Translate this page into:
Relapse of post-kala-azar dermal leishmaniasis – An emerging concern in the endemic area

*Corresponding author: Debopriya Paul, Department of Dermatology and STD, All India Institute of Medical Sciences, Patna, Bihar, India. debopriyapaul11@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh S, Paul D, Kumari P. Relapse of post-kala-azar dermal leishmaniasis – An emerging concern in the endemic area. CosmoDerma. 2024;4:134. doi: 10.25259/CSDM_142_2024
Dear Sir,
Post-kala-azar dermal leishmaniasis (PKDL) is a complication that can occur post-resolution of visceral leishmaniasis (VL), which is caused by Leishmania donovani, often serving as a reservoir for the spread of infection. The incidence of PKDL varies in Sudan (60%) and the Indian subcontinent (5–10%), with Bihar being highly endemic. In India, it usually occurs within 2–7 years after VL, while in Sudan, it can occur concomitantly or within 3–6 months. The interval between VL and PKDL is often influenced by prior treatment. It may present in 10–23% of cases without prior VL.[1] We present here an interesting case of relapse of PKDL in a patient who earlier completed treatment with complete resolution.
A 57-year-old male from Bihar was diagnosed with VL based on a prolonged episode of fever, hepatosplenomegaly, and a positive serum rK-39 rapid immunochromatographic test (ICT). He was treated with sodium stibogluconate for 3 weeks in 2004. In 2006, he noticed asymptomatic hypopigmented patches on his trunk and extremities. PKDL diagnosis was made and treated again with sodium stibogluconate (20/kg/day) for 2 months, resulting in complete resolution of the skin lesions. Five years later, he developed new hypopigmented, asymptomatic macules over his trunk and extremities. The swelling with occasional pain over hands and reddish nasal swelling with erosion at the angle of the mouth for the past 5 and 2 months, respectively.
On examination, a papulonodular lesion on the nasal septum, multiple normoanesthetic hypopigmented macules over the trunk and limbs, and nodular lesions with erosion and crusting over the fingers were noted [Figure 1]. Relevant investigations showed positive rK-39 ICT and Leishman–Donovan (LD) bodies on slit-skin smear [Figure 2], while other hematological tests were normal and viral markers were negative. The final diagnosis of relapse of PKDL was made and treated with miltefosine (50 mg, twice daily for 90 days), with complete resolution in the nodular lesions [Figure 3] but persistent hypopigmented patches over the thighs [Figure 4] at 12 months of follow-up, suggesting no complete clinical cure but parasitological cure which was confirmed by negative slit-skin smear for LD bodies.
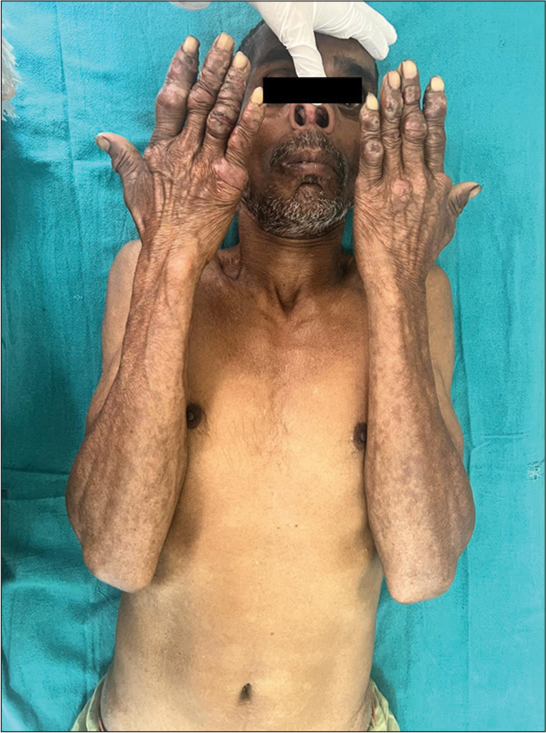
- Firm nodular lesions featuring erosions and crusting on fingers, a papule on the columella, and hypopigmented macules.
