Translate this page into:
Role of autologous platelet-rich plasma with collagen in storage of skin graft

*Corresponding author: Ravi Kumar Chittoria, Department of Plastic Surgery, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. drchittoria@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Chittoria RK, Amrutha JS, Yadav CN. Role of autologous platelet-rich plasma with collagen in storage of skin graft. CosmoDerma. 2024;4:38. doi: 10.25259/CSDM_213_2023
Abstract
Skin grafting remains the ideal method for the treatment of majority of wounds. Raw area following burns may require a lot of skin graft coverage. Grafting and regrafting may also be required subsequently. We treated a post-electric burn patient with skin grafting for the raw area over both hands and abdomen, and the excess graft was stored and reapplied. This article discusses the method of preservation of skin grafts and their usage in further grafting.
Keywords
Autologous platelet-rich plasma
Collagen
Skin graft
Storage
INTRODUCTION
Skin grafting is one of the most common procedures performed in plastic surgery. In case of burn injuries, the need for grafting in extensive areas occurs. Graft loss during the initial postoperative days is a common problem. The excess skin graft which was harvested can be stored so that if any graft loss happens, it can be used. Various methods for the storage of skin graft prevail. This is a case report demonstrating the unique way of preserving skin grafts with the help of autologous platelet-rich plasma (APRP) and collagen.
CASE REPORT
A 27-year-old male presented to the plastic surgery emergency department with postburn raw area over both hands and abdomen with no known comorbidities. He had sustained electric burns with second degree burns over both upper limb, abdomen, chest, and both lower limbs.
Serial debridement was done. Once wound bed preparation done [Figure 1], it is planned for skin grafting. Graft harvested from right thigh and kept over the raw area on both hand and lower abdomen [Figure 2]. Patient’s blood was drawn, and APRP was prepared. The steps in APRP preparation are as follows:
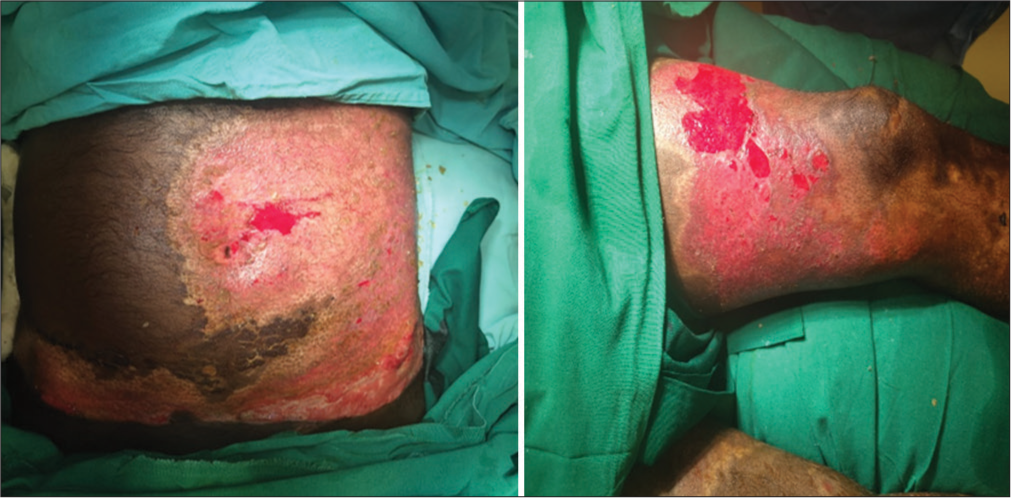
- Wound bed after preparation.

- Split skin graft applied over raw area over left thigh.
Step 1: A 10 mL of the patient’s venous blood was taken and heparinized
Step 2: Centrifugation at 3000 RPM for 10 min
Centrifugation of the blood sample will separate it to three distinct portions in the bottle. The upper portion contains plasma and platelets. The middle portion is buffy coat with white blood cell (WBC) and some platelets, and the lower portion contains red blood cell (RBC).
Step 3: The upper layer of the three layers is taken using a sterile needle and syringe
Step 4: Re-centrifugation at 4000 RPM for 10 min.
At the end of 10 min, the content is separated into two layers. The bottom layer is the plasma rich in platelets and was aspirated using a sterile needle and syringe.
