Translate this page into:
Role of low dose oral minoxidil in the treatment of hair loss: A review

*Corresponding author: Dr. Sajin Alexander MBBS MD(DVL) FRGUHS (Aesthetic Dermatology), Specialist Dermatologist, Department of Dermatology, Schieffelin Institute of Health-Research and Leprosy Centre, Vellore, Tamil Nadu, India. sajinalexander@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Alexander S, Mysore V, Hirevenkangoudar AL. Role of low dose oral minoxidil in the treatment of hair loss: A review. CosmoDerma 2021;1:38.
Abstract
Low-dose oral minoxidil (OM) has increasingly been used by many doctors around the world as a treatment option for hair loss. Sufficient data regarding its effect and side effect profile are lacking. An online search was done on PUBMED and GOOGLE SCHOLAR for articles that used OM as a treatment option for hair loss. Doses ranging from 0.25 to 5 mg have been used for treatment in various studies. Good compliance and tolerability have been noticed with low-dose OM therapy. Adverse effects are few and are mild with hypertrichosis being the most common adverse effect in a majority of the studies, the risk of which increases with an increase in dosage of the drug.
Keywords
Oral minoxidil
Low dose oral minoxidil
Alopecia
Androgenetic alopecia
Hair loss
INTRODUCTION
A common treatment advised by dermatologists all around the world, topical minoxidil (TM) is an FDA approved medication for patterned hair loss.[1] However, in cases of non-responders and in patients developing irritant or allergic contact dermatitis to the topical formulation, oral minoxidil (OM) can be an option of choice.[2]
Originally developed to treat hypertension, it was found to produce hypertrichosis in more than half of the cases treated.[3] Hair growth was noticed over the face, chest, arms, back, and much to the pleasure of many bald patients, over the scalp as well.[4]
Even though FDA approved for resistant hypertension,[5,6] OM is not approved for hair loss but has been used by many for the same.[2]
METHODOLOGY
A literature search for published articles was done using PUBMED and GOOGLE SCHOLAR using the keywords, “OM AND alopecia,” “OM AND androgenetic alopecia (AGA),” “OM AND AGA,” “OM AND hair loss,” “Low dose OM AND hair loss,” “Low dose minoxidil AND alopecia,” “OM AND adverse effects.”
The search resulted in 27 studies related to OM, of which fourteen studies were concerning patterned hair loss and eight studies related to its use in alopecia other than AGA. Articles which contained only a fleeting mention of OM were removed from our study due to insufficient data.
PHARMACOKINETICS
The chemical name of Minoxidil is piperidino-pyrimidine derivative. The chemical structure is 2, 4-primidinediamine, 6-(1-piperidinyl)-, 3-oxide (C9H15N5O)[7]
Once taken orally, 95% of the drug is absorbed from the GIT and appears in the plasma within 15–30 min with a peak concentration seen in 30–60 min. Within the body, nearly 90% of the drug is metabolized in the liver by conjugation with glucuronic acid at the N-oxide position in the pyrimidine ring and conversion to other polar metabolites.
There is no adaptive increase in drug metabolism with repetitive/chronic dosage noticed with the whole body clearance rate of the drug being 7–10 days after chronic drug administration.[8]
The kidney is the major excretory organ for minoxidil and its metabolites with ≥90% of the administered drug[8] excreted in urine as unchanged and as a metabolite (glucuronide conjugate) within 4 days. It does not cross the blood-brain barrier.[7]
MECHANISM OF ACTION
Knowledge of how OM affects human hair growth is little as the majority of the studies are limited to the response of TM in treating AGA.[9] The main step is the sulfation of minoxidil to its active form of minoxidil sulfate by the enzyme sulfotransferases (phenol sulfotransferase) through hepatic sulfonation.[10] In non-responders to TM, with outer root sheath (ORS) sulfotransferase enzyme level of <0.4 Optical density (OD)405, OM can be used as an alternative.[11] Having said that, recently it has been shown that ORS sulfotransferase enzyme can also be used as an enzymatic assay to detect nonresponders even to OM (ORS sulfotransferase level - <0.254 OD405).[12]
Oral administration induces relaxation of vascular smooth muscle through the opening of sarcolemmal ATP2-dependent potassium channels and thus reduces blood pressure (BP).[13]
Minoxidil has several effects which may promote hair growth:
Prolongation of the kenagen phase (latent period of the hair cycle-the time between the shedding of telogen hair and the onset of the next anagen). It has been suggested that minoxidil has a key role in cell proliferation as potassium channel activity is required for moving to the G1 phase of the cell cycle from the latent post telogen phase.
