Translate this page into:
A monocentric, single-group study to evaluate the effect of pramoxine containing moisturizing cream in participants with pruritis

*Corresponding author: Devesh Kumar Joshi, Department of Medical Affairs, Dr. Reddy’s Laboratory Ltd., Hyderabad, Telangana, India. deveshkj@drreddys.com
-
Received: ,
Accepted: ,
How to cite this article: Bhagat S, Motlekar S, Bhagotham S, Samal AR, Choudhury AA, Joshi DK, et al. A monocentric, single-group study to evaluate the effect of Pramoxine containing moisturizing cream in participants with pruritis. CosmoDerma 2023;3:87.
Abstract
Objectives:
The objective of the study was to evaluate the effectiveness of a blend of moisturising cream with anesthetic agent for the treatment of pruiritis.
Materials and Methods:
A prospective, single-group, and single-center trial to assess the efficacy and acceptability of moisturizing cream in patients with pruritis was carried out. The study included patients who were diagnosed at least once at one site with chronic pruritis with dryness, chronic itch for more than 6 weeks (H/o Itch every day), and itch score on visual analogue scale (VAS) (mild-to-moderate – equal to or <7 on VAS). P = 0.05 or lower indicated statistical significance.
Results:
A total of 32 patients were included in the study. Following the application of moisturizing cream, the percentage reduction in pruritis severity ranged from 26.3% at 3 min to 66.9% at 8 h. The mean itch score at baseline was 5.922 ± 0.908, while at 3 min, it was 4.366 ± 2.034. At 8-h post-application, it was 2.781 ± 1.460. The P-value for all the itch scores after application of the moisturizing cream right from 3-min to 8-h post-application was 0.001 which is highly significant.
Conclusion:
In patients with chronic pruritis, moisturizing cream with pramoxine as the topical local anesthetic significantly decreased the mean itch score. The onset of action began as soon as 3 min after application, and over the course of 8 h, a noticeable decrease in the severity of pruritis was seen in this study. The cream was well tolerated with no adverse effects in this study.
Keywords
Pramoxine hydrochloride
Moisturizing cream
Pruritis
Itching
Local anesthetic
INTRODUCTION
Pruritis is a dermatological condition characterized by dryness of the skin, along with an intense desire to scratch.[1] Pruritis may present as a standalone symptom or in conjunction with some other psychological or systemic diseases.[2,3] Majority of the people experience itching at some point in their lives, which can range anywhere between few seconds, due to an insect bite, to more than 6 weeks, as in the case of patients with chronic pruritis. In addition, due to the negative impact on the individual’s sleep, focus, and attention span, pruritis significantly affects the quality of life and compromises the overall patient well-being.[4,5]
Although many therapeutic options are available for the management of pruritis, paucity in the identification of factors involved in the genesis of pruritis has rendered its treatment, a challenge for clinicians. However, even the development of efficient treatment approaches has been hindered in part by lack of understanding of the pathological processes involved.[6] Pruritis is typically characterized by two important components, namely, dryness of the skin and itching. There is not yet a treatment for itch that is widely accepted. Instead, a customized strategy is implemented in the management of pruritis. The majority of the medications for topical therapy in pruritis (emollients) target the skin dryness, but do not address the itching component. Likewise, steroids and calcineurin inhibitors alleviate the itching, but fail to manage the skin dryness. Hence, there are no medications that effectively suppress both skin dryness and itching in pruritis.[7] In this regard, local anesthetics have also been used to treat pruritis and have been proven to be potent antipruritic medications for patients who suffer from persistent refractory pruritis.[8]
A formulation that would help to reduce the skin dryness and decrease the itching would therefore be ideal to manage the patients with chronic pruritis. Shea butter, mango butter, cocoa butter, aloe butter, cyclomethicone, dimethicone, propylene glycol, glycerine, and zinc oxide are the ingredients in moisturizer cream along with pramoxine hydrochloride, specifically intended to be used in chronic pruritis. This unique combination of four butters (Shea, Aloe, Cocoa, and Mango), emollient, and zinc oxide not only helps to moisturize and protect the skin but also contributes to improve the natural moisturizing capacity of the skin along with its barrier properties. Topical anesthetic and antipruritic, pramoxine, an excipient is an important constituent of moisturizing cream. Being a local anesthetic, pramoxine hydrochloride numbs the skin where it is applied and helps to minimize the sensation of itching and pain. By temporarily numbing the skin, pramoxine hydrochloride provides temporary relief from itching caused by insect bites, minor skin irritations, sunburn, and more. Pramoxine may be effective in reducing itching, thereby easing pruritis and calming the dry skin when combined with moisturizers, emollients, and humectants. Therefore, it was decided to investigate the tolerability and effectiveness of moisturizing cream in patients with chronic pruritis with respect to onset and total duration of anti-pruritic action.
