Translate this page into:
Platelet-rich plasma in interventional dermatology and trichology: How far have we come?

*Corresponding author: Suruchi Garg, Director and Chief Consultant, Aura Skin Institute, Chandigarh-160009, Punjab, India gargsuruchi01@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Garg S, Bansal A. Platelet-rich plasma in interventional dermatology and trichology: How far have we come? Cosmoderma 2021;1:12.
Abstract
Platelet-rich plasma (PRP) contains several growth factors and cellular adhesion molecules which promote wound healing, angiogenesis and accelerate the rejuvenation of skin and hair follicles. With its proven regenerative and regrowth potential in a plethora of conditions, PRP has been deemed as the “futuristic elixir.” Current evidence suggests that PRP effectively stimulates angiogenesis, collagen as well as elastin regeneration, and is a safe, easy to prepare, minimally invasive technique with limited downtime, and negligible risk of allergic/hypersensitivity reactions owing to its autologous nature. It has shown excellent results when utilized as monotherapy or in combination with microneedling or ablative lasers in acne scars, post-burn or post-traumatic scars, melasma, striae distensae, chronic ulcers, and lichen sclerosus. PRP injections or PRP combined with microneedling are increasingly being utilized for skin rejuvenation and recently have been utilized to provide non-invasive face lifts. A novel technique combining non-cultured epidermal cell suspension suspended in PRP results in superior repigmentation outcomes in case of vitiligo. Use of PRP alone or in combination with hair transplant in androgenetic alopecia is another well-researched indication and its use has been successfully extrapolated to indications such as alopecia areata, chronic telogen effluvium, and cicatricial alopecia.
In spite of its established efficacy in such a vast number of indications, PRP should be used with utmost caution. These growth mediators exert their own endocrine, paracrine, and enzymatic effects, the complete influence of which still remains a mystery and only years of experience, in the times to come will unravel the absolute power of our “mighty dragon warrior.”
Keywords
Platelet rich plasma
Interventional dermatology
Trichology
New indications
Elixir
INTRODUCTION
Platelet-rich plasma (PRP) also known as autologous platelet gel, plasma-rich growth factors (GFs), or platelet-concentrated plasma is an autologous concentration of platelets present in a small volume of plasma. It contains several GFs and cellular adhesion molecules which promote wound healing, angiogenesis, and accelerate the rejuvenation of skin and hair follicles (HFs).[1,2] The unearthing of the role of PRP in wound healing and repair paved the way for its utilization in various fields of medicine and surgery including dentistry, maxillofacial surgery, orthopedic trauma and sports injuries, cardiovascular, gastrointestinal, and plastic surgery. PRP has now become an indispensable tool in cutaneous and aesthetic surgery [Box 1].[2,3] This article hopes to shed light on the rewarding journey of PRP and discuss the indications, techniques, modifications of PRP in interventional dermatology and trichology.
| Advantages of PRP in cutaneous and aesthetic surgery[4] |
|---|
| • Tissue regeneration and rejuvenation • Induction of cell differentiation • Extracellular matrix formation • Recruitment of other cells to the site of injury • Increase in collagen production • Increase skin thickness and overall skin health • Nonallergenic, autologous physiological product • Eliminates donor transmissible infections • Biological glue for tissue adhesion, especially in skin flaps, grafts |
The rationale behind PRP – Understanding how it works
Ideal platelet concentration
PRP is obtained from the centrifugation of the patient’s own blood to yield a therapeutically effective concentration of platelets in a small volume of plasma.[2,3] Normal platelet counts in blood range between 1,50,000/µl and 3,50,000/µl. Enhancement of wound repair and regeneration has been demonstrated with PRP containing 1,000,000 platelets/µl, in a 5-mL volume of plasma.[5] However, the optimal platelet concentration for the induction of angiogenesis in human endothelial cells is 1.5 million platelets per microliter which is equivalent to 5–10 times the mean platelet levels, and concentrations higher or lower than this are considered detrimental to the process of vascular remodeling.[6] Several terms although incorrect, have been variously used in literature to describe PRP [Table 1].[6]