Action on smooth muscles of the peripheral artery - It opens the potassium channels leading to hyperpolarization of the cell membrane and inhibits calcium entry thus leading to hair growth.
Minoxidil also causes a delay in the hydrolysis of cAMP through inhibition of phosphodiesterase, thus resulting in vasodilatory action[6,14] [Figure 1].
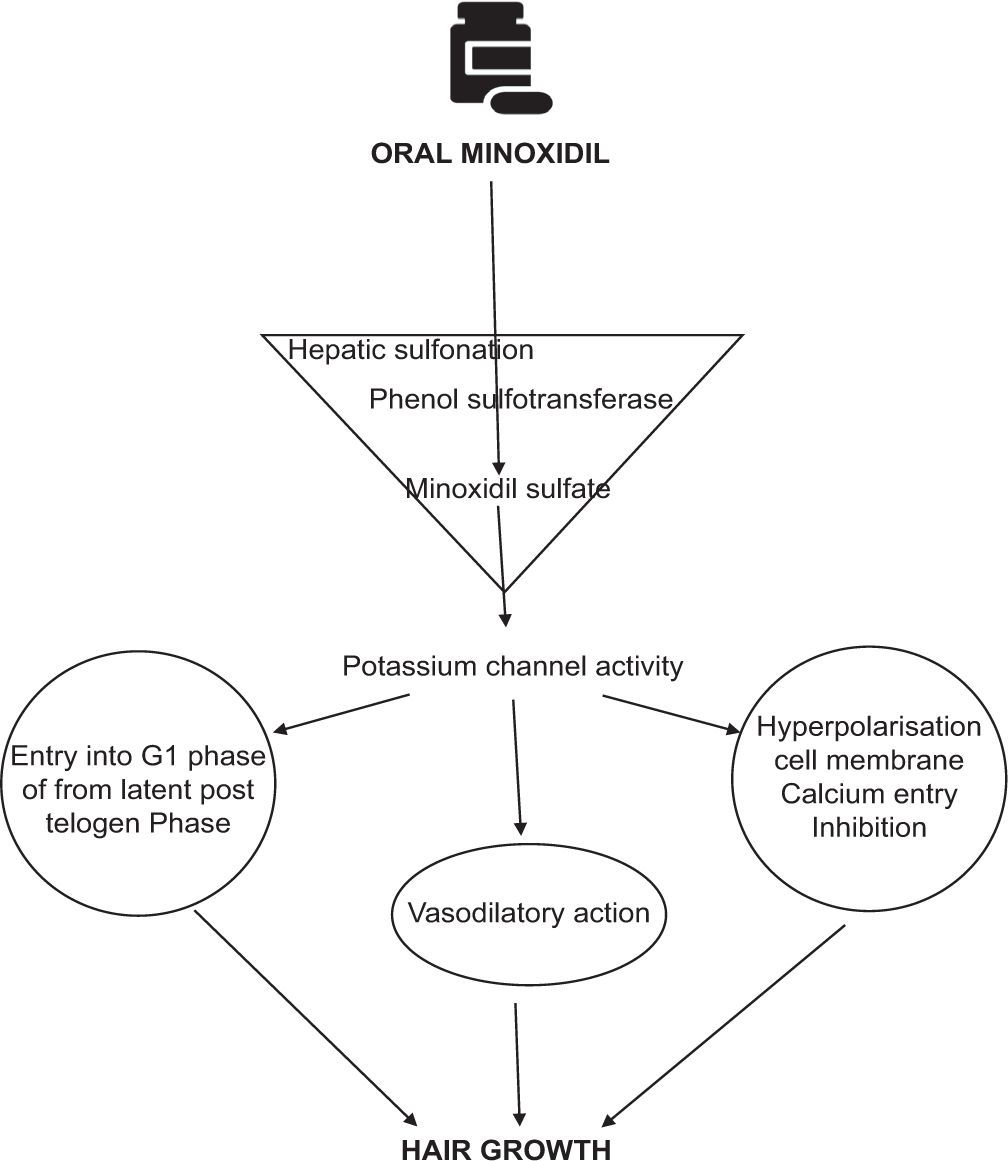
- Mechanism of action of Oral Minoxidil in Hair growth.
