Translate this page into:
A double whammy of bullous pyoderma gangrenosum and deep vein thrombosis following COVID-19 infection

*Corresponding author: Nirma Joy, Department of Dermatology, Al Azhar Medical College, Thodupuzha, Kerala, India. nirmajoy@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Joy N, Sobhanakumari K, Abraham A, Mathews H, George E. A double whammy of bullous pyoderma gangrenosum and deep vein thrombosis following COVID-19 infection. CosmoDerma. 2024;4:117. doi: 10.25259/CSDM_72_2024
Abstract
Bullous pyoderma gangrenosum (PG) is a rare variant presenting with hemorrhagic bulla and is usually associated with hematological malignancies. COVID-19 infection has various clinical manifestations, with one-fourth of patients developing deep vein thrombosis (DVT). There are a few reports of PG following COVID-19 infection or vaccination. We report a case of bullous PG, and skin lesions worsened due to concurrent DVT and COVID-19 infection, prolonging its healing time and physical disability.
Keywords
Bullous pyoderma gangrenosum
COVID-19 infection
COVID-19 vaccine
Deep vein thrombosis
INTRODUCTION
Bullous pyoderma gangrenosum (PG) is a rare variant of PG presenting with rapidly progressive superficial and hemorrhagic bulla which is often associated with hematological malignancy. Different cutaneous manifestations of COVID-19 are described nowadays, and DVT is perceived as one of the frequent findings.[1] We report a case of bullous PG, in whom skin lesions worsened while on treatment due to concurrent COVID-19 infection and DVT, prolonging its healing time.
CASE REPORT
A 60-year-old female presented with multiple painful ulcers over both legs and feet of one month duration. Initially, it started as a single painful bulla above the right ankle with no history of trauma. Similar new lesions appeared on the next day and rapidly progressed to necrotic ulcers. She had no history of associated fever, joint pain, altered bowel habits, post-menopausal bleeding, and loss of weight. She received two doses of the COVISHIELD (ChAdOx1nCov-19) vaccine four months and one month before the episode. She was on antidiabetics, antihypertensives, statins, and anti-platelets for coronary artery disease, but there was no recent change in medication before onset. There was a family history of hypertension, diabetes mellitus, and dyslipidemia among all her siblings, and her elder and younger sister were under treatment for deep vein thrombosis (DVT). On general examination, the patient was afebrile, her blood pressure was elevated (160/90 mmHg), had bilateral pitting pedal edema, and her body mass index was raised (28.9).
On dermatological examination, there were multiple ulcers of varying size from 2 cm × 1 cm to 10 cm × 6 cm and intact tense and collapsed hemorrhagic bullae over bilateral feet and legs. The ulcers had irregular, violaceous, and undermined edges and the ulcer base was covered with eschar and yellowish slough [Figure 1]. The skin pathergy test was positive. There was no regional lymphadenopathy, and all mucosae and hair appeared normal. The examination of both breasts revealed no palpable mass. The examination of the respiratory system, cardiovascular system, gastrointestinal, and central nervous system was within normal limits. Fundus examination showed changes of Grade I hypertensive retinopathy.
