Translate this page into:
A rare case of progressive neonatal pemphigus vulgaris treated successfully with intravenous immunoglobulin

*Corresponding author: Dr. Palvi Singla, Department of Internal Medicine, Maimonides Medical Center, Brooklyn, New York, United States. palvisingla7@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singla P, Kaur S. A rare case of progressive neonatal pemphigus vulgaris treated successfully with intravenous immunoglobulin. CosmoDerma. 2024;4:140. doi: 10.25259/CSDM_161_2024
Abstract
Pemphigus vulgaris is a rare autoimmune blistering disorder of the skin and mucous membranes, particularly during pregnancy. The transplacental transfer of maternal immunoglobulin G autoantibodies against desmoglein-3 to the fetus can result in neonatal pemphigus vulgaris (NPV). Although NPV is typically transient and benign, resolving without specific treatment or long-term sequelae, its impact on both the pregnancy and the fetus underscores the importance of early recognition. Prompt diagnosis is crucial to differentiate NPV from other blistering conditions, such as infections, epidermolysis bullosa, incontinentia pigmenti, and porphyrias, which require specific interventions. We present a case of a male neonate who developed blisters on the first day of life, which later involved mucosal surfaces, and responded well to treatment with intravenous immunoglobulin.
Keywords
Neonatal pemphigus vulgaris
Intravenous immunoglobulin
Pemphigus vulgaris
Autoimmune disease
Preterm infant
INTRODUCTION
Neonatal pemphigus vulgaris (NPV) is a rare disease resulting from transplacental passage of immunoglobulin G (IgG), mainly class 4 maternal autoantibodies, against desmoglein.[1] NPV is usually seen soon after the child’s birth, characterized by mucocutaneous erosions, and heals spontaneously after 2–3 weeks.[2] Here, we present a case of progressive NPV in a 1-day-old premature infant treated with intravenous immunoglobulin (IVIG) and a mother with severe pemphigus vulgaris (PV).
CASE SERIES
Case 1: NPV