EFFICACY
OM is a U.S. FDA-approved medication for hypertension but not approved for hair loss[2] and has been given a grade D recommendation by the Japanese Dermatological Association due to its side effect profile and lack of clinical trials.[5,15] Nevertheless, several studies have been conducted all over the world with various doses for treatment of alopecia and an increasing number of data show that OM can be considered as a possible therapeutic option for AGA.[2,16-20]
Due to its side effect profile, recently many authors have propounded a low dose OM regimen for treating AGA. In patients not responding to or are resistant to conventional therapy the search for other treatment avenues has been discouraging. As a result, many are revisiting the use of OM as a plausible option in the management of alopecia.
Our search revealed 14 studies in relation to OM therapy in patterned hair loss and 8 studies concerning its use in alopecia due to other causes. Three studies combined OM with other drugs to increase efficacy and compliance. The various studies and features of these studies have been explained in [Tables 1 and 2]. The dosage used in these studies ranged from 0.25 mg to 5 mg with a majority of them using doses <2.5 mg to avert any untold side effects. Most of these have been conducted over of 6 months with daily dosing the rule and lower doses being offered to women compared to males. One such study utilized 0.25 mg OM for the treatment of patterned hair loss in 100 females. OM was combined with 25 mg spironolactone in this study to avoid fluid retention that may arise due to the former. The study demonstrated significant improvement with a very low side effect profile (8%) after 12 months of therapy.[16] The same cannot be said about patterned hair loss in males where similar dosage as above only resulted in mild improvement suggesting the need for higher doses in men.[16,21] The above statement was reaffirmed in another study where higher doses led to better response in men.[22]
| Study | Dosage (mg) | Duration (months) | n* | Results |
|---|---|---|---|---|
| Sinclair[16] | 0.25 mg+Tab. Spironolactone 25 mg | 12 | 100 | Mean reduction in hair loss severity score was 1.3 from baseline value of Sinclair 2.79 (range 2–5), Mean reduction in hair shedding score was 2.6 from baseline value 4.82 (range 1–6) |
| Jimenez-Cauhe et al.[17] | 2.5 mg 5 mg |
>6 | 16 | 37.5% - Marked improvement 62.5% - Good improvement |
| Beach[18] | 1.25 mg | 6 | 16 | Decreased hair shedding - 37.5% Increased scalp hair growth - 28% |
| Lueangarun et al.[19] | 5 mg | 6 | 30 | Improvement seen in all patients, Remarkable improvement 43%, Significant improvement in total hair count (Vertex>Frontal) |
| Pirmez and Salas-Callo[21] | 0.25 mg | 6 | 25 | Improvement noticed in Frontal scalp: New hair growth-52% Total hair density-60% |
| Improvement noticed in occipital scalp: New hair growth - 40% Total hair density - 40% |
||||
| Therianou et al.[25] | 1 mg | 17 | 9 | Good improvement |
| Ramos et al.[23] | 1 mg | 6 | 52 | Total hair density - 12% increase OM superior to TM therapy |
| Olamiju B et al.[38] | 2.5 mg+Tab. Spironolactone 50 mg | 3 | 6 | 5 patients demonstrated a 1-grade improvement and 1 patient maintained the same grade but clinically was slightly improved |
| Panchaprateep and Lueangarun[39] | 5 mg | 6 | 30 | Significant increase in total hair counts from baseline notice Assessment of the vertex area revealed 100% improvement with 43% of patients showing excellent improvement |
| Vastarella et al.[40] | 1.25 mg | 6 | 12 | 58% had marked improvement while 42% had mild improvement |
| Jha et al.[41] | 1.25 mg | 6 | 32 | Marked improvement - 43.3% Mid improvement - 40.6% |
