Translate this page into:
Capecitabine-induced palmar-plantar erythrodysesthesia

*Corresponding author: Yamini Sihag, Department of Dermatology, All India Institute of Medical Sciences, Rajkot, Gujarat, India. yaminisihag174@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sihag Y, Tyagi S. Capecitabine-induced palmarplantar erythrodysesthesia. CosmoDerma. 2024;4:83. doi: 10.25259/CSDM_43_2024
A 68-year-old male with colon carcinoma developed redness and swelling over the hands shortly after the first cycle of capecitabine. It was associated with pain and burning sensation which resulted in difficulty in carrying out routine activities. On examination, well-defined blanchable erythema with edema was noted over the palms with prominent involvement of the thenar and hypothenar eminences [Figure 1]. The dorsum of the hands showed focal erythema with hyperkeratotic plaques over the proximal interphalangeal joints and distal interphalangeal joints [Figure 2]. Diagnosis of palmarplantar erythrodysesthesia was made, and the patient was started on emollients, topical steroids, and pregabalin. There was a significant improvement in the patient’s symptoms by 2–3 weeks.
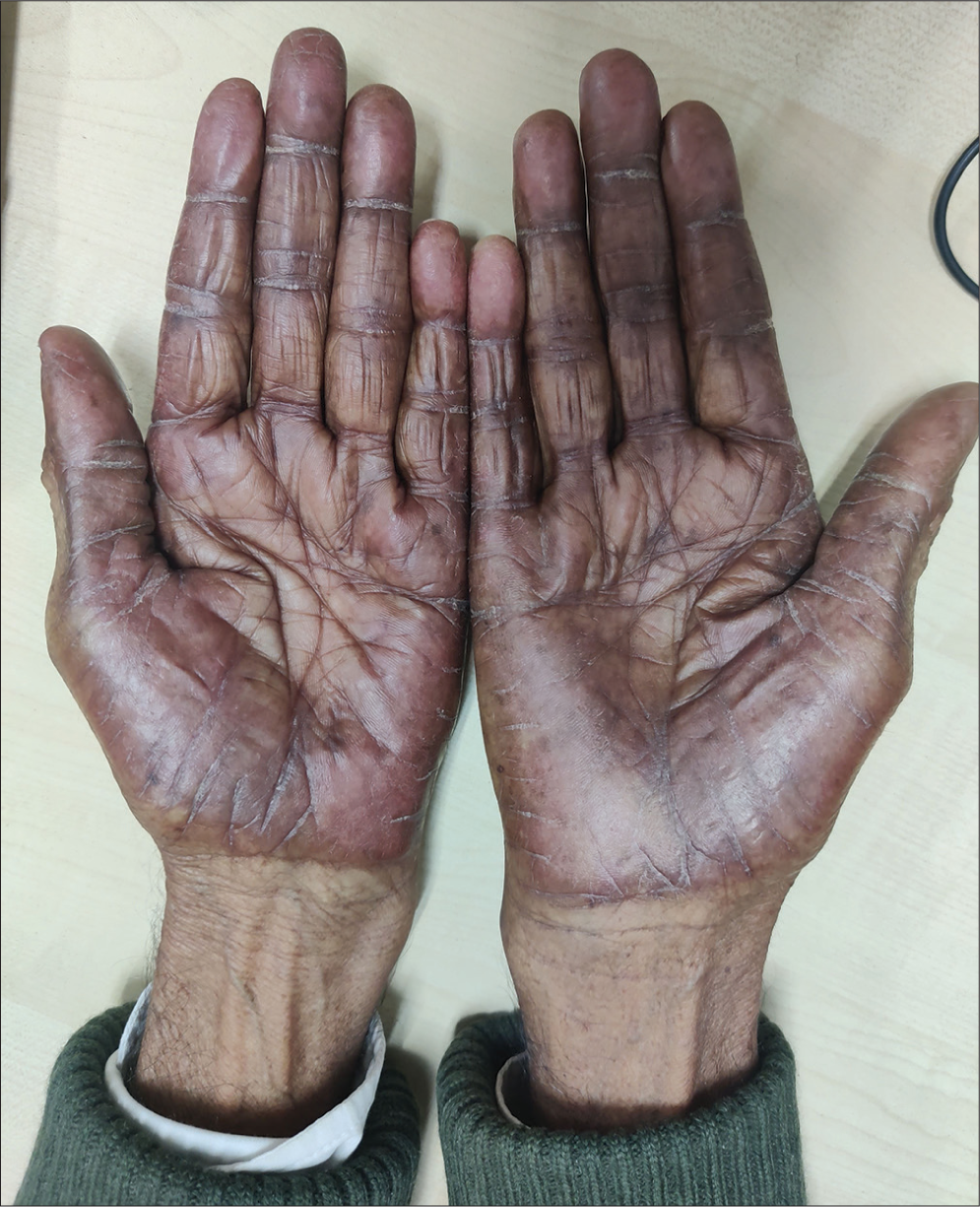
- Well-defined erythema over the palms with more involvement of thenar and hypothenar eminence.