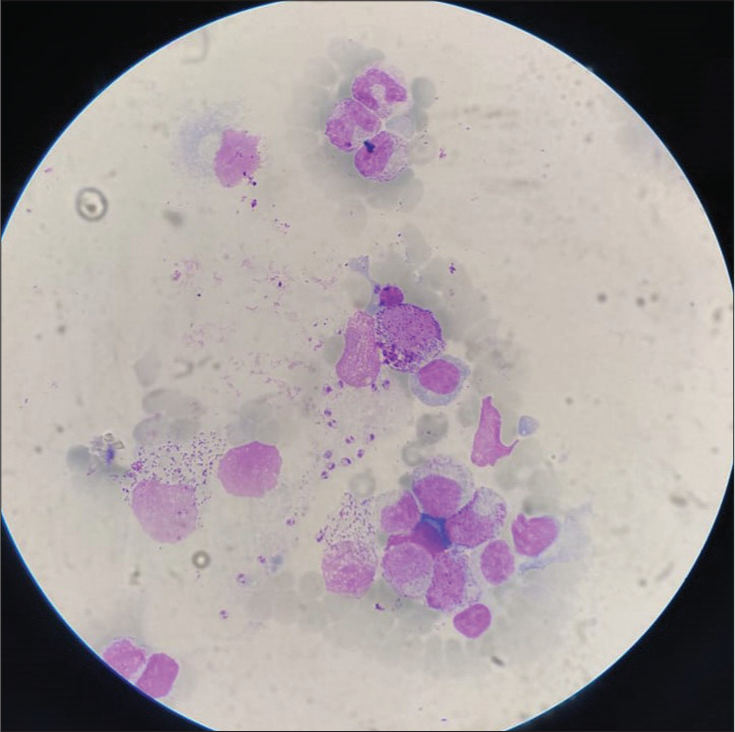
- Leishman-Donovan bodies on Giemsa’s stain following slit-skin smear. Stain used: Giemsa; Magnification: ×4.
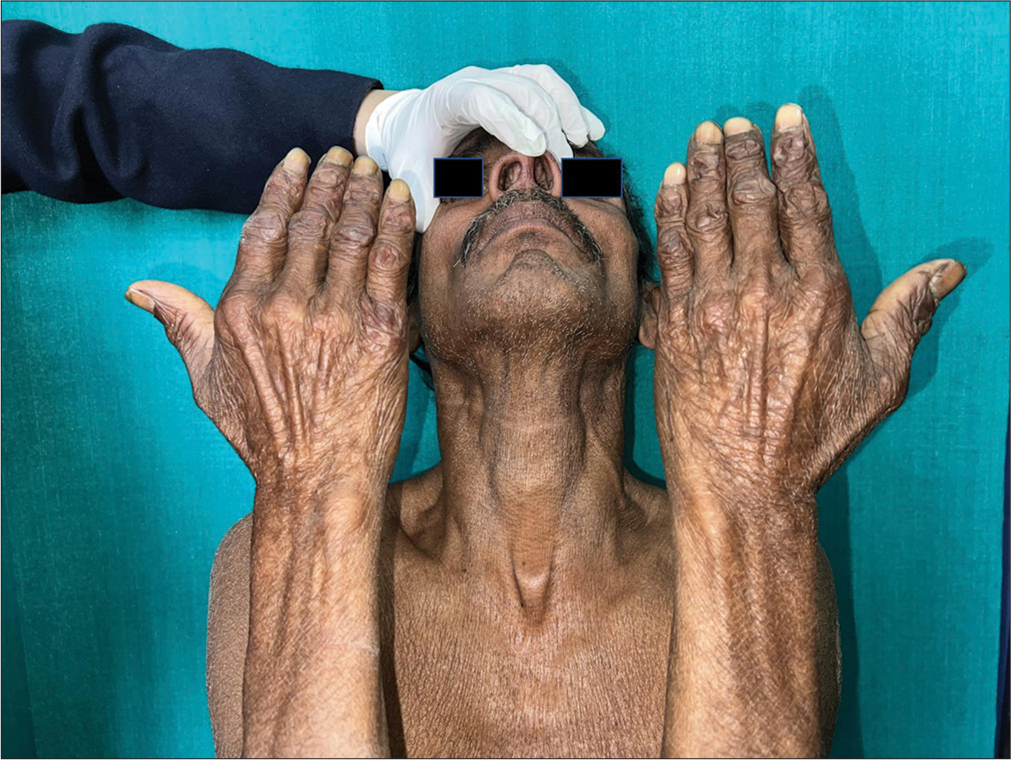
- Resolution of nodular lesions observed at 12 months after miltefosine therapy in a relapse case of post-kala-azar dermal leishmaniasis post-sodium stibogluconate therapy.