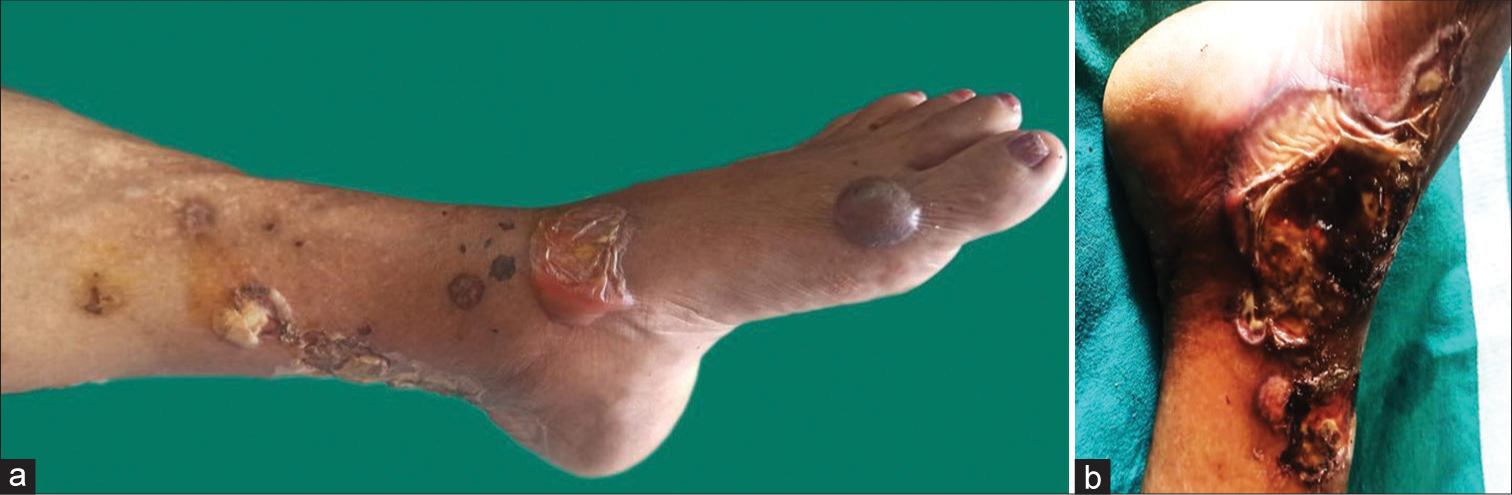
- (a and b) Multiple variable sized ulcers and hemorrhagic bullae over legs.
Routine laboratory testing showed elevated total count (20,600 cells/cumm) with neutrophilia (N 85% L15%), elevated erythrocyte sedimentation rate (120 mm/hr), C-reactive protein (CRP) levels (192 mg/L), elevated blood sugar (fasting blood sugar:211 mg/dL, postprandial blood sugar: 382 mg/dL), and altered renal function test (Serum urea: 103 mg/dL, Serum creatinine: 1.3 mg/dL). The peripheral smear showed neutrophilic leukocytosis with no abnormal blast cells. On investigation, levels of electrolytes, liver function test, lactate dehydrogenase, antinuclear antibody, myeloperoxidase antineutrophil cytoplasmic antibody, cytoplasmic antineutrophil cytoplasmic antibody, thyroid function test, thyroid-stimulating hormone receptor antibody, thyroid peroxidase antibody, bleeding time, clotting time, viral markers, venereal disease research laboratory test, rheumatoid factor, anti-cyclic citrullinated peptide antibody, carcinoembryonic antigen test, and cancer antigen 19.9 were all within normal levels. Examination of chest x-ray, x-ray of both ankles, x-ray spine, electrocardiogram, and Mantoux test revealed no significant findings. The pus culture from skin lesions showed growth of Klebsiella pneumonia, and the culture for fungus and mycobacteria was negative. Histopathological examination of skin biopsy showed non-specific findings with predominant neutrophilic infiltrate within the dermis [Figure 2]. The ultrasound examination of the abdomen and pelvis showed Grade 1 fatty liver, thickened endometrium with few subendometrial cysts. Based on the patient’s clinical examination and investigations, bullous PG was diagnosed. She was started on intravenous steroids, intravenous antibiotics, and other supportive measures including wound dressing, slough removal, compresses, and control of comorbidities. There was a dramatic improvement of lesions and symptoms within a week and, hence, was discharged on a tapering dose of oral steroids for PG, along with treatment for other comorbidities. After one month, she presented with a sudden onset of lower limb edema and pain along with lower abdominal pain, nausea, fever, respiratory discomfort, and decreased urine output. On clinical examination, there was diffuse leg swelling, tenderness, ill-defined erythema, and pitting pedal edema, more over the left leg than the right leg, and there was an increase in the size and depth of existing ulcers. On investigation, there was leukocytosis (total count: 17,400 cells/cumm), with neutrophilia (N85% L15%), altered renal function (serum urea: 108 mg/dL, serum creatinine: 1.9 mg/dL), raised D-dimer, and CRP. Astonishingly, she was COVID-19 antigen and reverse transcription-polymerase chain reaction positive. She gave no prior history of COVID-19 infection. The Doppler scan of the left lower limb showed near total to complete thrombosis of the venous system including common iliac, external iliac, common femoral, superficial femoral, popliteal, anterior and posterior tibial vein along with diffuse subcutaneous, and intramuscular edema showing features of cellulitis. There was growth of Pseudomonas aeruginosa on culture from skin lesions. No abnormality was seen on evaluating antiphospholipid antibodies, protein C, protein S, antithrombin level, factor V, and coagulation profile. She was started on intravenous antibiotics and heparin and then continued on oral dabigatran, rivaroxaban along with oral steroids. The lesions took nine months after the event to heal with cribriform scarring [Figure 3]. The patient came for a regular follow-up in three months after wound healing, there was no relapse and was continued on rivaroxaban.