| Rodrigues-Barata et al.[42] | 0.25–2 mg | 9 | 148 | Stabilization of alopecia noticed in 20.3% while 79.7% had clinical improvement. Of these, 64.2% presented with mild improvement and 15.5% with marked improvement. The clinical improvement was higher in more advanced stages of alopecia. |
| Jha et al.[43] | Group A: 1.25 mg+PRP Group B: 2.5 mg+PRP therapy | 6 | A=47 B=48 |
Marked improvement on GCP seen in 40.4% and 58.3% patients in Group A and B respectively. Patient satisfaction score between both groups were statistically significant with Group B patients having a better satisfaction score. |
| Indication | Study | n* | Dosage (mg) | Findings |
|---|---|---|---|---|
| AA | Fiedler-Weiss et al.[24] | 65 | 5 mg twice daily | Some form of improvement:80% Cosmetically meaningful improvement in 18% |
| Wambier et al.[44] | 12 | Females: 2.5 mg Males: 5 mg Combined with Oral tofacitinib 10 mg |
Median final percentage regrowth-93% | |
| TE | Perera and Sinclair[26] | 36 | 0.25–2.5mg | Improvement-92% Reduction of mean HSS from baseline to 12 months–2.58 (range 1–6) |
| Permanent CIA | Yang and Thai[27] (Case report) |
1 | 1 mg | Regrowth of significant amount of hair noticed after 1 year Cosmetically meaningful hair lengthening noticed Good patient satisfaction |
| Monilethrix | Sinclair[28] (Case series) |
2 | Case 1: 0.25 mg | Case 1: Significant increase in hair density, length with reduced breakage |
| Case 2: 0.25–0.5 mg | Case 2: Significant increase in hair density and significant reduction in hair shedding | |||
| Loose anagem hair syndrome | Cranwell and Sinclair[29] | 1 | 0.5 mg | Dramatic improvement in hair density and length |
| LPP | Vano-Galvan et al.[22] | 51 | Males: 2.5 mg Females: 1 mg |
Hair thickness improved-39% Hair thickness remained stable-53% Worsening of hair thickness-8% Better results with diffuse than patchy type LPP |
| Lichen planopilaris FFA Traction alopecia Central centrifugal cicatricial alopecia Chemotherapy Induced alopecia |
Beach et al.[45] | 51 | 1.25 mg | Increased scalp hair growth (33/51; 65%) and decreased hair shedding (14/51; 27%) |
| Frontal Fibrosing alopecia | Pirmez and Abraham[46] | 7 | 0.5–2.5 mg | Cosmetically acceptable regrowth noticed in both physician and patient after 6 months |
Higher doses of 5 mg given as a single dose or divided over a day for treatment of patterned hair loss revealed dramatic clinical improvement but the associated side effects were far more frequent than those seen in lower doses. However, these side effects were mild, tolerable, and reversible.[17,19]
In another study, 1.25 mg/day of OM was given for 3 months resulting in increased scalp hair growth and decreased hair shedding in 28% and 33%, respectively. The interesting aspect of this study was that except for two all other subjects were females and while hypertrichosis was noticed in 39% of patients, none deferred from continuing the therapy revealing good compliance and tolerability by patients. About 78% of patients continued therapy even 6 months post-study period.[18]
Ramos et al. reported that 1 mg OM was superior to the TM 5% and that it could be used as an alternative to TM.[23] The above conclusion supports the findings of Fiedler-Weiss et al. who compared OM and TM in the treatment of alopecia areata.[24] Reasons for this are:
Better convenience as swallowing a tablet is easy compared to applying lotions
Increased cosmesis as OM didn’t produce any residue unlike after TM application
Low cost of purchase for the same period
Co-therapy was made possible for people with visible bald patches where commercial fibers could be used to enhance the fullness of hair without any hassle.