A male infant, born at 31 + 3 weeks of gestation with a birth weight of 1.7 kg, was admitted to the neonatal intensive care unit at 5 h of life due to respiratory distress and prematurity. On the first day of life, a flaccid bulla developed on his left arm, which ruptured, leaving an erosion. Over the following days, additional bullae appeared on the scalp, face, abdomen, and lower limbs [Figure 1a and b]. The oral and genital mucosae were unaffected. Differential diagnoses included NPV, herpes simplex, congenital syphilis, and epidermolysis bullosa. Informed consent was obtained from the parents before clinical photographs were taken.
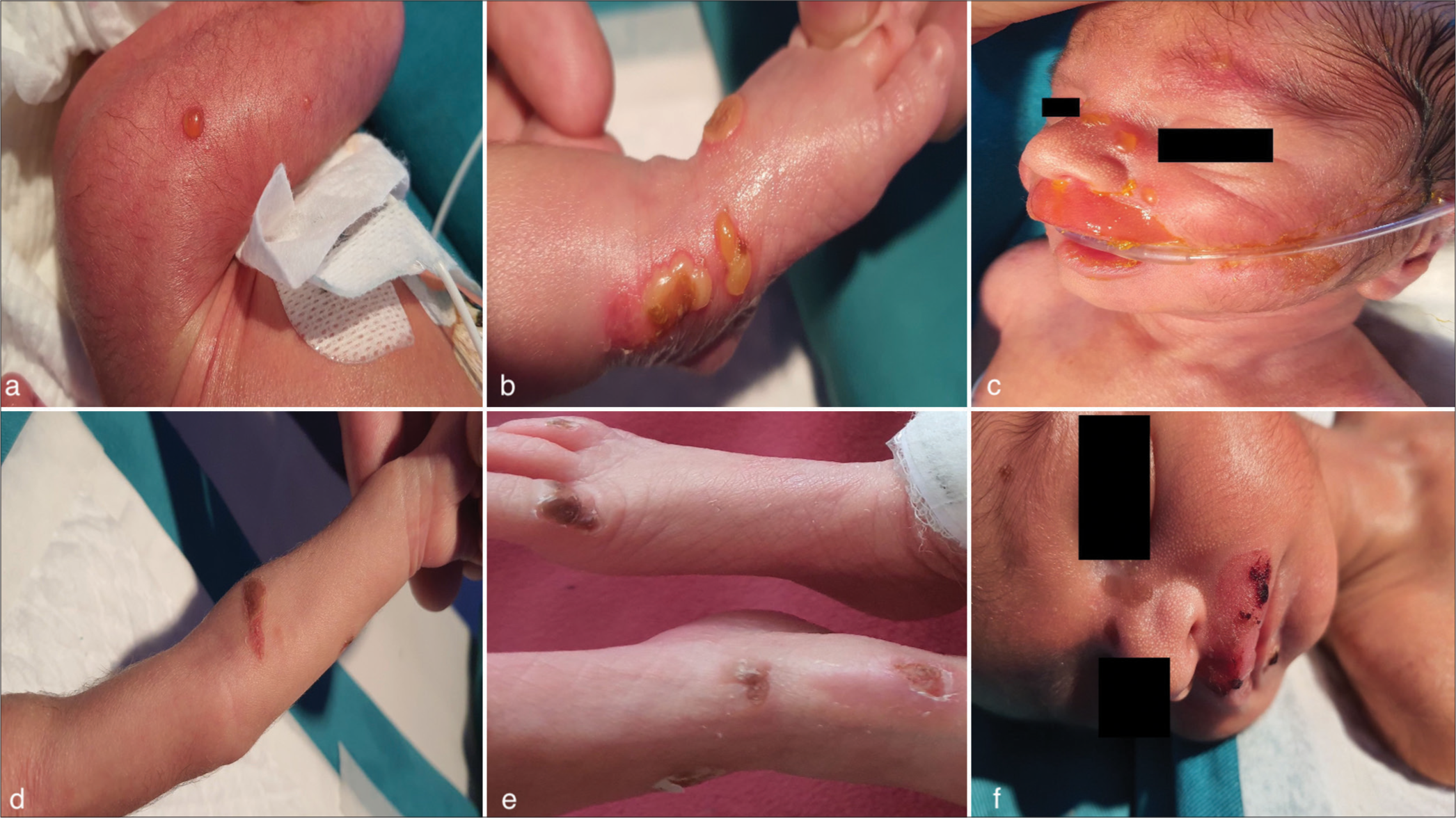
- Pre- and post- intravenous immunoglobulin (IVIG) images of neonatal pemphigus vulgaris lesions (a) Neonate presenting with multiple blisters on the left thigh and (b) left foot. (c) Worsening lesions involving the nose, upper lip, and oral mucosa. (d) Healed arm lesions on day 10 post-birth, (e) healed lesions on bilateral feet, and (f) healed facial lesions following IVIG therapy.
A Tzanck smear from a bulla revealed acantholytic cells, and the mother’s Venereal Disease Research Laboratory test was negative. The parents declined a skin biopsy. A septic screen performed on the infant was negative, leading to discontinuation of antibiotics after five days. NPV was diagnosed as the final diagnosis.
Management included barrier nursing, warm saline compresses, and close monitoring. Despite these measures, the bullae continued to increase in number and size, especially on the face, with involvement of the oral and nasal mucosa. Oral erosions necessitated the insertion of an orogastric tube for feeding. The worsening of lesions in the upper lip and oral cavity prompted the initiation of specific therapy. Given the infant’s premature status and the likelihood of high maternal antibody transfer, IVIG was administered at 500 mg/kg on day 6 of life. The infant responded well, with complete resolution of lesions by the fourth day of treatment [Figure 1c-f]. There was no recurrence at follow-up.