MATERIAL AND METHODS
We designed a single-center, prospective, interventional, and single-group study to evaluate the effectiveness and tolerability of moisturizing cream in patients suffering from chronic pruritis. The study details, including potential risks and benefits, were explained to the patients by the Principal investigator/Co-investigator before screening for the study. All volunteer queries were cleared by the Principal investigator/Co-investigator and willing patients were consented for the study.
The patients diagnosed with chronic pruritis with dryness, chronic itch for more than 6 weeks (H/o Itch everyday), itch score on visual analogue scale (VAS) (mild-to-moderate – ≤7 on VAS), and on at least one site were included in the study. Patients attending the clinic outpatient department with chief complaints of pruritis were assessed for eligibility by the principal investigator following which, the study details were explained to patients. Patients’ demographics, physical examination, and vitals were checked and recorded. Baseline scores for patient’s self-assessment for itching using VAS were recorded. Thereafter patients were instructed to use the moisturizing cream (test product) on affected areas of the body where they experienced itching.
The effectiveness of moisturizing cream was assessed with the help of a questionnaire that was given to patients, wherein they were asked to record the effect of moisturizing cream (test product), on itching before application of test product and at 3 min, 5 min, 10 min, 15 min, 30 min, 60 min, 90 min, 2 h, 4 h, 6 h, and 8 h post-application. Patients were trained on how to fill in the VAS questionnaire by the study personnel. They were asked to remain at the study center for the entire study duration, post-application of moisturizing cream. VAS of all time points were recorded by the patients. Patients were advised to inform the study team if they needed to reapply the moisturizing cream (test product) or when the itching restarted after product application. This period was considered as itch free period preceding the next time of moisturizing cream application.
Statistical analysis was carried out using SPSS version 10.0, the continuous variables were summarized by treatment group using the summary statistics. Wilcoxon Signed rank test was used to assess the two-sided level of significance. P < 0.05 was statistically significant. The sample size was calculated using the level of significance as 0.05%, power (%) as 80.0%, and average onset of action (μ) = 5 min. The standard deviation (SD) was considered as 1 min, and the precision was considered as 0.5 min. Assuming the expected population SD to be 1, and employing t-distribution to estimate sample size, the study would require a sample size of n = 31 cases to estimate a mean with 95% confidence, 80% power, and a precision of 0.5. Thirty-two patients were included to get 31 complete cases.
RESULTS
A total of 32 patients were included in the study. The study was completed with all 32 patients and there were no dropouts. Data were then analyzed for all 32 patients. In this study, the age of the patients ranged from 19 to 49 years with the average age being 38.17 years. About 18.75% of the patients were males and 81.25% of the patients were females [Table 1].