| Terminology | Differences from PRP |
|---|---|
| Fibrin glue | PRP, however, contains only the same concentrations of these cell adhesion molecules as does a normal blood clot (200 µg 400 µg/mL). Therefore, PRP is not a fibrin glue. |
| Platelet concentrate | A platelet concentrate is a solid composition of platelets without plasma, which would therefore not clot. |
| Platelet gel | PRP is nothing more than a human blood clot with increased platelet numbers. The clot by virtue of its cell adhesion molecules has additional biologic activity, whereas a gel does not. |
Abbreviation: PRP – platelet rich plasma
Activation of PRP
Contact with coagulation triggers leads to platelet activation, degranulation of their alpha granules, and active secretion of several pre-formed GFs including platelet derived growth factor (PDGF), transforming growth factor-beta (TGF-β ), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), hepatocyte growth factor (HGF) and fibroblast growth factor (FGF).[2,3] The release of GFs occurs within 10 minutes of platelet activation and more than 95% of the secretion is completed within 1 hour, thus warranting the application of PRP within 10 minutes of clot initiation.[7,8]
Release of growth factors
The secreted GFs bind to their particular transmembrane receptors present over adult mesenchymal stem cells, osteoblasts, fibroblasts, endothelial cells and epidermal cells. This initiates several downstream signal-transduction pathways responsible for cell proliferation, formation of matrix components as well as osteoid and collagen synthesis, thereby enhancing the natural wound-healing and regeneration process [Figure 1]. PRP is also rich in fibrin, fibronectin and vitronectin, which are cell adhesion molecules required for cell migration and provide a supporting framework for the bone, connective tissue, and epithelial migration.[9,10]

- Mechanism of action of PRP.
-
Abbreviations: PRP – platelet-rich plasma; PDGF – platelet derived growth factor; TGF-β and TGF-β2 – transforming growth factor-beta; VEGF – vascular endothelial growth factor; EGF – epidermal growth factor; HGF – hepatocyte growth factor; FGF – fibroblast growth factor.
Classification of PRP
Based on the cell composition (such as leukocytes) and the fibrin architecture, PRP has been classified into four categories by Dohan Ehrenfest et al. [Table 2].[11,12]
| Type of PRP | Preparation | Components |
|---|---|---|
| P-PRP (pure platelet-rich plasma) | After the first slow spin centrifugation, only the superficial buffy coat layer (containing platelets) is pipetted out and prepared for next centrifugation | Leucocyte poor and have a low-density fibrin network on activation |
| L-PRP (leucocyte- and platelet-rich plasma) | After the first slow spin centrifugation, the entire buffy coat layer along with residual RBCs is pipetted out and prepared for next centrifugation | L-PRP consists of most of the platelets, along with leucocytes and some residual RBCs, suspended in fibrin-rich plasma |
| P-PRF (pure platelet-rich fibrin) or P-PRF matrix | When P-PRP is mixed with activator and allowed to incubate for some time, a stable PRFM clot is formed. P-PRP is mixed with an activator and a specific separator gel is used |
Leucocyte poor and with a fibrin network which is high-density |
| L-PRF (leucocyte- and platelet-rich fibrin) | Blood is collected without any anticoagulant and immediately centrifuged. A natural coagulation process then occurs and three layers are formed no biochemical modification of the blood, i.e., no anticoagulants, thrombin or CaCl2are required |
With leucocytes and with a fibrin network which is high-density RBC base layer, acellular plasma top layer and L-PRF clot in the middle, which harvests platelet and leucocyte growth factors into the fibrin matrix When pressed between two gauzes, the PRF clot becomes a strong membrane |
Preparation of PRP
PRP is obtained from the patients’ own blood and a 30 mL venous blood sample will yield 3–5 mL of PRP depending on the baseline platelet count, device used, and the technique applied. The blood is collected in an anticoagulant containing vial to prevent platelet activation prior to its use.