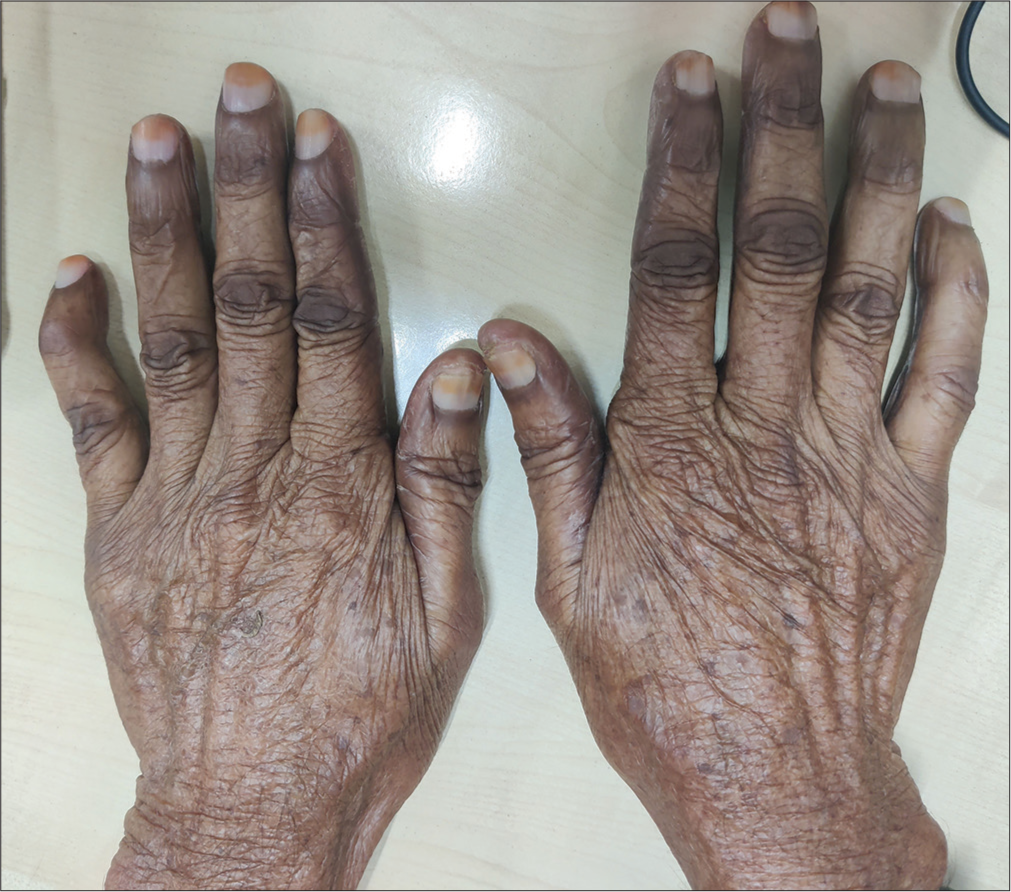
- Focal areas of erythema and hyperkeratotic plaques over periarticular area.
Palmar-plantar erythrodysesthesia, also known as hand and foot syndrome, is a major dose-dependent adverse effect of capecitabine, seen in 22–77% of cases. It is hypothesized to occur secondary to toxic accumulation of capecitabine in eccrine glands of palm and soles which lead to cellular injury, that is, keratinocyte apoptosis and thinning of stratum corneum.[1,2] This translates clinically as erythema, with or without edema, desquamation, and fissuring. Severe cases may develop blisters and ulceration, hampering their quality of life.[2] Based on severity, it can be classified into three grades:[1]
Grade I: Minimal skin changes or dermatitis (erythema, edema, and hyperkeratosis), no pain.
Grade II: Moderate skin changes (scaling, blisters, bleeding, cracking, and erosion) with pain, relative limitation.
Grade III: Severe skin changes (scaling, blisters, bleeding, fissures, erosions, and hyperkeratosis) with pain.
Palmar-plantar erythrodysesthesia is a dose-dependent condition that is generally observed after a median of three cycles of chemotherapy. This case presented relatively early after starting capecitabine and peculiarly with erythema and swelling rather than macular hyperpigmentation which is characteristic of palmar-plantar erythrodysesthesia in the skin of color.[1] Management includes symptomatic treatment with emollients, topical steroids supported with cold compresses, and limb elevation. Systemic treatment and cessation of the offending drug is considered in severe cases.[2,3]
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Hand-foot syndrome caused by capecitabine: Incidence, risk factors and the role of dermatological evaluation. Ecancermedicalscience. 2022;16:1390.
- [CrossRef] [PubMed] [Google Scholar]
- Hand and foot syndrome secondary to capecitabine. Indian J Dermatol Venereol Leprol. 2014;80:427-30.
- [CrossRef] [PubMed] [Google Scholar]
- Hand-foot syndrome in cancer patients on capecitabine: Examining prevalence, impacts, and associated risk factors at a cancer centre in Malaysia. Support Care Cancer. 2024;32:345.
- [CrossRef] [PubMed] [Google Scholar]





