Translate this page into:
Trichoscopy basics: Tinea capitis revisited!

*Corresponding author: Anshuman Dash, Department of Dermatology and Sexually Transmitted Diseases, Vardhman Mahavir Medical College and Safdarjung Hospital, Delhi, India. anshu.sipun@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dash A, Bansal S. Trichoscopy basics: Tinea capitis revisited! CosmoDerma. 2025;5:53. doi: 10.25259/CSDM_17_2025
INTRODUCTION
Dermatophytoses or cutaneous fungal infections caused by dermatophytes have multifarious clinical manifestations. India is facing an epidemic-like scenario of dermatophytosis.[1] Tinea capitis (TC), or dermatophytosis of the scalp predominantly affects children; however, there has been a growing number of reports documenting its occurrence in adults.[2] Trichoscopy has emerged as a valuable bedside tool for clinicians in diagnosing patients with hair-loss and inflammatory scalp lesions, including TC. Herein, we describe the trichoscopic findings in various subtypes of TC.
EPIDEMIOLOGY
The epidemiology of TC varies greatly in different geographical locations across the world. The predominant organisms responsible are Trichophyton and Microsporum spp. The species vary across India, with Trichophyton violaceum common in Northern states, Trichophyton mentagrophytes in hilly areas, and Trichophyton rubrum in the southern part of the country.[3-5] The most common age group affected is 3–7 years.[6]
CLINICAL PATTERNS
Immediately after the transfer of fungal spores from one infected host, the spores adhere to the hair keratinocytes strongly. Depending on the species, the hyphae produce spores on the surface of the hair cortex (ectothrix) or within the hair cortex (endothrix). The two predominant types of TC described are inflammatory and non-inflammatory. Kerion, favus, abscess, and pustular forms are the inflammatory variants. Black-dot, gray-patch, seborrheic, alopecia-areata-like, and the glabrous type (adult TC) are the non-inflammatory variants. A 10%-potassium hydroxide (KOH) wet mount from plucked hair can reveal ectothrix or endothrix. The gold standard remains fungal culture where species identification is an added advantage. The sensitivity of KOH mount has been reported to be close to 50%.[7] However, sample collection, the examiner’s expertise, and disease severity may influence the KOH mount results. Culture is not always feasible and time-consuming. Trichoscopy is an easy bedside test that can be performed in all cases of suspected TC with high sensitivity and specificity (94% and 83%, respectively).[7] Since its first use by Slowinska et al. in the description of two cases of TC caused by Microsporum canis, numerous reports and studies have been published, reinforcing its utility in the diagnosis of TC.[8] Moreover, it can also be used for monitoring patients on treatment.
TRICHOSCOPIC SIGNS IN TC
An illustration [Figure 1] depicts the various trichoscopic findings associated with TC.
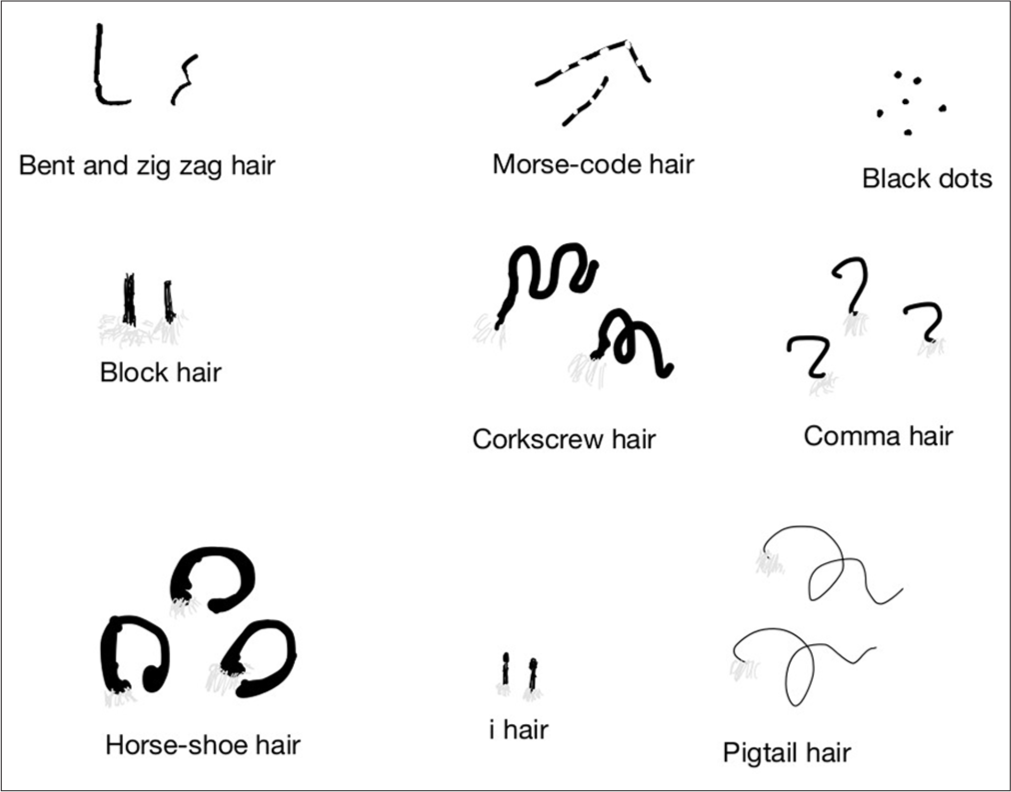
- An illustration depicting various trichoscopic signs in tinea capitis.