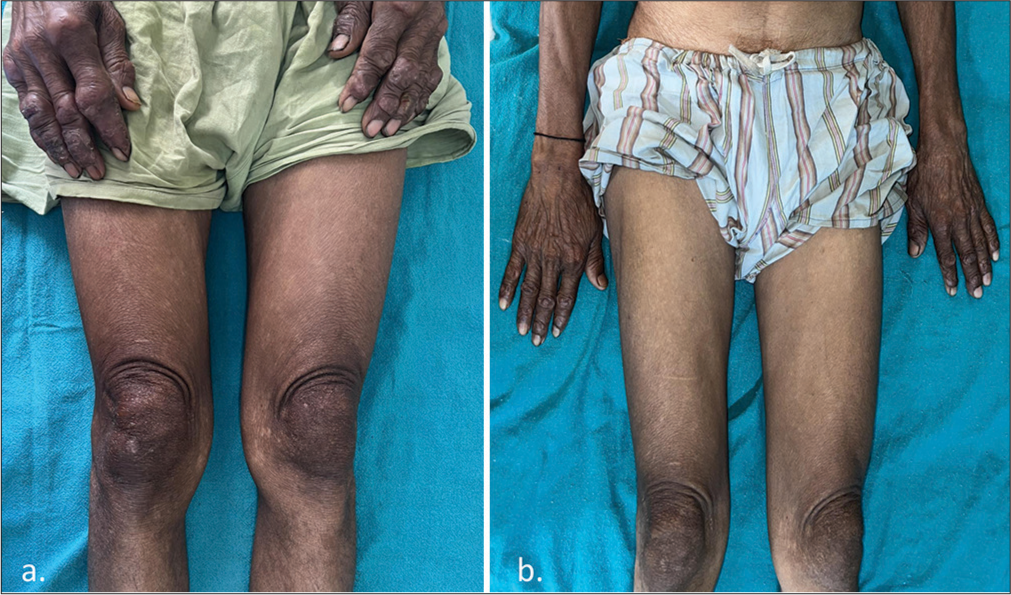
- (a) Baseline picture showing nodular lesions over the hands and hypopigmented macules over the thighs. (b) Complete resolution of nodular lesions with partial improvement in the hypopigmented macules over the thigh.
PKDL may have a polymorphic presentation, posing a diagnostic challenge. Common cutaneous manifestations include hypopigmented macules, erythematous papules/plaques, and papulonodular lesions associated with photosensitivity. Less frequent manifestations are ulceration, butterfly rash, micropapular, erythrodermic, annular, keloidal, xanthomatous, papillomatous, verrucous, and hypertrophic lesions. Asymptomatic macular lesions usually precede plaques/nodules, although exceptions may exist. Succulent/juicy plaques and nodules may develop de novo, primarily on the face, joints, and extremities. Severe cases may involve mucosal and upper respiratory airway lesions.[1] The rK-39 antigen positivity and demonstration of LD bodies in slit-skin smears can confirm the diagnosis; polymerase chain reaction on skin and blood samples can verify the diagnosis in cases of negative slit-skin smears.[2]
PKDL has been reported in the post-treated cases of VL with sodium antimony/stibogluconate (SAG/SSG), amphotericin B, miltefosine, or combination with amphotericin B probably because of a shorter duration of therapy or lower dosage which usually treats VL but not the dermal leishmaniasis.[2] In a study by Younis et al., 7 patients of PKDL who were treated more than 1 year prior had relapsed and treated again with combination therapy.[3] Based on our literature review [Table 1], SAG monotherapy was associated more with relapse as compared to other drug regimens, which correlated to our case also who was previously treated with SSG. According to the World Health Organization recommendation, 12 weeks of miltefosine is used to treat PKDL. However, studies have reported a 15–20% relapse rate at 12–18 months post-therapy [Table 1] and resistance to miltefosine.[1,2] In our case, the patient had reoccurrence to PKDL post-stibogluconate therapy, later showed complete resolution of nodular lesions with miltefosine therapy and partial resolution of hypopigmented macules at 1 year of follow-up. As there was a parasitological cure (confirmed by negative slit-skin smear for LD bodies) and no new lesions or progression of the existing lesions, the patient was kept on regular follow-up (every 6 months). If skin lesions recurred at any time, rescue therapy was planned, i.e., liposomal amphotericin B (2.5 mg/kg for 20 days) alone or in combination with immunotherapy based on the evidence from literature [Table 1].