Increased compliance and
Better tolerability[18]
Apart from AGA, low-dose OM has also been used in the treatment of other conditions associated with hair loss. We found eight such studies that used OM as a treatment option for indications other than AGA. Doses as low as 0.25–10 mg have been used in these studies. The different studies, indications, dosage, and outcomes are enumerated in Table 2. As noticed in Table 2, OM has been used in both non-cicatricial and cicatricial alopecia. Clinically evident improvement has been noticed in the form of hair regrowth and decreased hair shedding and has been noticed in all studies with good patient satisfaction in many of the studies. An improvement of 80% and 93% have been noticed by Fiedler-Weiss et al.[24] and Wambier CG et al.[44] in their respective studies when used for alopecia areata while an improvement of 92% in Telogen effluvium was noticed by Perera and Sinclair in his study on 36 subjects.[24,26] Granted that these studies have had many flaws such as small sample size and lack of control; however, the results, as well as the patient satisfaction scores, are significantly high enough to demand attention. Furthermore, the adverse effects noticed with doses ranging from 0.25 mg to 5 mg in these studies were very minuscule.
Regarding adverse effects, a dose-dependent relation has been noticed with adverse effects more common in the higher dosage of 5 mg and least in lesser dosages.[16-19,21]
Hypertrichosis is the most common effect with 4% risk at 0.25 mg, 37.5% at 1.25 mg, and 93% at 5 mg.[16,18,19] First noticed on the temples, glabella, forehead, or along with the sideburns, it is reversible on discontinuation.[42,43]
Hypotension has been noticed to occur in few studies as well.[16,22] Fleishaker et al. in his study concluded that doses ranging from 2.5 mg to 10 mg had little effect on BP in normotensive subjects. Nevertheless, measurement of baseline BP of all patients planning to be started on OM is recommended and in patients with values <90/60 mmHg, the addition of 50 mg of sodium chloride to the dose can be considered.[16,31]
Salt and water retention in the form of swelling of the feet, lower legs, hands or face, and rapid weight gain can occur.[30] This risk varies from 4% at doses of 0.25 mg to 10% at doses of 5 mg. The addition of 25 mg of spironolactone has been shown to mitigate this risk while potentiating the effect of minoxidil; however, caution is advised as it can, in theory, worsen postural hypotension.[13,16]
ECG changes noticed include T-wave flattening or inversion, other T-wave alterations and other ST wave changes which are improved within 24 h of withdrawal of the drug. These changes have been reported in only two studies where low-dose OM was used.[4,19,38]
Cardiac manifestations have rarely been reported in the literature and are unseen with low dose OM (tachycardia, congestive cardiac failure, pericardial effusion, and pulmonary hypertension).[4]
Other adverse effects noted but uncommonly seen include headache, rash, nausea, gynecomastia, bullous eruptions, drowsiness, fatigue, polymenorrhea, and itchy eyes.[4,7]
Three cases of Stevens-Johnson syndrome due to OM have been reported in the literature. Two of these three cases transformed into toxic epidermal necrolysis and eventually culminated in death while one of the patients subsequently improved on cessation of the drug. These patients were on OM to manage hypertension and all three had a history of underlying chronic renal impairment before the initiation of minoxidil therapy.[32] Since the kidney is the main route of excretion of minoxidil, the hypothesis is that there is defective detoxification of minoxidil sulfate which triggers an immune response and an inability to clear these metabolites leading to an accentuation of the immune response.[7,32-35] Having said that the adverse effects associated with the use of low-dose OM for the treatment of alopecia were considered mild and insignificant in six studies while there were no adverse effects noticed in five studies. These studies used doses ranging from 0.25 mg to 2.5 mg while studies with higher doses were associated with more frequent side effects [Table 3].[25,27-29,38-41,45,47]
