
Adalimumab-induced palmoplantar pustulosis in a patient with inflammatory bowel disease

*Corresponding author: Arunachalam Narayanan, Department of Dermatology and STD, Jawaharlal Institute of Postgraduate Medical Education and Research, Gorimedu, Puducherry, India. narayanan359@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Narayanan A, Ramesh N, Mithin Kumar B, Thappa DM. Adalimumab-induced palmoplantar pustulosis in a patient with inflammatory bowel disease. CosmoDerma 2022;2:58.
Dear Sir,
Anti-TNF-α agents like adalimumab have been a part of dermatologists’ treatment armamentarium in patients with psoriasis, psoriatic arthritis, and hidradenitis suppurativa. They are also used to treat inflammatory bowel disease. However, these agents often cause paradoxical adverse effects including the onset of palmoplantar pustulosis. We report a case of adalimumab-induced palmoplantar pustulosis
A 23-year-old male patient presented with pustular lesions on the bilateral palms and soles for 25 days. He was a known case of inflammatory bowel disease for which he was on treatment with adalimumab since December 2019. Initially, the patient was administered adalimumab 80 mg every 2 weeks, followed by adalimumab 40 mg every 2 weeks subcutaneously. The patient received his last dose of adalimumab 2 weeks before presentation to our outpatient clinic. There was no previous history of psoriasis. Examination revealed multiple pustules over well-defined, erythematous, scaly plaques on his bilateral palms and soles with dried lakes of pus [Figures 1a and c]. Gram’s stain from the pustule revealed only neutrophils. Histopathology revealed neutrophils in the epidermis forming microabscesses of Kogoj. The patient had a score of 7 (probably adverse drug reaction) on the Naranjo Adverse Drug Reaction Probability Scale. Based on the clinical and histopathological findings, we made a final diagnosis of adalimumab-induced palmoplantar pustulosis and treatment with a patient with cream clobetasol propionate 0.05%. The patient came for follow-up after 2 weeks with significant resolution of his palmoplantar lesions [Figures 1b and d]. Inadvertent administration of adalimumab resulted in the recurrence of lesions at the same sites and this made medical gastroenterologists to agree for stopping this drug. The patient did not develop any flares of pustules during the follow-up of 2 months.
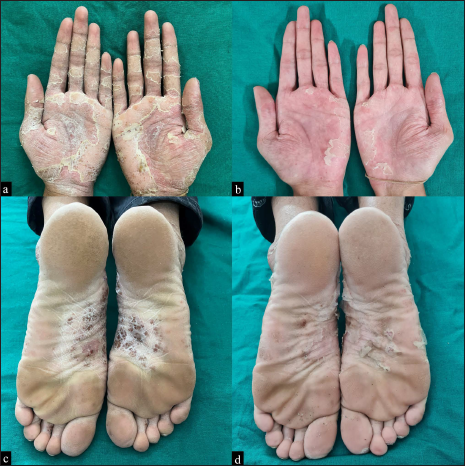
- Multiple pustules over well-defined, erythematous, scaly plaques with dried lakes of pus on his bilateral palms (a) at baseline and (b) after treatment with topical clobetasol propionate 0.05% for 2 weeks. Similar lesions over the soles (c) at baseline and (d) after treatment with topical clobetasol propionate 0.05% for 2 weeks.
Palmoplantar pustulosis is a chronic inflammatory skin disorder presenting with sterile pustules on palms and/or soles. It is classified into Type A and Type B.[1] Type A is rarely associated with psoriasis and Type B is usually associated with plaque psoriasis. While Type A is characterized by vesicles preceding pustules, Type B presents with vesicles without pustules. Smoking, emotional stress, and TNF-α inhibitors are known to induce and exacerbate palmoplantar pustulosis.[2]
Biologic-induced palmoplantar pustulosis occurs usually in the fifth decade.[3] There is a female preponderance.[1] The most common indication for administration of biologicals in such cases was rheumatoid arthritis, ankylosing spondylitis, psoriasis/psoriatic arthritis, and inflammatory bowel disease.
The most common TNF-α inhibitor associated with palmoplantar pustulosis is adalimumab. In a systematic review by Lu et al., the mean latency period before the onset of pustular lesions after adalimumab administration was 7.8 months (range: 0.3-36.5 months).[3] In our patient, the latency period was 27 months. Topical corticosteroids are used to control palmoplantar pustulosis. If it isn’t controlled or the lesions are severe, switching the biologic agent should be considered.[4] Other agents used to treat adalimumab-induced palmoplantar pustulosis are cyclosporine, phototherapy, and methotrexate. The mean time to attain complete resolution was found to be 4.2 months (range: 0.5-11 months).[3] Among patients treated with topical corticosteroids, 60% achieved complete remission in 3.5 months.[3]
Paradoxical reactions following biologic therapy are uncommon. They induce psoriasis-like lesions in 2-5% of patients.[5] These reactions occur due to imbalances in cytokine activities. Genetic polymorphisms also play a role. It is hypothesized that TNF-α inhibition induces IFN-α overproduction by plasmacytoid dendritic cells leading to T-cell activation and paradoxically increased production of TNF-α.[6] Other hypothesized causes include increased expression of CXCR3 chemokine which induces migration of auto-reactive T-cells toward the skin.[5,6]
In the current age, where there is widespread and long-term use of biologicals in the treatment of various diseases, dermatologists should be aware of their conventional and paradoxical side effects. This helps in early detection, appropriate treatment, and switching biologics when necessary.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflict of interest
Devinder Mohan Thappa is the Editor-in-Chief of this journal.
References
- Palmoplantar pustulosis: Current understanding of disease definition and pathomechanism. J Dermatol Sci. 2020;98:13-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features, etiologic factors, associated disorders, and treatment of palmoplantar pustulosis: The Mayo Clinic Experience, 1996-2013. Mayo Clin Proc. 2017;92:1351-8.
- [CrossRef] [PubMed] [Google Scholar]
- Biologic therapies associated with development of palmoplantar pustulosis and palmoplantar pustular psoriasis: A systematic review. Int J Dermatol 2022
- [CrossRef] [PubMed] [Google Scholar]
- Adalimumab biosimilar-induced severe paradoxical palmoplantar pustulosis in a patient with psoriasis and psoriatic arthritis successfully treated with Ixekizumab. Dermatol Ther (Heidelb). 2022;12:605-9.
- [CrossRef] [PubMed] [Google Scholar]
- TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis: Classical vs. paradoxical. The Yin-Yang of TNF and Type I interferon. Front Immunol. 2018;9:2746.
- [CrossRef] [PubMed] [Google Scholar]