Comma-shaped hairs
These are short, c-shaped hairs resembling the punctuation mark, comma. Fungal hyphal invasion causes the hair shaft to weaken with subsequent bending and fracture giving rise to the characteristic comma shape.[8] It has been reported to be the most common finding in many studies and is associated with both endothrix and ectothrix infections.[9-11] It can be occasionally seen in alopecia areata and trichotillomania as well.
Corkscrew hairs
These are specific forms of comma-hair in the shape of a corkscrew, first described in African-American children.[12] Later on, these were found to be present even in white-skinned patients and Indians. It is a highly specific and sensitive marker for TC and is associated with endothrix infections more commonly than ectothrix.[8] [Figure 2].
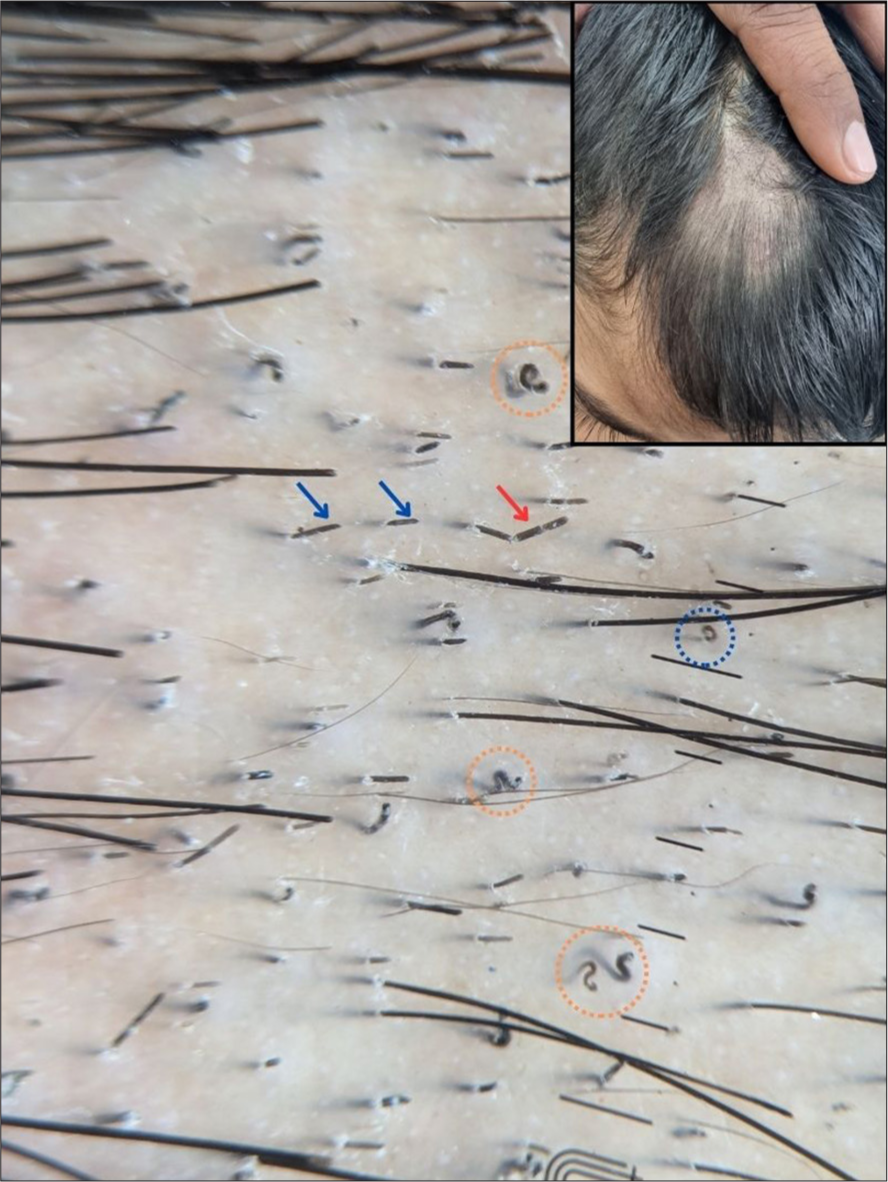
- Trichoscopy showing corkscrew hairs (orange circle), horse-shoe hair (blue circle), Morse-code hair (red arrow), and block hairs (blue arrows). The inset shows the clinical image of the same patient (×20, DermLite DL5, polarized, contact, dry).