Case 2: Maternal PV
The infant’s mother was also admitted with worsening skin and oral erosions over the preceding 10 days. She had a one-year history of painful oral ulcers and occasional blisters with erosions on the body but had not received specific treatment. On examination, she had multiple flaccid bullae with erosions and crusting on her skin [Figure 2a]. Oral and genital mucosae exhibited multiple ulcers with whitish pseudomembranes. Nikolsky’s sign was positive, and the bulla spread sign demonstrated an angulated extension of the lesions.
Routine laboratory investigations, including a complete blood count, renal and liver function tests, and erythrocyte sedimentation rate, were normal. However, her urine analysis revealed 10–15 red blood cells/hpf, 18–20 pus cells/hpf, and proteinuria. A perilesional skin biopsy was performed, and direct immunofluorescence showed intercellular deposition of IgG and C3, confirming the diagnosis of PV [Figure 2b and c].
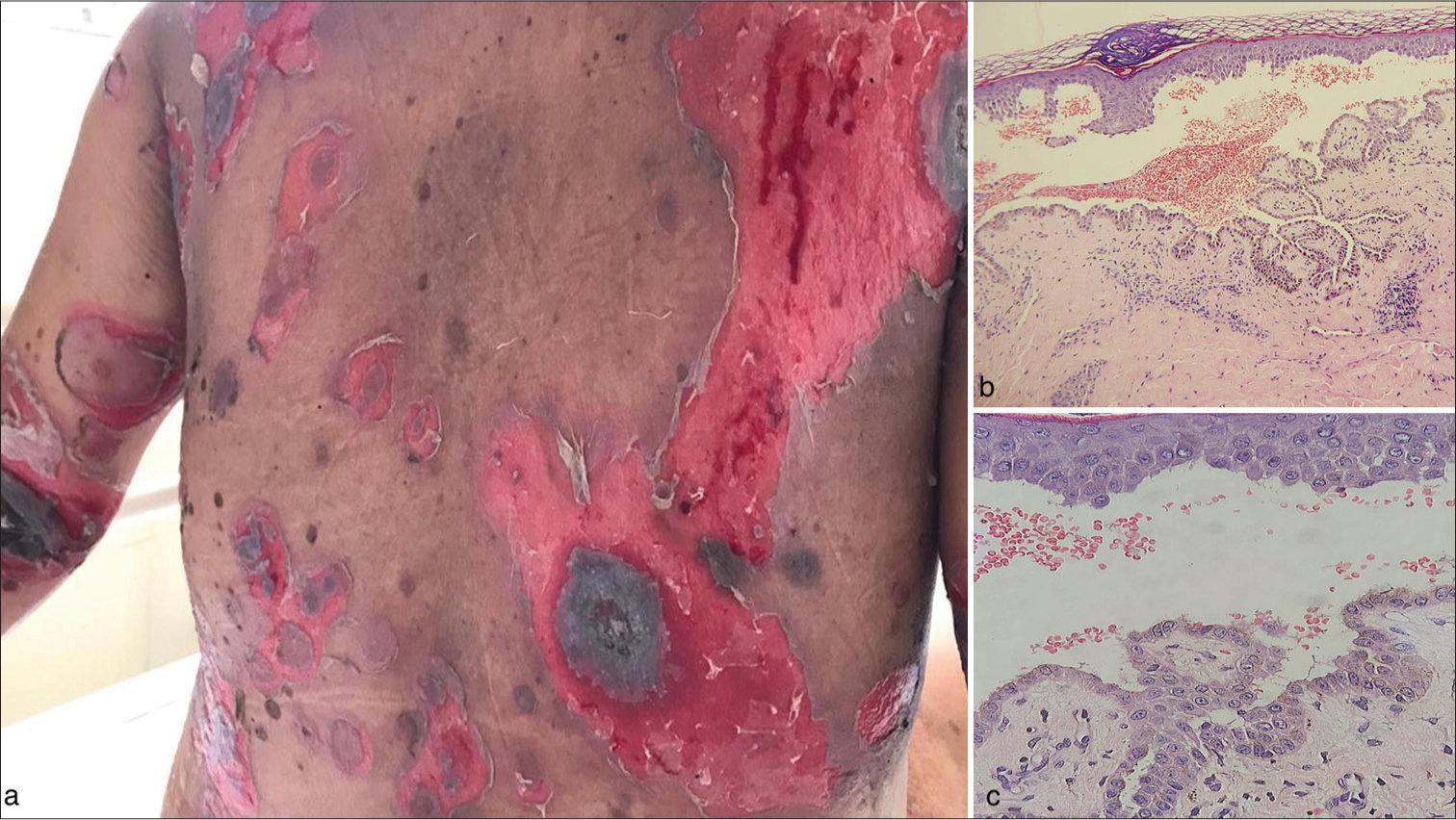
- Clinical and histopathological findings in the postpartum mother with pemphigus vulgaris. (a) Multiple erosions with brown-black crusting and blisters over the trunk in the postpartum mother. (b) Histopathology of a skin lesion showing a suprabasal blister with acantholysis and inflammatory cell infiltrates (H&E, ×200 magnification (c) ×400 magnification). H&E: Hematoxylin and eosin.