| Study | Dose | n* | Adverse effects |
|---|---|---|---|
| Sinclair[16] | 0.25 mg+Tab. Spironolactone 25 mg | 100 | Mild Urticaria-2%, Hypotension-2%, Facial hypertrichosis-4% |
| Jimenez-Cauhe et al.[17] | 2.5 mg 5 mg |
16 | Mild-29.3% Hypertrichosis-24.3%, Pedel edema-4.8%, Hair shedding-2.4% |
| Beach[18] | 1.25 mg | 16 | Mild Hypertrichosis-37.5% |
| Lueangarun et al.[19] | 5 mg | 30 | Hypertrichosis-93%, Pedal edema-10%, ECG alteration-10% |
| Pirmez and Salas-Callo[21] | 0.25 mg | 25 | Hypertrichosis-20%, Hair shedding-16%, Pedal edema-4% |
| Therianou et al.[25] | 0.5 mg | 9 | 2 cases of mild hypertrichosis |
| Ramos et al.[23] | 1 mg | 52 | Pre tibial oedema-4%, Hypertrichosis-27% |
| Fiedler-Weiss et al.[24] | 5 mg twice daily | 65 | Hypertrichosis-17%, Fluid retention, Headache, Depression |
| Perera and Sinclair[26] | 0.25–2.5mg | 36 | Facial hypertrichosis-39%, Transient postural, Dizziness-5.6%, Ankle oedema-2.8% |
| Yang and Thai[27] (Case report) |
1 mg | 1 | No adverse effects noticed after 1 year of therapy |
| Sinclair[28] (Case series) |
Case 1: 0.25 mg Case 2: 0.25–0.5mg |
2 | Case 1: No adverse effects noticed after 2 years of therapy Case 2: No adverse effects noticed after 18 months of therapy |
| Cranwell and Sinclair[29] | 0.5 mg | 1 | No adverse effects mentioned |
| Vano-Galvan et al.[22] | Males: 2.5 mg Females: 1 mg |
51 | Adverse effects-mild-37% Hypertrichosis-27.5%, Postural hypotension-5.9%, Tachycardia-3.9%, Weight gain-1.9% |
| Olamiju and Craiglow[38] | 2.5 mg | 6 | None-Treatment was well tolerated and without any adverse effects |
| Beach et al.[45] | 1.25 | 51 | Lightheadedness-4, Palpitations-2, Ankle edema-1, Hypertrichosis-22, Skin Urticaria-1, Other Paresthesia-1 |
| Sanabria et al.[47] | 0.5–5 mg | 435 | Hypertrichosis-54%, Headache-10%, Insomnia-7%, Edema-9%, Dizziness-7%, Palpitations-4%, Facial edema-1% |
| Panchaprateep and Lueangarun[39] | 5 mg | 30 | Hypertrichsosis-93%, ECG findings-20%-premature ventricular contraction (occasional), T-wave inversion, Pedal edema-10% |
| Vastarella et al.[40] | 1.25 mg | 12 | Mild hypertrichosis-25%, Postural hypotension-8.3%, Peripheral edema-25% |
| Jha et al.[41] | 1.25 mg | 32 | Hypertrichosis and peripheral edema in 1 patient |
Contraindications to OM include pheochromocytoma or previous hypersensitivity reactions to ingredients, hypotension, cardiac comorbidities, and pregnancy or breast feeding.[20]
Considered as Pregnancy Category-C drug, rare reports of fetal malformations have been reported.[36] Use of OM has also been studied in pediatric patients in a review article by Lemes et al. where they concluded that data for use in hair growth was insufficient and that adverse effects were a cause of concern in this age group. However, they also commented that although TM was the preferred choice, in special circumstances such as hair shaft disorders, cicatricial alopecia, precocious, and severe AGA, or in extensive AA, OM may be considered in very low doses.[37]
With regards to combination therapy, we found three studies where OM was combined with other medications such as oral tofacitinib and spironolactone. Oral spironolactone was used in two studies, 25 mg combined with 0.25 mg of OM in one study for the treatment of AGA while 50 mg of spironolactone was combined with 2.5 mg of OM in another. The purpose of combining the former drug with OM in both these studies was to allay fluid retention that may arise as a result of the use of OM. None of the above two studies developed any symptoms or signs suggestive of fluid retention in their respective studies.[16,36]
One study combined OM with oral tofacitinib 10 mg in the treatment of alopecia areata where they noticed a median percentage improvement of 93% in hair regrowth.