Black dots
They represent broken or dystrophic hairs in TC that are fractured just at the level of emergence from the scalp. These are important as markers of disease activity but are non-specific, and commonly seen in alopecia areata and trichotillomania.
Morse-code-like hairs
Also known as bar-code hairs, these are represented by multiple thin white bands or sheaths across the hair shaft. They are formed due to the presence of fungal colonies causing transverse perforation of the hair shaft.[8] It is considered very specific for ectothrix infections, particularly by Microsporum spp.[13] [Figures 2 and 3].
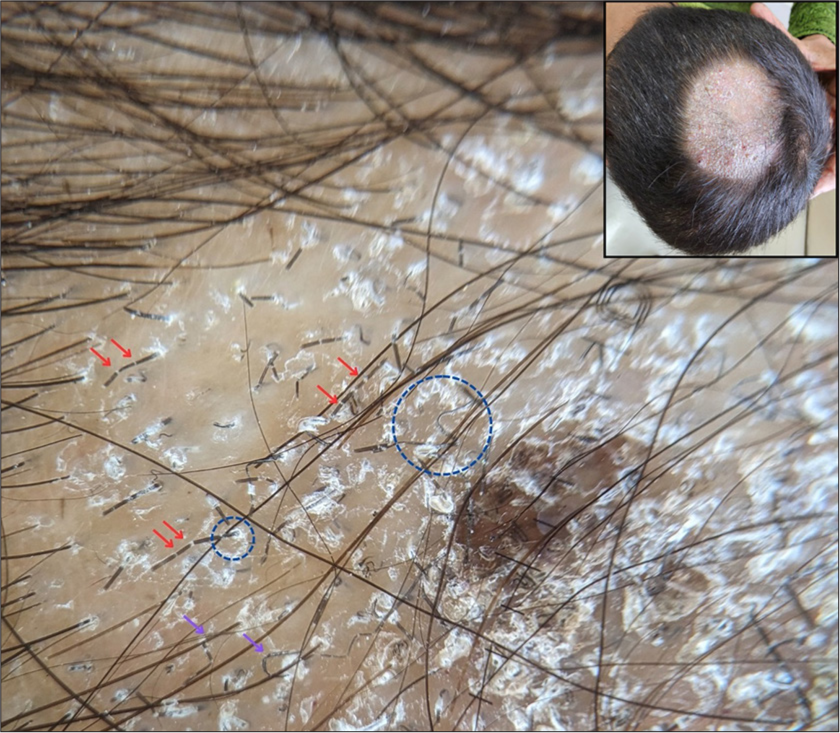
- Trichoscopy showing diffuse and perifollicular scaling (blue circle) along with Morse-code hairs (red arrows) and zigzag hairs (purple arrows). The inset shows the clinical image of the same patient (×20, DermLite DL5, polarized, contact, dry).
Zigzag hairs
Several incomplete transverse fractures across the hair shaft give rise to hairs with multiple acute angle bends forming a “Z” shape. They have been reported more commonly with ectothrix infections, although some studies from India have detected them in endothrix as well[8,14] [Figure 3].
Bent hairs
These are hairs where there is bending of the hair shaft with homogeneous pigmentation.[7] In contrast to comma hairs, the length of the hair remains normal. These are also more frequent in ectothrix.
Block hairs
These are dystrophic short hairs with a horizontal distal end seen in the endothrix[8] [Figure 2].
“i” hair
Block hairs that have a prominent distal end, resembling the English alphabet, “i” are designated as “i” hairs.[15] Apart from TC, both block and “i” hairs may be seen in alopecia areata and trichotillomania [Figure 4].