The mother was started on monthly dexamethasone pulse therapy, along with IV linezolid (600 mg twice daily), IV clindamycin (600 mg twice daily), and supportive care. Her cutaneous and mucosal lesions showed improvement, with a reduction in the number of new lesions. She is currently in the first phase of steroid pulse therapy and is being followed regularly.
DISCUSSION
PV is characterized by the production of antibodies against desmoglein 1 and 3 by memory B-cells.[3] In NPV, IgG autoantibodies formed in the mother cross the placenta, resulting in disease in the newborn. The first case of NPV was reported by Ruocco et al. in 1975.[4] Clinical manifestations include cutaneous blisters, erosions at birth, mucosal lesions, and extensive skin exfoliation. Although rare during pregnancy,[5] PV can have significant impacts, including premature births, stillbirths (10%), perinatal mortality (12%), and abortions (9.7%).[6] NPV occurs in 30–45% of children born to mothers with pemphigus.[7] The disease may either commence or worsen with the progression of pregnancy, particularly in the last trimester, or immediately after delivery.[8,9] In this case, the mother’s condition exacerbated during the third trimester.
The prognosis for NPV is generally excellent, with lesions typically resolving by 3 weeks of life. No case has been reported where NPV persists into adulthood or progresses into adult PV.[2,10,11] Treatment options include expectant management or systemic corticosteroids with or without antibiotics. However, the use of corticosteroids in neonates is discouraged due to their potential adverse effects on neurodevelopment, such as increasing the risk of cerebral palsy in preterm infants.[12] Corticosteroids can impair brain myelination, cell division, somatic growth, and long-term behavioral outcomes, meaning most infants treated with postnatal steroids are affected to some degree.[12,13] In this case, IVIG was chosen to avoid these risks and control the rapid progression of the disease. IVIG works by saturating neonatal Fc receptors, leading to accelerated degradation of pemphigus autoantibodies, reducing them by 70% after one week of a single IVIG cycle.[14,15]
There is a notable male predominance in neonatal pemphigoid diseases (M:F ratio = 4.6), which was also observed in our case. Zhao et al. reviewed cases of NPV and found a high proportion of premature births (51.5%), consistent with this case where the infant was born at 34 weeks of gestation. The infant in this report had a low birth weight of 1.7 kg, which aligns with previous findings, where 29.4% of NPV infants were born with low birth weight.[16]
To date, 36 case reports of NPV have been published, with most showing spontaneous resolution of lesions without specific treatments like IVIG. Only one case, by Ibrahim et al., described the use of systemic steroids in a premature NPV case.[16-21] Factors that predict poor fetal outcomes include increased maternal disease activity, high-dose corticosteroid use during pregnancy, and elevated antepartum maternal autoantibody titers, the latter of which we were unable to measure due to financial constraints for the patient.[22]
While NPV often resolves on its own, it remains difficult to determine if improvements in this case were due to IVIG or natural disease resolution. However, IVIG is beneficial as it neutralizes maternal autoantibodies, leading to rapid lesion clearance without the risks associated with immunosuppressive therapies. IVIG has a favorable safety profile but is expensive and may not be widely available. In addition, its effects are temporary, requiring close follow-up and potentially repeat doses, with limited large-scale evidence to fully endorse its use in NPV.