CONCLUSION
Low dose OM appears to be a tolerable[42,43] and effective alternative to TM in patients not responding to the latter. However, it has to be stressed at this time that evidence for OM is low and concerns over systemic side effects remain and larger studies involving greater sample size should be done in the future for a better understanding of its role in hair loss.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:136-41.
- [CrossRef] [Google Scholar]
- Safety concerns when using novel medications to treat alopecia. Expert Opin Drug Saf. 2018;17:1115-28.
- [CrossRef] [Google Scholar]
- Stability of an extemporaneously compounded minoxidil oral suspension. Am J Health Syst Pharm. 2018;75:309-15.
- [CrossRef] [Google Scholar]
- Lysyl hydroxylase inhibition by minoxidil blocks collagen deposition and prevents pulmonary fibrosis via TGF-β1/Smad3 signaling pathway. Med Sci Monit. 2018;24:8592-601.
- [CrossRef] [Google Scholar]
- Hair Transplantation In: Ch. 11 (1st ed). New Delhi: Jaypee Brothers Medical Publishers Pvt. Ltd.; 2016. p. :71-3.
- [Google Scholar]
- Minoxidil and its use in hair disorders: A review. Drug Des Devel Ther. 2019;13:2777-86.
- [CrossRef] [Google Scholar]
- Pharmacology and pharmacokinetics of minoxidil. J Cardiovasc Pharmacol. 1980;2(Suppl 2):S93-106.
- [CrossRef] [Google Scholar]
- Minoxidil: Mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-94.
- [CrossRef] [Google Scholar]
- Sulfation of minoxidil by human liver phenol sulfotransferase. Biochem Pharmacol. 1990;40:1027-32.
- [CrossRef] [Google Scholar]
- Novel enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatol Ther. 2014;27:171-3.
- [CrossRef] [Google Scholar]
- Oral minoxidil bio-activation by hair follicle outer root sheath cell sulfotransferase enzymes predicts clinical efficacy in female pattern hair loss. J Eur Acad Dermatol Venereol. 2020;34:e40-1.
- [CrossRef] [Google Scholar]
- RF-oral minoxidil for female pattern hair loss and other alopecias. Actas Dermosifiliogr (Engl Ed). 2019;110:861-2.
- [CrossRef] [Google Scholar]
- Clinical utility and validity of minoxidil response testing in androgenetic alopecia. Dermatol Ther. 2015;28:13-6.
- [CrossRef] [Google Scholar]
- Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version. J Dermatol. 2018;45:1031-43.
- [CrossRef] [Google Scholar]
- Female pattern hair loss: A pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57:104-9.
- [CrossRef] [Google Scholar]
- Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J Am Acad Dermatol. 2019;81:648-9.
- [CrossRef] [Google Scholar]
- Case series of oral minoxidil for androgenetic and traction alopecia: Tolerability and the five C's of oral therapy. Dermatol Ther. 2018;31:e12707.
- [CrossRef] [Google Scholar]
- Efficacy and safety of oral minoxidil 5 mg daily during 24-week treatment in male androgenetic alopecia. J Am Acad Dermatol. 2015;72:AB113.
- [CrossRef] [Google Scholar]
- Oral minoxidil: A possible new therapy for androgenetic alopecia. J Cutan Med Surg. 2020;24(Suppl 3):88-9.
- [CrossRef] [Google Scholar]
- Very-low-dose oral minoxidil in male androgenetic alopecia: A study with quantitative trichoscopic documentation. J Am Acad Dermatol. 2020;82:e21-2.
- [CrossRef] [Google Scholar]
- Oral minoxidil improves background hair thickness in lichen planopilaris. J Am Acad Dermatol. 2020;84:1684-6.
- [CrossRef] [Google Scholar]
- Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss: A randomized clinical trial. J Am Acad Dermatol. 2020;82:252-3.