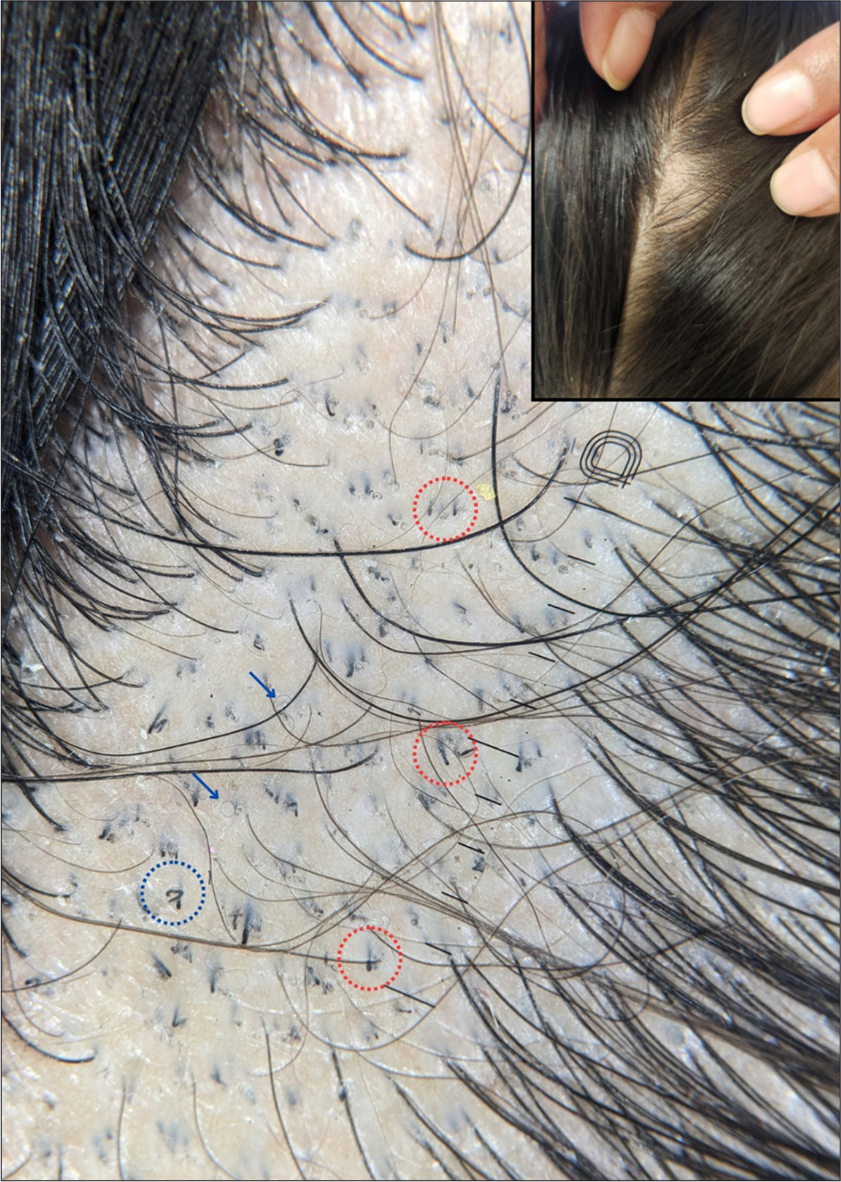
- Trichoscopy showing i-hairs (red circles), pigtail hairs (blue arrows), and horse-shoe hair (blue circle). The inset shows the clinical image of the same patient (×20, DermLite DL5, polarized, contact, dry).
Horse-shoe hairs
It has been reported as a novel finding associated with endothrix infections and is considered to be a variant of comma-hair. These are U-shaped hairs with arms of equal length resembling a horse-shoe[14] [Figures 2 and 4].
Pigtail hairs
These are short-curved vellus hairs, formed from regrowing hairs found in lower frequencies in patients with TC. These have also been reported with alopecia areata[11] [Figure 4].