| Study | Type of study | Sample size | Drug regimen | Follow-up (in days) post treatment) | Relapse |
|---|---|---|---|---|---|
| Younis et al. 2023[3] | RCT | 55 in each | Group A: Paromomycin (20 mg/kg for 14 days) plus Miltefosine at allometric dose for 42 days Group B: Liposomal amphotericin B - 20 mg/kg on days 1, 3, 5, and 7 plus Miltefosine at allometric dose for 28 days |
365 | Group A: 1/55 (1.8%) Group B: 11/55 (20%) |
| Pandey et al. 2021[4] | RCT | 50 in each | Group A: Liposomal amphotericin B- 5 mg/kg biweekly for 3 weeks Group B: Miltefosine-2.5 mg/kg/day for 12 weeks |
365 days | Group A: 12/47 (25.5%) Group B: 6/46 (13.0%) |
| Rabi Das et al. 2017[5] | RCT | 25 in each | Group A: Amphotericin B - 0.5 mg/kg/day for 20 infusions and total 3 cycles at 15-day interval. Group B: Amphotericin B -1 mg/kg alternate day for 20 infusions and total 3 cycles at 15-day interval. |
365 days | Group A: 1/23 (4.3%) Group B: 1/22 (4.5%) |
| Sundar et al. 2013[6] | RCT | 18 in each | Group A: Miltefosine -2.5 mg/kg/day for 12 weeks Group B: Miltefosine -2.5 mg/kg/day for 8 weeks |
365 days | Group A: 1/15 (6.6%) Group B: 3/16 (18.8%) |
| Musa et al. 2008[7] | RCT | 15 in each | Group A: Four weekly doses of 100 g Alum/ALM+BCG (one-tenth of the 0.1 mL dose used for TB vaccination), plus SSG (20 mg/kg) daily for 40 days. Group B: SSG (20 mg/kg) daily for 40 days plus placebo |
90 days | Group A: No relapse Group B: 2/15 (13.3%) |
| Thakur et al. 1997[8] | RCT | 11 in each | Group A: Amphotericin B deoxycholate - 1 mg/kg/day for 20 day and total 3 cycles at 20-day interval. Group B: Sodium antimony gluconate - 20 mg/kg/day for 20 days and repeated after every 20 days for 6 times or till resolution of symptoms. |
365 days | Group A: No relapse Group B: 4/11 (36.4%) |
RCT: Randomized controlled trial, SSG: Sodium stibogluconate, BCG: Bacillus Calmette-Guerin, TB: Tuberculosis, PDKL: Post-kala-azar dermal leishmaniasis
Therefore, in endemic areas like the eastern part of India, it is necessary to establish or define long-term follow-up post-therapy to detect all the relapse cases of PKDL. It is also high time to consider combination therapy such as paromomycin plus miltefosine or liposomal amphotericin B plus miltefosine along with immunotherapy (alum-precipitated autoclaved Leishmania major plus Bacille Calmette-Guerin) to improve the cure rate, reduce cases of relapse, and prevent the development of drug resistance.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Post Kala-Azar dermal leishmaniasis: Clinical features and differential diagnosis. Indian J Dermatol. 2021;66:24-33.
- [CrossRef] [PubMed] [Google Scholar]
- Monitoring the long-term effectiveness of miltefosine in Indian post-Kala-Azar dermal leishmaniasis. Am J Trop Med Hyg. 2024;110:656-62.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of paromomycin/miltefosine/liposomal amphotericin B combinations for the treatment of post-Kala-Azar dermal leishmaniasis in Sudan: A phase II, open label, randomized, parallel arm study. PLoS Negl Trop Dis. 2023;17:e0011780.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, open-label study to evaluate the efficacy and safety of liposomal amphotericin B (AmBisome) versus miltefosine in patients with post-Kala-Azar dermal leishmaniasis. Indian J Dermatol Venereol Leprol. 2021;87:34-41.
- [CrossRef] [PubMed] [Google Scholar]
- To evaluate efficacy and safety of amphotericin B in two different doses in the treatment of post Kala-Azar dermal leishmaniasis (PKDL) PLoS One. 2017;12:e0174497.
- [CrossRef] [PubMed] [Google Scholar]
- Oral miltefosine for Indian post-Kala-Azar dermal leishmaniasis: A randomised trial. Trop Med Int Health. 2013;18:96-100.
- [CrossRef] [PubMed] [Google Scholar]
- Immunochemotherapy of persistent post-Kala-Azar dermal leishmaniasis: A novel approach to treatment. Trans R Soc Trop Med Hyg. 2008;102:58-63.
- [CrossRef] [PubMed] [Google Scholar]
- Amphotericin B is superior to sodium antimony gluconate in the treatment of Indian post-Kala-Azar dermal leishmaniasis. Ann Trop Med Parasitol. 1997;91:611-6.
- [CrossRef] [PubMed] [Google Scholar]