| S. No. | Parameters | Mean±SD |
|---|---|---|
| 1. | Age (years) | 38.17±7.42 |
| 2. | Gender (male/female) | (6/26) |
| 3. | Temperature (degree celsius) | 36.1±0.3 |
| 4. | Systolic blood pressure (mm of Hg) | 115.22±9.8 |
| 5. | Diastolic blood pressure (mm of Hg) | 78.03±6.97 |
| 6. | SpO2(%) | 98.22±1.39 |
| 7. | Pulse rate (bpm) | 83.94±11.33 |
SD: Standard deviation, SpO2: Oxygen saturation
A questionnaire assessment was done at the beginning and at 3 min, 5 min, 10 min, 15 min, 30 min, 60 min, 90 min, 2 h, 4 h, 6 h, and 8 h post-application of the moisturizing cream to evaluate its effectiveness and tolerability. The percentage reduction in the intensity of pruritis ranged from 26.3% at 3 min to 66.9% at 8 h post-moisturizing cream application. The mean itch score at baseline was 5.922 ± 0.908, while at 3-min post-application, it was 4.366 ± 2.034 and at 8-h post-application, it was 2.781 ± 1.460 [Table 2]. The P-value for all the itch scores after application of the moisturizing cream right from 3-min to 8-h post-application was 0.001 which is highly significant. [Figure 1] represents the reduction in mean itch score following application of the moisturizing cream.
| S. No. | Time point | Mean itch score (Mean±SD) | Percentage reduction in the mean itch score as compared to baseline (%) | P-value |
|---|---|---|---|---|
| 1. | Baseline | 5.922±0.908 | ||
| 2. | 3 min | 4.366±2.034 | 26.3 | 0.001 |
| 3. | 5 min | 4.400±2.080 | 25.7 | 0.001 |
| 4. | 10 min | 4.281±2.143 | 27.7 | 0.001 |
| 5. | 15 min | 4.250±2.178 | 28.2 | 0.001 |
| 6. | 30 min | 4.109±2.115 | 30.6 | 0.001 |
| 7. | 1 h | 3.734±1.942 | 36.9 | 0.001 |
| 8. | 90 min | 3.291±1.822 | 44.4 | 0.001 |
| 9. | 2 h | 3.044±1.626 | 48.6 | 0.001 |
| 10. | 4 h | 2.781±1.460 | 53.0 | 0.001 |
| 11. | 6 h | 2.459±1.241 | 58.5 | 0.001 |
| 12. | 8 h | 1.959±1.38 | 66.9 | 0.001 |
SD: Standard deviation
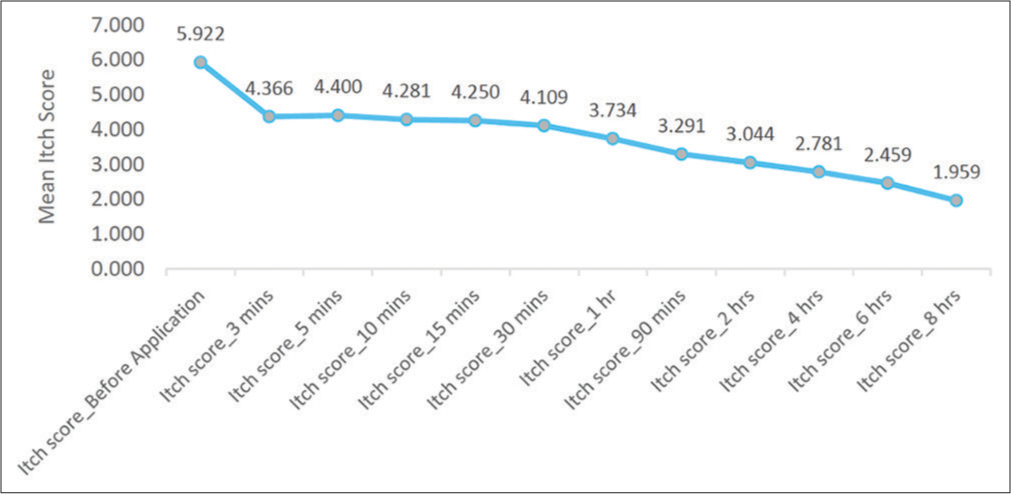
- Change in the mean itch severity over a duration of 8 h.