Perifollicular and diffuse scaling
Common findings in TC are seen more commonly with ectothrix infections[7] [Figure 3]. Several studies have concurred that TC should not be diagnosed on the basis of a single trichoscopic finding. Brasileiro et al. observed that a combination of six trichoscopic features (broken, black-dot, comma, corkscrew, and zigzag hair in combination with perifollicular scaling) was essential to making the diagnosis.[16] Another Indian study concluded that four features (short-broken hair, black-dot, comma-hair, and perifollicular scaling) are sufficient for diagnosing with a 98.97% sensitivity.[14]
Trichoscopy helps in differentiating between the causative organisms. Microsporum spp. causing ectothrix frequently shows Morse-code such as hairs, zigzag hairs, bent hairs, and diffuse scaling, whereas corkscrew and comma-hairs are more commonly observed with endothrix infections caused by Trichophyton spp.[8,14] A recent study correlating trichoscopic findings with mycological culture reports found that features such as corkscrew and comma hairs had 94.9% specificity and 96.4% positive predictive value for Trichophyton tonsurans infection, whereas, Morse-code and zigzag hairs had 85.4% specificity and 65.3% positive predictive value for M. canis infection. The identification of the dermatophyte species is important from a therapeutic perspective.[17] Griseofulvin is recommended for Microsporum spp. while terbinafine is considered first-line for Trichophyton infections.[18] Furthermore, trichoscopy has been utilized for monitoring treatment efficacy. Dystrophic hair signs such as comma hairs, corkscrew hairs, Morse-code-like hairs, and black dots are the first to disappear within four weeks of initiating treatment. In contrast, perifollicular and diffuse scaling take longer to resolve, persisting until around 12 weeks of therapy.[19,20]
CONCLUSION
Trichoscopy is a validated and easy-to-perform bedside tool for the accurate diagnosis of TC. The knowledge of the specific trichoscopic signs helps in the initial diagnosis and timely initiation of correct treatment, often eliminating the need for direct microscopy or fungal culture.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- The unprecedented epidemic-like scenario of dermatophytosis in India: I. Epidemiology, risk factors and clinical features. Indian J Dermatol Venereol Leprol. 2021;87:154-75.
- [CrossRef] [PubMed] [Google Scholar]
- Adult tinea capitis in China: A retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-88.
- [CrossRef] [PubMed] [Google Scholar]
- Tinea capitis in the pediatric population: A study from North India. Indian J Dermatol Venereol Leprol. 2010;76:527-32.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and laboratory characteristics of a tinea capitis outbreak among novice buddhist monks. Pediatr Dermatol. 2017;34:371-73.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical study of tinea capitis in Northern Karnataka: A three-year experience at a single institute. Indian Dermatol Online J. 2013;4:22-6.
- [CrossRef] [PubMed] [Google Scholar]
- Tinea capitis in infants: Recognition, evaluation, and management suggestions. J Clin Aesthet Dermatol. 2012;5:49-59.
- [Google Scholar]
- Trichoscopy of tinea capitis: A systematic review. Dermatol Ther (Heidelb). 2020;10:43-52.
- [CrossRef] [PubMed] [Google Scholar]
- Comma hairs: A dermatoscopic marker for tinea capitis: A rapid diagnostic method. J Am Acad Dermatol. 2008;59:S77-9.
- [CrossRef] [PubMed] [Google Scholar]
- Can dermoscopy serve as a diagnostic tool in dermatophytosis? A pilot study. Indian Dermatol Online J. 2019;10:530-5.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy in Tinea capitis: A prospective study on 43 patients. Med Cutan Iber Lat Am. 2014;42:18-22.
- [Google Scholar]
- Trichoscopic and clinico-morphological evaluation of tinea capitis. Indian Dermatol Online J. 2024;15:437-42.
- [CrossRef] [PubMed] [Google Scholar]
- Corkscrew hair: A new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147:355-6.
- [CrossRef] [PubMed] [Google Scholar]
- A single typical trichoscopic feature is predictive of tinea capitis: A prospective multicentre study. Br J Dermatol. 2019;181:1046-51.
- [CrossRef] [PubMed] [Google Scholar]
- Trichoscopy as a diagnostic tool for tinea capitis: A prospective, observational study. Int J Trichology. 2020;12:68-74.
- [CrossRef] [PubMed] [Google Scholar]
- Newly described features resulting from high-magnification dermoscopy of tinea capitis. JAMA Dermatol. 2015;151:308-10.
- [CrossRef] [PubMed] [Google Scholar]
- Trichoscopy as an additional tool for the differential diagnosis of tinea capitis: A prospective clinical study. Br J Dermatol. 2016;175:208-9.
- [CrossRef] [PubMed] [Google Scholar]
- Trichoscopy patterns of tinea capitis and their correlation with mycological culture results. J Am Acad Dermatol. 2023;88:166-7.
- [CrossRef] [PubMed] [Google Scholar]
- British association of dermatologists' guidelines for the management of tinea capitis 2014. Br J Dermatol. 2014;171:454-63.
- [CrossRef] [PubMed] [Google Scholar]
- Follow-up of tinea capitis with trichoscopy: A prospective clinical study. J Eur Acad Dermatol Venereol. 2017;31:e478-80.
- [CrossRef] [Google Scholar]
- Morse code-like hairs in tinea capitis disappear after successful treatment. Int J Dermatol. 2018;57:e150-1.
- [CrossRef] [Google Scholar]