CONCLUSION
This case highlights the potential of intravenous immunoglobulin (IVIG) as a safe and effective treatment for progressive neonatal pemphigus vulgaris, particularly in premature infants. While spontaneous resolution is common, IVIG may offer a viable alternative to corticosteroids, minimizing associated risks and promoting rapid recovery. Further research is needed to establish standardized treatment guidelines for such cases.
Ethical approval
The Institutional Review Board approval is not required
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Pemphigus and pregnancy. Analysis and summary of case reports over 49 years. Saudi Med J. 2015;36:1033-8.
- [CrossRef] [PubMed] [Google Scholar]
- Maternal medicine: Medical problems in pregnancy, dermatological disease in pregnancy (1st ed). UK: Elsevier Health Sciences; 2007. p. :276.
- [Google Scholar]
- Neonatal pemphigus vulgaris with extensive mucocutaneous lesions from a mother with oral pemphigus vulgaris. Br J Dermatol. 2002;147:801-5.
- [CrossRef] [PubMed] [Google Scholar]
- A congenital acantholytic bullous eruption in the newborn infant of a pemphigus mother. Ital Gen Rev Dermatol. 1975;12:169-74.
- [Google Scholar]
- Pemphigus vulgaris in pregnancy: A case report and review of literature. Hum Reprod. 2000;15:1195-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus vulgaris in pregnancy: Analysis of current data on the management and outcomes. Obstet Gynecol Surv. 2009;64:739-49.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus vulgaris during pregnancy-a case report. J Pak A Dermatol. 2011;21:301-3.
- [Google Scholar]
- Congenital epidermolysis bullosa acquisita: Vertical transfer of maternal autoantibody from mother to infant. Arch Dermatol. 2011;147:337-41.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus vulgaris and pregnancy: Risk factors and recommendations. J Am Acad Dermatol. 1993;28(5 Pt 2):877-9.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal pemphigus vulgaris associated with mild oral pemphigus vulgaris in the mother during pregnancy. Br J Dermatol. 1998;139:500-3.
- [CrossRef] [PubMed] [Google Scholar]
- The adverse neuro-developmental effects of postnatal steroids in the preterm infant: A systematic review of RCTs. BMC Pediatr. 2001;1:1.
- [CrossRef] [PubMed] [Google Scholar]
- Postnatal steroid treatment and brain development. Arch Dis Child Fetal Neonatal Ed. 2004;89:96F-100.
- [CrossRef] [PubMed] [Google Scholar]
- Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. 2005;115:3440-50.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of pemphigus with intravenous immunoglobulin. J Am Acad Dermatol. 2002;47:358-63.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal autoimmune blistering disease: A systematic review. Pediatr Dermatol. 2016;33:367-74.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal pemphigus vulgaris characterized by tensive blisters. World J Pediatr. 2020;16:633-4.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal pemphigus in an infant born to a mother with pemphigus vulgaris: A case report. Rev Paul Pediatr. 2019;37:130-4.
- [CrossRef] [PubMed] [Google Scholar]
- A case of neonatal pemphigus vulgaris with co-existing BP180 autoantibodies. Pediatr Dermatol. 2020;37:241-3.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal pemphigus vulgaris: A case report. Arch Pediatr Infect Dis. 2020;8:e101153.
- [CrossRef] [Google Scholar]
- Pemphigus vulgaris in a pregnant woman and her neonate. BMJ Case Rep. 2012;2012:bcr0220125850.
- [CrossRef] [PubMed] [Google Scholar]
- Do safe and effective treatment options exist for patients with active pemphigus vulgaris who plan conception and pregnancy? Arch Dermatol. 2008;144:783-5.
- [CrossRef] [Google Scholar]