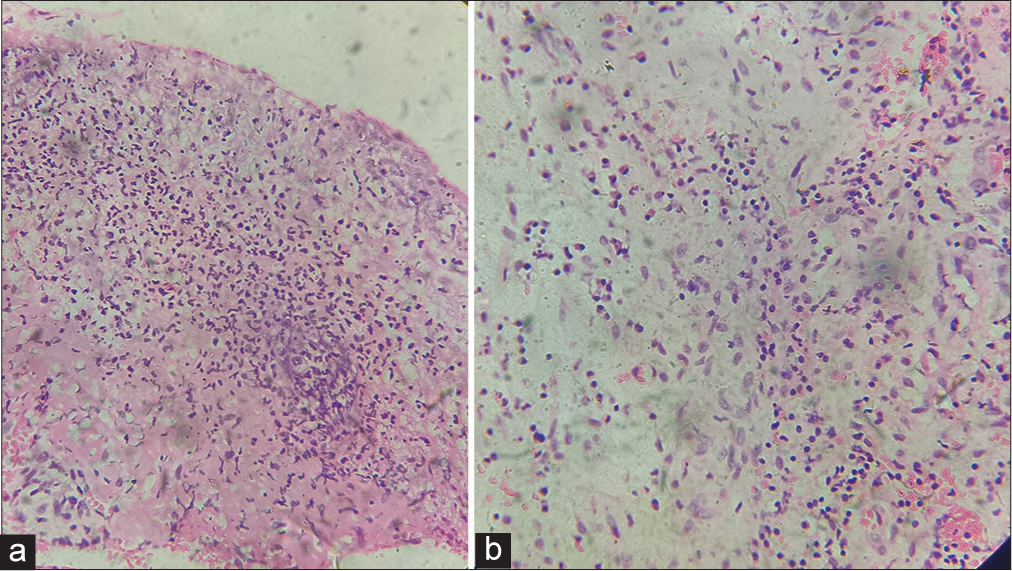
- (a and b) Histopathological findings with intense dermal infiltrates and neutrophil predominance. (a, H&E X100, b H&E X200).