The initial patient assessment has been outlined in [Box 2]. The single spin technique utilizes only one centrifugation step and volume immediately above the erythrocyte layer is collected. It is a faster method with reduced decay of GFs due to minimal damage to platelet architecture, lower volume of required blood, and a platelet yield similar to the double spin technique (DST).[12–14]
| Initial patient assessment[3,4,15] |
|---|
|
Pre-operative checklist • A proper informed consent • History regarding platelet dysfunction, bleeding issues, recent illnesses, and localized infections • Ensure aseptic conditions • Local anesthesia – injectable or topical • Patient must be free of (for at least two weeks prior to the procedure) ■ Anti-platelet drugs like aspirin or ■ Other non-steroidal anti-inflammatory drugs • Avoid smoking and alcohol intake as they may diminish stem cell release |
|
Absolute contraindications • Platelet dysfunction syndrome • Critical thrombocytopenia • Hemodynamic instability • Septicemia • Local infection at the site • Anticoagulation therapy • Hypofibrogenemia • Chronic liver disease |
|
Relative contraindications • Consistent use of NSAIDs within 48 hours of procedure • Corticosteroid injection at treatment site within 1 month • Systemic use of corticosteroids within 2 weeks • Use of tobacco • Recent fever/illness • Cancer-especially hematopoietic or bone • Hemoglobin < 10 g/dL • Platelet count <105/μL. • Active autoimmune conditions not associated with thrombocytopenia |
In the DST, PRP is generally prepared using the PRP method or the buffy-coat method [Box 3].[12] PRP can be activated by the addition of calcium gluconate, 10% calcium chloride, and/ or thrombin. Nonactivated PRP utilizes host dermal collagen and thrombin as endogenous activators. The concentrated platelets remain viable for up to 8 hours.[12]
| PRP method[12] |
| 1. Obtain whole blood by venepuncture in acid citrate dextrose (ACD) tubes 2. Centrifuge the blood using a “soft” spin. 3. Transfer the supernatant plasma containing platelets into another sterile tube (without anticoagulant). 4. Centrifuge tube at a higher speed (a hard spin) to obtain a platelet concentrate. • The lower 1/3rd is platelet-rich plasma (PRP) • Upper 2/3rd is platelet-poor plasma (PPP). • At the bottom of the tube, platelet pellets are formed 5. Remove PPP and suspend the platelet pellets in a minimum quantity of plasma (2–4 mL) by gently shaking the tube. |
| Buffy coat method[12] |
| 1. Whole blood should be stored at 20–24°C before centrifugation. 2. Centrifuge whole blood at a “high” speed. 3. Three layers are formed because of its density: • Bottom layer consisting of RBCs • Middle layer consisting of platelets and WBCs • Top PPP layer. 4. Remove supernatant plasma from the top of the container. 5. Transfer the buffy-coat layer to another sterile tube. 6. Centrifuge at low speed to separate WBCs or use leucocyte filtration filter. |
Various commercially available PRP systems are now being employed which enable the preparation of ready to use platelet-rich suspensions.[12] The authors employ a commercially available PRP kit (Renew Cell Kit) and REMI cold centrifugation machine. Two hourglass vials [Figure 2] each containing 13.5 mL of whole blood mixed with 1.5 mL of acid citrate dextrose-A (ACD-A) solution are used and centrifuged for 4 min at 3,200 rpm (revolutions per minute) and 1,320 residual centrifugal force (rcf). The hourglass configuration facilitates the separation of highest density component of blood, i.e., RBCs. Out of these two vials, a total of 5–6 mL of buffy coat along with PRP is harvested and injected as per requirement. It gives 3–5 times the concentration of the baseline platelet count, equivalent to 8–15 lakh platelets per microliter.