Excess graft harvested was taken. The APRP was sprayed over the dermal side, and a dry collagen sheet was applied over it. These grafts were wrapped in saline-soaked gauze [Figure 3] and kept in a sterile container. It is stored in the refrigerator at 4°C.
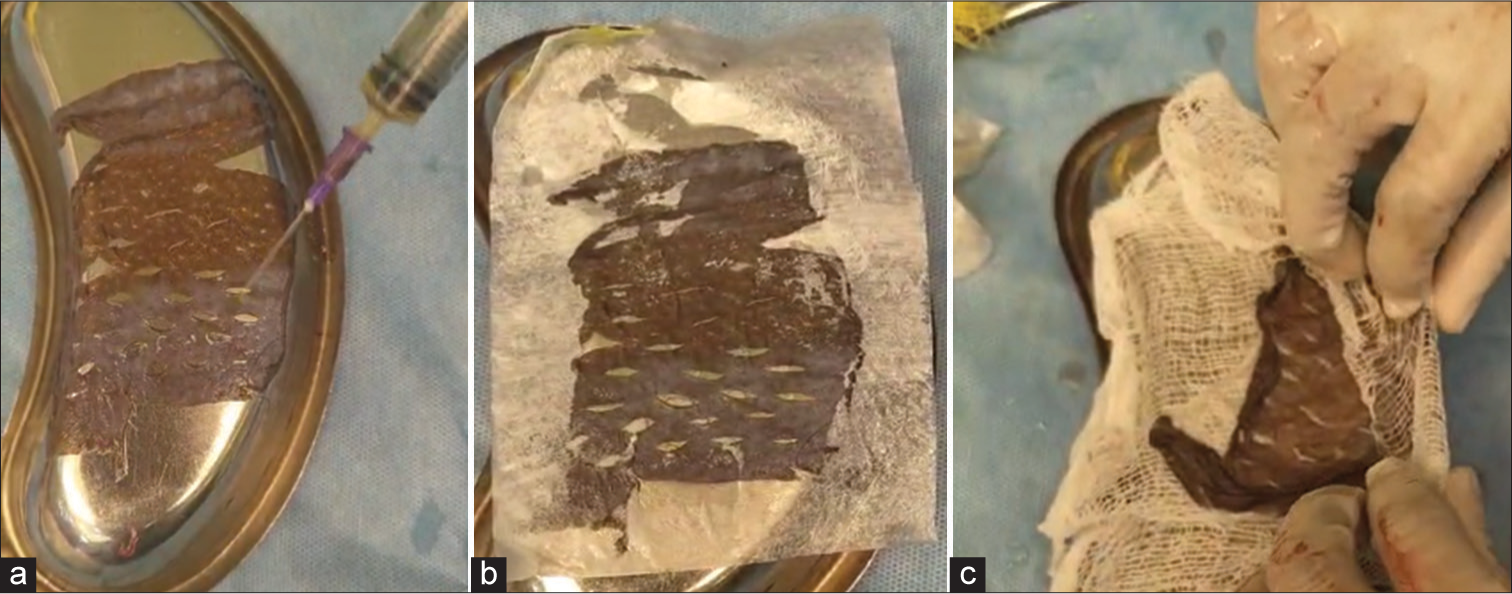
- Steps in graft storage - (a) autologous platelet-rich plasma application with (b) collagen sheet placement and (c) wrapping of graft in saline soaked gauze.
The graft site opened on post-operative day five, and graft loss areas were assessed [Figure 4]. The stored grafts were taken out and applied over the graft-lost areas [Figure 5].