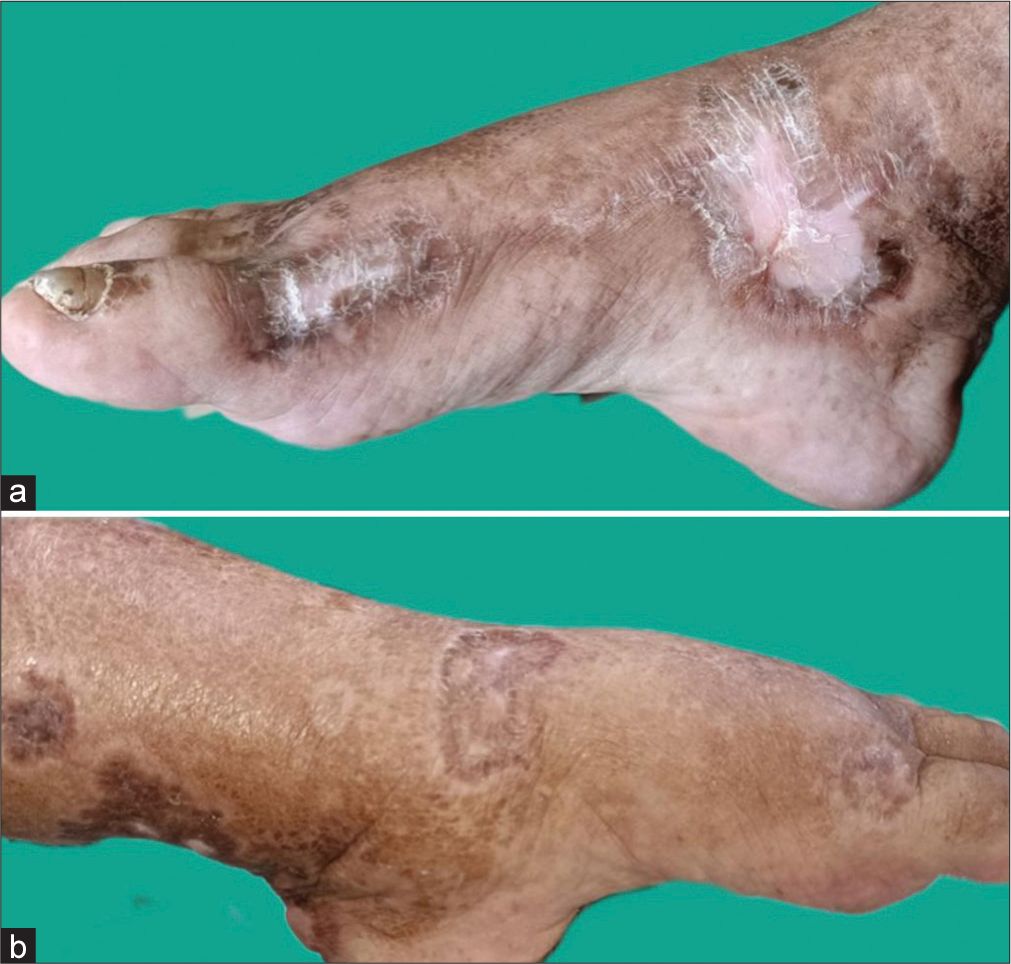
- (a and b) Lesions healed with cribriform scarring over left and right leg.
DISCUSSION
The PG was first described by Brunsting et al. in 1930 as a rare, neutrophilic ulcerative disorder of the skin.[2] The incidence is thought to be approximately 0.63/100,000 with the median age, at presentation, of 59 years.[3] The sex incidence ranges from being equal, to females being predominantly affected in up to 76% of cases.[4] Most cases are of classic ulcerative type (approximately 85%), other subtypes include bullous, vegetative, pustular, peristomal, and superficial granulomatous variants with subtypes sometimes transitioning from one form to another.[5] Bullous PG was initially described by Perry and Winkelmann in 1972.[6] This type is characterized by painful inflammatory bulla that breaks down into rapid, progressive, but superficial ulcers without undermining, mostly appearing over arms and face.[7] In contrast, our patient presented with hemorrhagic bullae progressing to ulcers with undermined and violaceous edges over both lower limbs.
A hematological malignancy should be sought as these are identified in up to 70% of cases. Bullous PG may precede, occur concurrently, or be diagnosed after a malignancy. The associated diseases include hematological malignancy (acute myeloblastic leukemia, chronic myeloblastic leukemia, myelodysplastic syndrome, multiple myeloma, and myeloid metaplasia), inflammatory bowel disease, rheumatoid arthritis, and Klinefelter’s disease.[8] Our patient had no associated diseases on clinical examination and investigations by a multidisciplinary team and was kept under regular follow-up to look for the development of any relevant signs and symptoms. Based on the diagnostic criteria created following a Delphi consensus exercise using the RAND/UCLA Appropriateness method,[9] our patient fulfilled one major criterion and seven minor criteria [Table 1].