DISCUSSION
Advances in our knowledge related to the mechanisms and pathological processes involved in pruritis are the foundation for new treatments. However, treatment is usually symptomatic, without curing the underlying systemic condition. Some treatments, such as emollients and antihistamines, if used individually have a negligible overall effect in pruritis. However, given their affordability and potential for pain alleviation, they are frequently incorporated into different formulations. Interestingly, topical anesthetics have also been demonstrated to be effective antipruritic medicines for individuals with chronic refractory pruritis across the medical literature. In this regard, we decided to investigate the effectiveness of moisturizing cream in treating pruritis. After using the moisturizing cream, there was a significant decrease in the mean itch score, demonstrating effectiveness of the combination of the moisturizing cream in reducing the severity of pruritis.
The article by Purnamawati et al. emphasizes the clinical utility of moisturizers to support health worker’s recommendation and promotion of appropriate moisturizer application, particularly for therapeutic purposes to alleviate symptoms from various kinds of dermatitis.[9] In a double-blind, randomized, vehicle-controlled, and crossover study (n = 25), researchers looked at the effectiveness of 0.2% hydrocortisone and 1.0% lidocaine in treating pruritis ani.[10] For a week, patients sprayed the affected areas 2–3 times/day with the spray. The severity of pruritis significantly decreased, with just one patient suffering from mild unpleasant effects, such as a light transient stinging sensation. Another double-blind, randomized, and controlled trial (n = 28) evaluated the effectiveness of 1% pramoxine lotion in treating uremic pruritis in patients with end-stage renal illness who were reliant on hemodialysis.[11] It was noticed that the topical lotion reduced the VAS for itching in patients allocated in the treatment group by 61%, which was significantly higher than the 12% reduction discovered in the control group. In addition, the treatment group’s rate of symptom drop was quicker than the control groups with no negative effects. These results are consistent with pramoxine’s efficacy profile in our study which led to a 66.9% decrease in the mean pruritis score at 8 h compared to baseline values.
Pramoxine was successful in reducing pruritis in 57% of the patients in a major clinical trial including 200 patients with diverse itchy dermatoses, wherein cream formulations were more successful and favored over gel formulations.[12] Between 24 female individuals, lactic acid and pramoxine showed a substantial increase in skin hydration (P = 0.001) and dry and itchy skin (P = 0.001).[13] Another study demonstrated that pramoxine formulations containing ceramide offered comparable relief from atopic dermatitis (24.6% reduction in mean itch severity at 2 min after application and 58% reduction 8 h later) to ceramide-containing creams containing hydrocortisone (18.5% and 59.7% reduction, respectively), over an 8-h period.[14] Findings from these clinical trials have been consistent to those in our study, wherein the percentage reduction in the intensity of pruritis ranged from 26.3% at 3 min to 66.9% at 8-h post-moisturizing cream application and the mean itch score ranged from 5.922 ± 0.908 at baseline to 2.781 ± 1.460 at 8-h post-application. These results show that topical anesthetics have the potential to be an additional antipruritic therapy for patients whose symptoms are not satisfactorily alleviated by standard therapy.