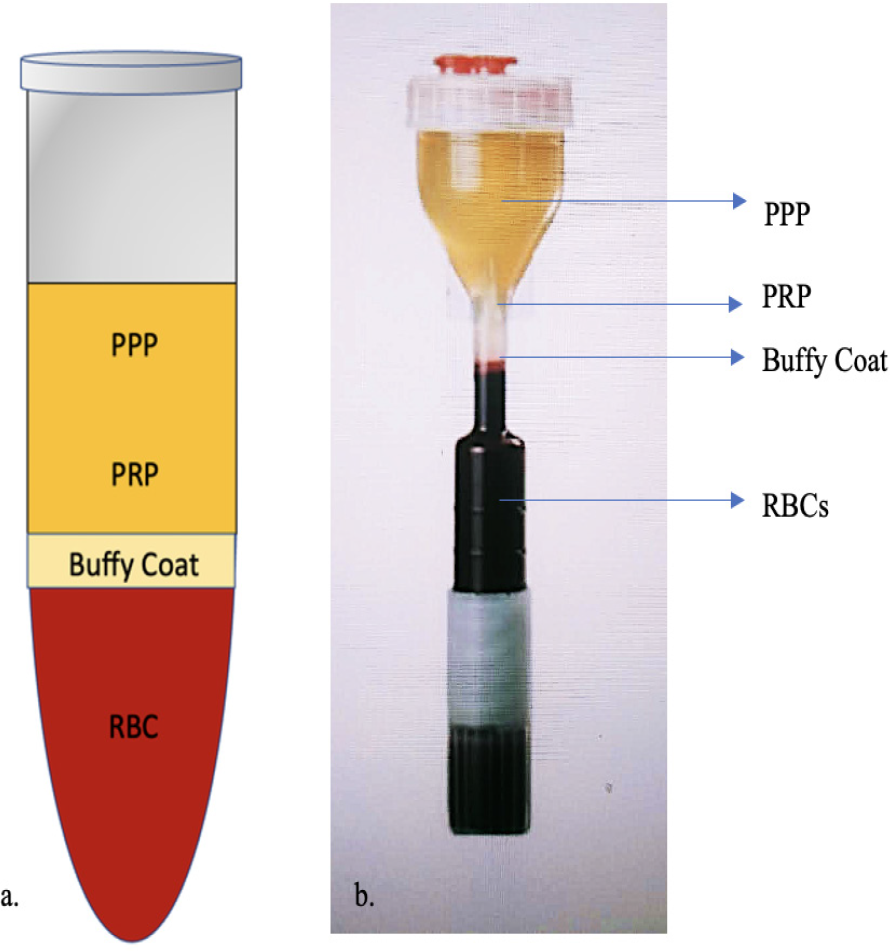
-
Components of PRP: (a) Normal acid citrate dextrose (ACD) vial; (b) Hour glass tube: In the single-spin method, the lower portion of the plasma layer is collected as PRP and to increase the platelet concentration of PRP, the plasma and superficial buffy coat can be collected and a second centrifugation can be performed.
-
Abbreviations: PPP – platelet-poor plasma; P-PRP – pure platelet-rich plasma; RBC – red blood cells.
Plasmapheresis, the basic principle in the formation of PRP occurs based on the principle of Stokes law, according to which the settling velocity of particles in a liquid in response to gravity is approximately proportional to their diameter. Thus, platelets remain suspended in the liquid component (plasma) of blood, whereas the larger particles, RBCs and WBCs, settle more rapidly and become separated from the platelets by gravity and the hourglass configuration of the system used facilitates the faster separation of blood components due to variable diameter of the tube experienced by the molecules from the center of the centrifuge.
Adverse effects
The most common adverse effects of PRP include skin discoloration and bruising, pain at the injection site, allergic reaction (rarely reported), and blood clot (vessel damage during injection), and infection.[4]
Post-procedure recommendations
Photoprotection for at least 2 weeks and post-procedure hydration of the skin are recommended to enhance the overall outcome.[1]
PRP in interventional dermatology
1. Difficult scars
A. Atrophic acne scars
Mechanism
Significant improvement in scars especially, post-acne scars, by the use of PRP has been demonstrated. PRP enhances wound repair and tissue regeneration via the stimulation of collagen as well as elastin synthesis. It expedites the healing and recovery, greatly reducing the post-procedure downtime.[4]
Techniques
Microneedling (MN) and PRP Microneedling may be combined with injectable or topically applied PRP. Studies utilize activated PRP prepared by using a DST with treatment sessions every 2–4 weeks for a total of 3–6 sessions.[16] Several studies comparing the use of MN alone versus MN with PRP report a statistically significant difference in the two groups, with greater improvement noted in the latter.[17,18] Although few studies report less impressive additive benefits with PRP, most studies recommend an overall higher patient satisfaction with treatments utilizing PRP.[16,19,20]
Laser ablation and PRP injections A systematic review assessing 7 studies (amongst a total of 167 patients) evaluating fractional ablative laser treatment with PRP in acne scarring, reported added benefits in 5 of these 7 studies and improvements in erythema, edema, and pain in 6 of the 7 studies. The PRP was administered via intradermal injections with 2 or 3 sequential treatment sessions 1 month apart.[16,21]
Laser ablation and topical PRP The microscopic pores created by fractional laser treatment enhance transepidermal delivery of topical PRP rendering it as effective as intradermal injections of PRP, according to the findings of a study by Gawdat et al.[22] This combination also reduced the post-procedure side effects, enhancing the overall patient experience, with the added advantage of being painless.[23]
PRP may be combined with fat grafting for soft tissue augmentation in traumatic scars and contour defects. Data suggests that fat grafts can be admixed with PRP in treating traumatic scars, and further can be followed by fractional laser resurfacing to give best results as PRP results in overall improved survival and quality of fat grafts.[24,25]
As per the author’s experience, combination of microneedling radiofrequency (MNRF) with PRP produces good results with a uniform glow, decreased downtime, faster recovery, reduced rates of post-inflammatory hyperpigmentation(PIH) as well as a reduced incidence of edema and crusting. Furthermore, the beneficial effects of PRP continue long after the treatment with ongoing improvement in the skin appearance and texture.