- [CrossRef] [Google Scholar]
- Evaluation of oral minoxidil in the treatment of alopecia areata. Arch Dermatol. 1987;123:1488-90.
- [CrossRef] [Google Scholar]
- How safe is prescribing oral minoxidil in patients allergic to topical minoxidil? J Am Acad Dermatol 2020:S0190-962230567-3.
- [CrossRef] [Google Scholar]
- Treatment of chronic telogen effluvium with oral minoxidil: A retrospective study. F1000Res. 2017;6:1650.
- [CrossRef] [Google Scholar]
- Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. Australas J Dermatol. 2016;57:e130-2.
- [CrossRef] [Google Scholar]
- Treatment of monilethrix with oral minoxidil. JAAD Case Rep. 2016;2:212-5.
- [CrossRef] [Google Scholar]
- Loose anagen hair syndrome: Treatment with systemic minoxidil characterised by marked hair colour change. Australas J Dermatol. 2018;59:e286-7.
- [CrossRef] [Google Scholar]
- Hair restoration surgery in Asians In: Imagawa K, ed. Is There a Place for Oral Minoxidil? An Overview. Tokyo: Springer; 2010. p. :73-7.
- [CrossRef] [Google Scholar]
- The pharmacokinetics of 2.5-to 10-mg oral doses of minoxidil in healthy volunteers. J Clin Pharmacol. 1989;29:162-7.
- [CrossRef] [Google Scholar]
- Fatal toxic epidermal necrolysis associated with minoxidil. Pharmacotherapy. 2009;29:460-7.
- [CrossRef] [Google Scholar]
- Disposition of minoxidil in patients with various degrees of renal function. J Clin Pharmacol. 1989;29:798-802.
- [CrossRef] [Google Scholar]
- Minoxidil-induced Stevens-Johnson syndrome. Arch Intern Med. 1981;141:1515.
- [CrossRef] [Google Scholar]
- Stevens-Johnson syndrome associated with oral minoxidil: A case report. J Nephrol. 2007;20:91-3.
- [Google Scholar]
- Medical treatments for male and female pattern hair loss. J Am Acad Dermatol. 2008;59:547-68.
- [CrossRef] [Google Scholar]
- Topical and oral minoxidil for hair disorders in pediatric patients: What do we know so far? Dermatol Ther. 2020;33:e13950.
- [CrossRef] [Google Scholar]
- Combination oral minoxidil and spironolactone for the treatment of androgenetic alopecia in adolescent girls. J Am Acad Dermatol. 2021;84:1689-91.
- [CrossRef] [Google Scholar]
- Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: An open-label and global photographic assessment. Dermatol Ther. 2020;10:1345-57.
- [CrossRef] [Google Scholar]
- Efficacy and safety of oral minoxidil in female androgenetic alopecia. Dermatol Ther. 2020;33:e14234.
- [CrossRef] [Google Scholar]
- Efficacy and safety of very-low-dose oral minoxidil 1.25 mg in male androgenetic alopecia. J Am Acad Dermatol. 2020;83:1491-3.
- [CrossRef] [Google Scholar]
- Low-dose oral minoxidil for female pattern hair loss: A unicenter descriptive study of 148 women. Skin Appendage Disord. 2020;6:175-6.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma with low dose oral minoxidil (1.25mg versus 2.5mg) along with trichoscopic pre-and post-treatment evaluation. J Cosmet Dermatol 2021
- [CrossRef] [Google Scholar]
- Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. J Am Acad Dermatol. 2021;85:743-5.
- [CrossRef] [Google Scholar]
- Low-dose oral minoxidil for treating alopecia: A 3-year North American retrospective case series. J Am Acad Dermatol. 2021;84:761-3.
- [CrossRef] [Google Scholar]
- Eyebrow regrowth in patients with frontal fibrosing alopecia treated with low-dose oral minoxidil. Skin Appendage Disord. 2021;7:112-4.
- [CrossRef] [Google Scholar]
- Adverse effects of low-dose oral minoxidil for androgenetic alopecia in 435 patients. J Am Acad Dermatol. 2021;84:1175-8.
- [CrossRef] [Google Scholar]