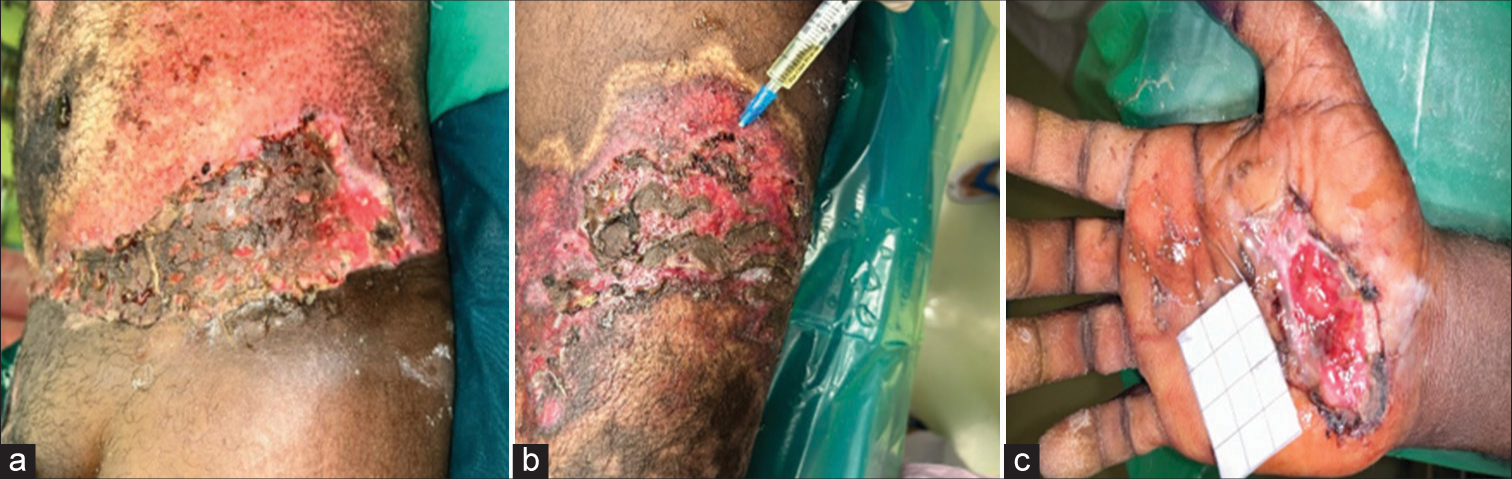
- Areas of graft loss in (a) abdomen, (b) left thigh, and (c) right hand during first dressing.

- Stored graft applied.
Skin grafting of the recipient site is done with the graft taken from the right thigh. The extra skin graft, which is available, needs to be stored in a physiological medium for the survival of the cells. Autologous platelet-rich plasma ensures nourishment and improved quality and survival of the skin graft. In our case, after 10 days, we used the stored skin graft for regrafting the graft loss on the recipient area. Regrafted skin take was good on post-operative day five. No complication was noted with this procedure.
DISCUSSION
Skin grafts are delicate tissues that require careful storage to maintain their viability and increase the chances of successful grafting. The choice of storage method depends on factors such as the type of graft, duration of storage, and available resources. Here are some common skin graft storage methods:
Refrigeration
Temperature: Skin grafts are stored in a refrigerator at temperatures between 2°C and 8°C (36–46°F).
Duration: Refrigeration is suitable for short-term storage, typically up to five days.
Method: The graft is usually placed in a sterile container or wrapped in sterile gauze soaked in a preservative solution before refrigeration.
Freezing (Cryopreservation)
Temperature: Grafts are frozen at temperatures below −70°C (−94°F).
Duration: Cryopreservation allows for extended storage periods, potentially months or even years.
Method: Skin grafts are carefully prepared, often using cryoprotective solutions to protect cells during freezing. Specialized equipment is used for controlled freezing and thawing. The most common technique of cryopreservation involves the use of glycerol as a cryoprotectant medium along with antimicrobial agents and storage at a temperature of −80°C.[1]
Dermatome humidification
Use: This method is typically used for grafts obtained using a dermatome (a surgical instrument for skin grafting).
Method: The graft is placed in a humidified environment using saline-soaked gauze or sterile moistened sponges until it is ready for grafting.
Skin bank
Use: Some medical facilities maintain skin banks where donated cadaveric skin is processed, preserved, and made available for grafting.
Method: Skin banks use specialized methods and equipment for long-term storage and preservation including cryopreservation techniques. Glycerolization or cryopreservation methods for the long-term storage of allografts are employed in tissue banks.[2]
Amniotic membrane
Use: Amniotic membrane grafts, derived from placental tissue, are used in certain wound care applications.
Method: These grafts are often cryopreserved at very low temperatures until needed for grafting.
Epidermal suspension
Use: Epidermal suspension grafts, which consist mainly of epidermal cells, can be stored as a suspension.
Method: The epidermal cells are suspended in a suitable medium and stored under controlled conditions.