B. Post-burn scars
Mechanism of action
PRP helps improve the pigmentation, skin texture, and associated neuropathic pain in post-burn scars. The impaired healing process in burn wounds is augmented by the various GFs released by PRP resulting in neovascularization (NV) and neocollegenesis (NC).[26,27]
Technique
PRP can be injected into the scar using the “linear retrograde and fanning” technique with two or three retrograde injections, or “tunnelling”: a back-and-forth movement of the needle which first creates a tunnel, a track and then loads it with the solution. In a study utilizing monthly sessions of PRP for a total of three sessions amongst 19 patients, significant improvement was observed in itching, pigmentation, and pliability of scars. Improvement in pain was also experienced and may be attributed to nerve GFs which help in nerve healing and regeneration.[27]
Various treatment combinations such as fat grafting, CO2 laser, and PRP are associated with improvement in the post-burn scars.[28] As per the author’s experience, a cocktail treatment consisting of PRP, ablative Pixel Erbium: YAG laser, and MNRF in 5 patients of post-burn scars significantly improved pigmentation, vascularity, skin texture, thickness, and pliability [Figures 3 a–c]. There was appearance of new HFs in 4 out of 5 patients. This is based on the principle of regenerative medicine where laser and MNRF help in resurfacing old scarred skin, induce micro injuries which heal effectively with the support of GFs released by PRP.[29]

- A 17-year-old male with post-burn scarring over the lip. (a) Baseline photograph. (b) Four months after combination of follicular unit extraction (FUE) hair transplant with PRP therapy and pixel erbium YAG laser. (c) After 7 years, PRP therapy was associated with ongoing improvement. PRP was also injected into the lip to provide a volumizing effect and helped in improving the lip asymmetry.
C. Post-traumatic scars and post-surgical scars
In a study comparing the efficacy of three procedures for the treatment of traumatic scars, 60 patients were randomly allocated to one of the three groups (20 patients per group) – fat grafts mixed with PRP, non-ablative laser, or combination treatment with both procedures. Comparison of the groups showed that addition of PRP produced a significant difference in these treatment response and the most effective scar treatment was the combination of fat grafts mixed with PRP plus non-ablative laser resurfacing. This combination modality resulted in an increase of 22% in wound healing compared fat grafts and PRP and an increase of 11% compared with only non-ablative laser.[30] The authors concluded that the addition of PRP to a combination of fat grafting and non-ablative 1540-nm laser improves adipose tissue maintenance, survival, and more durable corrections.[30,31]
PRP applied to subcutaneous tissues of the wound before closure in 140 patients undergoing elective cesarean delivery, resulted in significant reduction in post-operative pain, enhanced the wound healing process as well as the quality of scar formation.[32] The study used the “Reduction in the edema ecchymosed discharge approximation” REEDA scale as the primary endpoint for assessing the changes in wound healing with a lower score indicating better healing. A greater reduction in the REEDA score was seen in the PRP group as compared to the control group (85.5% reduction in the PRP group; 72% in the control group) (P < 0.001).[32]