| Criteria* | Patient fulfillment |
|---|---|
| Major criterion | |
| 1. Biopsy of ulcer edge demonstrating neutrophilic infiltrate | Yes |
| Minor criteria | |
| 1. Exclusion of infection | Yes |
| 2. Pathergy: Skin | Yes |
| 3. Personal history of IBD or inflammatory arthritis | No |
| 4. Papule, pustule, or vesicle that rapidly ulcerates | Yes |
| 5. Peripheral erythema, undermining border, and tenderness at site of ulceration | Yes |
| 6. Multiple ulcerations | Yes |
| 7. Cribriform or wrinkled paper scars at healed sites | Yes |
| 8. Decrease in ulcer size after immunosuppressive treatment | Yes |
IBD: Inflammatory bowel disease, *1 major criterion and four out of eight minor criteria needed for diagnosis
The risk factors for the development of DVT in our patient included COVID-19 infection, positive family history, prolonged immobility, advancing age, systemic glucocorticoids, and associated comorbidities such as diabetes mellitus, hypertension, dyslipidemia, coronary artery disease, and obesity. According to a study by Ierardi et al., the prevalence of DVT in laboratory-confirmed COVID-19 patients was 25.5% and the mean age was 63 ± 15 years.[1] The incidence rate ratios of a first episode of DVT were 5.59 during the first week after COVID-19 and 7.44 during the second week and significantly increased up to 70 days.[10] The hypercoagulability of SARSCoV-2 involves thromboinflammation triggered by viral infection and infection of the endothelial cells through the angiotensin-converting enzyme 2 receptor leads to endothelial activation and dysfunction and contributes to thrombin generation and fibrin clot formation.[11] Serum CRP is the main variable associated with the presence of DVT, whereas other clinical or laboratory variables, age, or D-dimer are not independently associated with DVT.
The DVT can lead to increased pressure in the distal veins of the leg and finally cause venous hypertension. Fibrin gets excessively deposited around capillary beds in the dermis leading to elevated intravascular pressure. Dysregulation of various proinflammatory cytokines and growth factors such as tumor necrosis factor-α (TNF-α), transforming growth factor-beta, and matrix metalloproteinases can also lead to chronicity of ulcers.[12]
The mechanisms behind cutaneous manifestations in COVID-19 are still under investigation, but likely involve the indirect effects of immune system hyperactivity and hypercoagulability. Interleukin 6 (IL-6) plays a key role in the activation and accumulation of neutrophils and potentially plays a significant role in hyperinflammation in both COVID-19 and PG, which can initiate PG after COVID-19 infection.[12] The vaccines can trigger human antibody and Th1 cell response, which increases blood levels of IL-2 and TNF-α, contributing to the secretion and activation of IL-6 and JAK/STAT kinase pathway, which also has a role in the pathophysiology of PG. These immunological similarities might have caused the onset of PG following COVID-19 vaccination and exacerbation of existing PG lesions after COVID-19 infection. The details of reported cases of PG following COVID-19 vaccination or infection are summarized in Table 2.
| Study | Age | Sex | COVID-19 infection/vaccination |
Duration of onset after infection/vaccination | Site | Previous history of PG | Morphology | Treatment |
|---|---|---|---|---|---|---|---|---|
| Syed et al.[13] | 71 | Male | COVID-19 infection | 10 days | Penis, groin, buttocks, abdomen | No | Ulcerative PG | Oral prednisolone, infliximab |
| Barry et al.[14] | 27 | Male | First dose of BNT162b2 COVID-19 vaccination | 24 h | Lower leg, thigh, peri-anal area, hand | No | Ulcerative PG | Oral prednisolone |
| Mohd e t al.[15] | 36 | Male | Sinopharm BIBP COVID-19 vaccination | 3 days | Site of vaccination-left arm | Yes | Ulcerative PG | Oral cyclosporine, intravenous methylprednisolone |
| Clark and Williams[16] | 73 | Female | Second dose of tozinameran (BNT162b2) vaccine | 2 weeks | Previous healed PG scar–left lower leg | Yes | Ulcerative PG | Oral prednisolone, cyclosporine, adalimumab |