Topical anesthetics can cure a number of persistent pruritis diseases that have a neurogenic component. Small unmyelinated C fibers, which are also implicated in the transmission of pain sensation, are assumed to be responsible for transmitting neuropathic itch. The intensity of itching may be decreased in pruritic patients for this reason. Pramoxine is hypothesized to function by blocking this signaling pathway.[15] Topical anesthetics are simple to apply and have few adverse effects. Along with emollients, they not only reduce the itching sensation but also address the skin dryness, effectively targeting the itch scratch cycle. Itching and skin dryness being two important components of pruritis, go hand in hand together in the course of the disease. Persistent itching can give rise to skin dryness, while on the other hand, dry skin can predispose to increase the tendency to scratch, thereby leading to the itch sensation, subsequently initiating the itch scratch cycle. Moisturizing cream, by effectively addressing the skin dryness and the itch sensation, breaks this itch scratch cycle in chronic pruritis providing definitive long-term relief. Moisturizing cream is a unique blend of moisturizers and emollient in a humectant-based cream with an anti-itch agent which aids in providing moisture to the skin and treating itchiness with a quick healing time. Therefore, moisturizing cream acts as a promising option in chronic pruritis providing dual benefits of itch relief and intense moisturization that takes care of skin dryness. Considering these benefits of the pramoxine, it is recommended by the Indian consensus[16] and the European guidelines[7] as the first-line agent for the management of pruritis.
The moisturizing cream caused a significant reduction in the mean itch score in case of patients suffering from chronic pruritis. The onset of action was as early as 3-min post-application while a significant reduction in the intensity of pruritis was observed over a period of 8 h. There were no serious adverse events reported during the study period and the cream was very well tolerated.
CONCLUSION
In patients with chronic pruritis, moisturizing cream with pramoxine as the topical local anesthetic significantly decreased the mean itch score. The onset of action began as soon as 3 min after application, and over the course of 8 h, a noticeable decrease in the severity of pruritis was seen in this study. The cream was well tolerated with no adverse effects in this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Depression modulates pruritus perception: A study of pruritus in psoriasis, atopic dermatitis, and chronic idiopathic urticaria. Psychosom Med. 1994;56:36-40.
- [CrossRef] [PubMed] [Google Scholar]
- Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2006;21:3495-505.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic pruritus in psychiatric inpatients: An explorative study. Gen Hosp Psychiatry. 2008;30:344-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical classification of itch: A position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87:291-4.
- [CrossRef] [PubMed] [Google Scholar]
- Itch characteristics in Chinese patients with atopic dermatitis using a new questionnaire for the assessment of pruritus. Int J Dermatol. 2002;41:212-6.
- [CrossRef] [PubMed] [Google Scholar]
- European S2k guideline on chronic pruritus. Acta Derm Venereol. 2019;99:469-506.
- [CrossRef] [PubMed] [Google Scholar]
- The role of topical anesthetics in the management of chronic pruritus. J Dermatol Treat. 2017;28:338-41.
- [CrossRef] [PubMed] [Google Scholar]
- The role of moisturizers in addressing various kinds of dermatitis: A review. Clin Med Res. 2017;15:75-87.
- [CrossRef] [PubMed] [Google Scholar]
- PERINAL-a new no-touch spray to relieve the symptoms of pruritus ani. Int J Color Dis. 1993;84:184-7.
- [CrossRef] [PubMed] [Google Scholar]
- A pramoxine-based anti-itch lotion is more effective than a control lotion for the treatment of uremic pruritus in adult hemodialysis patients. J Dermatol Treat. 2009;20:76-81.
- [CrossRef] [PubMed] [Google Scholar]
- Tronothane hydrochloride (pramoxine hydrochloride) in the control of pruritus. Postgrad Med. 1954;16:453-5.
- [CrossRef] [PubMed] [Google Scholar]
- An evaluation of the moisturizing and anti-itch effects of a lactic acid and pramoxine hydrochloride cream. Cutis. 2004;73:135-9.
- [Google Scholar]
- Anti-pruritic efficacy of itch relief lotion and cream in patients with atopic history: Comparison with hydrocortisone cream. J Drugs Dermatol. 2017;16:243-7.
- [Google Scholar]
- Topical pramoxine in chronic pruritus: Where do we stand? Indian J Dermatol. 2021;66:576.
- [CrossRef] [PubMed] [Google Scholar]
- Management of pruritus in indian settings : An expert opinion management of pruritus in Indian settings : An expert opinion. Am J Dermatol Venereol. 2021;10:30-43.
- [Google Scholar]