As per the author’s experience, a cocktail of ablative pixel erbium YAG laser, PRP, and autologous fat transplant works on the principle of regenerative medicine and can be effectively utilized to treat post-traumatic scars. Ablative laser induces microinjuries on to the scarred skin, PRP stimulates wound healing, and the fat acts as a reservoir of adipocyte-derived stem cell while providing a cushioning effect. Furthermore, PRP may also promote regeneration of terminal nerve fibers.[33]
D. Striae Distensae (SD)
Mechanism of action
PRP acts by augmenting dermal elasticity via the stimulation of ECM and inducing synthesis of new collagen.[34]
Techniques
-
PRP injection monotherapy A recent comparative study evaluated the efficacy of PRP monotherapy, microdermabrasion (MD), and combination therapy with PRP and MD amongst 68 patients with SD. Treatment consisted of a maximum of 6 sessions at 2-week intervals. Significant clinical improvement was noted in those treated with PRP alone or PRP with MD when compared to MD alone, with no statistically significant difference in the outcomes between the groups.[35]
Studies comparing PRP monotherapy with topical tretinoin 0.05% cream applied once at night or tripolar radiofrequency found no statistically significant difference in the clinical outcome between the treatment groups.[36]
Carboxytherapy (CT) involves infusion of carbon dioxide gas via a subcutaneously placed needle. In a recent study comparing CT with PRP amongst 20 females with SD, significant clinical improvement was observed in all lesions, with no significant difference between treatment modalities.[37]
PRP with RF Intradermal radiofrequency (RF) device capable of delivering higher energy fluencies directly to the dermis is combined with injection of PRP through its needle electrode. Bipolar RF causes the denaturation of collagen and elastin fibers resulting in tissue contraction while PRP stimulates neocollagenesis (NC) leading to tightening of the dermal tissue.[34] In a study amongst 19 patients with SD, this combination demonstrated marked improvement in majority of the cases.[38]
PRP with ultrasound Very good or excellent improvement was seen at 2 months in majority of the 18 women with striae alba treated with plasma fractional radiofrequency and enhanced PRP penetration using acoustic wave ultrasound every 2 weeks for 4 sessions.[39]
PRP combined with pixel erbium YAG laser and MNRF result in significant improvement in the erythema, pigmentation, skin texture, and overall appearance in striae distensae, as observed by the author [Figure 4a,b].

- (a) A 25-year-old female with childhood onset nephrotic syndrome, undergoing corticosteroid therapy for a long duration (2 years) who presented with severe striae distensae. (b) Improvement in striae distensae in terms of erythema, texture and appearance, over the arms following three sessions of combination therapy with pixel erbium YAG laser, microneedling radiofrequency (MNRF) and PRP therapy,
2. Melasma
Mechanism of action
Recently, PRP has shown promising results in the treatment of melasma [Figure 5a,b] Inhibition of melanogenesis by GFs such as TGF-β1 as well the increase in skin volume resulting from PDGF mediated collagen synthesis and increase in the ECM components enhance the overall appearance and skin texture.[40,41] The pigmentary lightening effect may also be attributed to basement membrane healing stimulated by TGF-β released upon PRP activation.[42]

- (a) Baseline photograph of a 45-year-old female patient with melasma and baseline modified melasma area severity index (mMASI) score of 10. (b) Significant improvement in terms of patient satisfaction and reduction in mMASI to 6, after three sessions of MNRF and PRP injections.