| Rich et al.[17] | 44 | Female | COVID-19 infection | 3 months | Post-surgical-after reduction mammoplasty over breast | No | Ulcerative PG | Corticosteroids, mycophenolic acid, infliximab |
| Toyama et al.[18] | 29 | Male | Second dose of tozinameran (BNT162b2) vaccine | 1 month | Lower extremity | No | Ulcerative PG | Oral prednisolone |
| Hung et al.[19] | 46 | Male | First dose ChAdO × 1nCov-19 (Oxford-AstraZeneca) | 2 weeks | Hands, elbows, legs, feet | No | Bullous PG | Intravenous methylprednisolone, cyclosporine |
PG: Pyoderma gangrenosum
CONCLUSION
Bullous PG is a rare variant of PG, which may also present as deep ulcers with undermined borders over the lower extremities. They should be thoroughly scrutinized and followed up for associated diseases, especially hematological malignancies. As COVID-19 infection continues to wreak havoc across the world, even though there has been substantial progress in clinical research on clinical manifestations and pathophysiology, there are no published case reports on exacerbation of bullous PG following COVID-19 along with DVT. Early screening of serum CRP and thromboprophylaxis in high-risk patients can reduce the risk of DVT in COVID-19 infection. In this changing era of emerging infectious diseases, its impact on pre-existing diseases can cause varied clinical presentation, altered treatment response, and changed prognosis and outcome, demanding continuous education among health-care professionals.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Deep vein thrombosis in COVID-19 patients in general wards: prevalence and association with clinical and laboratory variables. Radiol Med. 2021;126:722-8.
- [CrossRef] [Google Scholar]
- Pyoderma gangrenosum: Clinical and experimental observations in five cases occurring in adults. Arch Dermatol. 1930;22:655-80.
- [CrossRef] [Google Scholar]
- Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: A retrospective cohort study. J Invest Dermatol. 2012;132:2166-70.
- [CrossRef] [Google Scholar]
- Pyoderma gangrenosum: A retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244-50.
- [CrossRef] [Google Scholar]
- Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412-8.
- [CrossRef] [Google Scholar]
- Bullous pyoderma gangrenosum and leukemia. Arch Dermatol. 1972;106:901-5.
- [CrossRef] [Google Scholar]
- Bullous pyoderma gangrenosum as the presenting sign of fatal acute myelogenous leukemia. Leuk Lymphoma. 2006;47:147-50.
- [CrossRef] [Google Scholar]
- Bullous pyoderma gangrenosum: A case report and review of the published work. J Dermatol. 2012;39:1010-5.
- [CrossRef] [Google Scholar]
- Diagnostic criteria of ulcerative pyoderma gangrenosum: A Delphi consensus of International expert. JAMA Dermatol. 2018;154:461-6.
- [CrossRef] [Google Scholar]
- Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: Nationwide self-controlled cases series and matched cohort study. BMJ. 2022;377:e069590.
- [CrossRef] [Google Scholar]
- The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb Res. 2020;194:101-15.
- [CrossRef] [Google Scholar]
- Venous leg ulcers: Pathophysiology and classification. Indian Dermatol Online J. 2014;5:366-70.
- [CrossRef] [Google Scholar]
- Pyoderma gangrenosum following COVID-19 infection. J Community Hosp Intern Med Perspect. 2021;11:601-3.
- [CrossRef] [Google Scholar]
- Pyoderma gangrenosum induced by BNT162b2 COVID-19 vaccine in a healthy adult. Vaccines (Basel). 2022;10:87.
- [CrossRef] [Google Scholar]
- COVID-19 vaccine: A possible trigger for pyoderma gangrenosum. Cureus. 2022;14:e25295.
- [CrossRef] [Google Scholar]
- Recurrence of pyoderma gangrenosum potentially triggered by COVID-19 vaccination. Cureus. 2022;14:e22625.
- [CrossRef] [Google Scholar]
- Post-surgical pyoderma gangrenosum in otherwise healthy patient with history of COVID-19. Breast J. 2021;27:671-4.
- [CrossRef] [Google Scholar]
- Pyoderma gangrenosum following vaccination against coronavirus disease-2019: A case report. Int J Dermatol. 2022;61:905-6.
- [CrossRef] [Google Scholar]
- Haemorrhagic bullous pyodermagangrenosum following COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2022;36:e611-3.
- [CrossRef] [Google Scholar]