Regardless of the storage method used, maintaining sterility throughout the process is crucial to prevent contamination. In addition, proper documentation of graft preparation, storage conditions, and handling procedures is essential for quality control and traceability.
The ideal way of preservation is one that is easy to carry out, safe for tissue, and does not cause any alteration in the biological properties of skin.[3] Roswell Park Memorial Institute 1640 solution was reported superior to other media including Eagle’s minimal essential medium, Euro-Collins preservation fluid, University of Wisconsin solution, Histidine tryptophan ketoglutarate solution, and saline.[4,5]
Storing graft in physiological saline-soaked gauze is widely used, as it is inexpensive as well as simple. However, it is inferior to other methods. Sommeling et al.[6] initially proposed using human plasma to store skin grafts. It is a natural source of growth factors. In platelets, cytokines and growth factors are stored in a-granules in their incomplete form. Of these, the most important is platelet-derived growth factor, transforming growth factor-beta 1, vascular endothelial growth factor, and epidermal growth factor.[7] Platelet-rich plasma plays a critical role in the repair and regeneration of different tissues through the activation and secretion of these growth factors and other cytokines stored in the alpha-granules of platelets. Platelet-rich plasma is believed to be a useful storage medium due to the increased concentration of essential cytokines and growth factors released by activated platelets. Steps of autologous platelet-rich plasma preparation as described by Li et al.[8,9] are as follows:
Step 1: A 10 mL of the patient’s venous blood was taken and heparinized.
Step 2: Centrifugation at 3000 RPM for 10 min.
After centrifugation, it is separated into three layers. The upper portion contains plasma and platelets. The middle portion is buffy coat with WBC and some platelets, and the lower portion contains RBC.
Step 3: The upper layer of the three layers is taken using a sterile needle and syringe.
Step 4: Re-centrifugation at 4000 RPM for 10 min.
At the end of 10 min, the content is separated into two layers. The upper two-third is platelet-poor plasma and lower one-third is platelet-rich plasma+ RBC-platelet clump.
The bottom layer is the plasma rich in platelets and was aspirated using a sterile needle and syringe.
Platelets remain active for five to seven days at room temperature and up to 10 days at 0–6°C.[2,10]
The limitation of our study is that a histopathological examination was not done, and in subsequent studies, we plan to do a histopathological examination also.
CONCLUSION
In conclusion, graft preservation using APRP and collagen can be an excellent method for the preservation of skin grafts. This is economical and can provide near physiological conditions for better survival of graft cells. This modification can prolong the graft quality and better graft take.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Skin graft storage and keratinocyte viability. Br J Plast Surg. 1993;46:292-5.
- [CrossRef] [PubMed] [Google Scholar]
- Toward a definition of “fresh” whole blood: An in vitro characterization of coagulation properties in refrigerated whole blood for transfusion: Clotting properties in fresh whole blood. Transfusion. 2011;51:43-51.
- [CrossRef] [PubMed] [Google Scholar]
- Current trends in the use of allograft skin for patients with burn and reflections on the future of skin banking in the United States. J Burn Care Rehabil. 1994;15:428-31.
- [CrossRef] [PubMed] [Google Scholar]
- The effects of saline and plasma on skin graft keratinocyte viability. Br J Plast Surg. 2000;53:418-9.
- [CrossRef] [PubMed] [Google Scholar]
- The use of platelet-rich plasma in plastic surgery: A systematic review. J Plast Reconstr Aesthet Surg. 2013;66:301-11.
- [CrossRef] [PubMed] [Google Scholar]
- Protocol for obtaining PRP for autologous use. Aesthetic Plast Surg. 2012;36:1254-9.
- [CrossRef] [PubMed] [Google Scholar]
- Subcutaneous injections of platelet-rich plasma. Plast Reconstr Surg. 2012;129:858-6.
- [CrossRef] [PubMed] [Google Scholar]
- The Israel National Skin Bank: Quality assurance and graft performance of stored tissues. Cell Tissue Bank. 2000;1:303-12.
- [CrossRef] [PubMed] [Google Scholar]
- Refrigerated platelets for the treatment of acute bleeding: A review of the literature and re-examination of current standards. Shock. 2015;44:616-7.
- [CrossRef] [PubMed] [Google Scholar]