Techniques
Studies comparing MN and microinjection techniques for delivery of PRP have reported them to be equally efficacious, although most of the patients experienced more pain as well downtime on the side treated with microinjections.[43] Better improvement was noted in the epidermal type of melasma than the mixed one.[42]
Ch et al. reported improvement in two cases of melasma treated with PRP in conjunction with Q switched Nd:YAG (1064 nm) and topical alpha arbutin.[44] Çayırlı et al. reported more than 80% improvement in melasma in a single patient receiving PRP injections for skin rejuvenation.[41]
3. Vitiligo surgery
Mechanism of action
PRP has a high concentration of GFs, particularly basic FGF (bFGF) and stem cell factor (SCF) which may stimulate melanocyte proliferation and repigmentation in the vitiligo lesions.[45,46] In addition PRP also contains PDGF and VEGF which enhance cellular proliferation, ECM formation, connective tissue healing, angiogenesis, and collagen synthesis, resulting in faster graft uptake, improved chances of survival, reduced risk of infection.[47] PRP enhances hair melanocyte reserves and may reverse leukotrichia. It contains natural trypsin inhibitors and also increases the viscosity of the suspension, thus preventing its run off. This results in better melanocyte proliferation, survival, and faster repigmentation.[47]
Techniques
Studies comparing non-cultured epidermal cell suspension (NCES) suspended in PRP versus NCES suspended in phosphate buffered saline, report better RP rate and patient satisfaction with the former.[45] Similarly, a combination of PRP with fraction carbon dioxide laser results in superior RP than intradermal PRP alone.[46] Recently, the authors proposed a new modified procedure as an effort to improve the outcome of vitiligo surgery using PRP as a suspending agent and erbium: yttrium aluminum garnet (Er:YAG) laser for ablating the recipient site, known as laser ablation on recipient area with PRP—enriched epidermal suspension transplant (LA-PEEST) [Figure 6a,b].[47]

- (a) Baseline photograph of a 20-year-old male with vitiligo. (b) Laser ablation on recipient area with PRP—enriched epidermal suspension transplant (LAPEEST) with near complete repigmentation (more than 90%) after 3 months.
4. Chronic ulcers
Mechanism of action
Autologous platelet-rich plasma is a novel treatment for cutaneous ulcers which persist for more than 6 weeks and show no tendency to heal after 3 or more months.[48] PRP contains GFs which promote mesenchymal cell recruitment, proliferation, and ECM synthesis, thus enhancing wound healing.[49] Furthermore, platelets exert an antimicrobial action and studies report decreased presence of infection in PRP-treated ulcers.[50]
Technique
Intralesional injection (ILI) PRP administered via ILI to the edges and floor of the wound, every week for 6 weeks or more depending on the response is an effective therapeutic option. In a study by Suryanarayan et al., 33 ulcers were treated with ILI of PRP for 6 weeks and percentage improvement in area was reported to be 91.7% in 7–9 weeks.[51] Large ulcers with longer duration were slow to heal, requiring prolonged treatment therapy. A reduction in wound area of 30% within 4 weeks of treatment is an appropriate predictor of healing and indicator of treatment efficacy.[48]
PRP gel Studies have utilized a combination of thrombin and calcium chloride to prepare a platelet activator fluid resulting in fibrin matrix formation which promotes cell permeation and ulcer healing, as early as 4 weeks post-PRP treatment.[52] Although the PRP gel formulation is easier to use, it is associated with certain drawbacks such as non-uniform distribution, possible loss of gel, restricted access to the wound surface as well as limited duration of effect.[48]
Platelet-rich fibrin matrix (PRFM) PRF belongs to a new generation of platelet concentrates with a simplified preparation technique [Box 4]. Ten milliliter of venous blood is drawn and added to a sterile centrifugation tube devoid of anticoagulant. Centrifugation is done at 3000 rpm for ten minutes and three layers are obtained: upper straw-colored PPP; lower fraction – RBCS; middle portion containing – the PRFM. The PPP layer is discarded and PRFM is separated from the RBCs at the base conserving a small RBC layer (around one mm). Middle membrane is applied on the debrided ulcer floor followed by placement of a secondary non-absorbable dressing. In a study amongst 7 Hansen patients with 9 trophic non-healing ulcers, PRFM was repeated once a week and complete closure of all ulcers was achieved in a maximum of 5 weeks with the mean percentage improvement in the area and volume of ulcer at the end of second sitting reported as 93.52% and 97.74%, respectively.[53]
| Advantages of PRFM[54] |
|---|
| • Neither requires anticoagulant nor bovine thrombin (nor any other gelling agent). • Higher platelet concentration when compared to platelet-rich fibrin (PRF). • Mean concentration of growth factors in the platelet concentrates was three times or more than that observed with conventional platelet-rich plasma. • Growth factors are released in a controlled manner over approximately 1 week |
Lipodermatosclerosus
PRP stimulates adipose tissue regeneration, neoangiogenic vascularization, and ulcer re-epithelization and studies have shown that intralesional subcutaneous injections of PRP result in significantly better RP and patient satisfaction.[54]
5. Skin rejuvenation and anti-ageing effects
Mechanism [Table 3]
| Effects of ageing | Role of PRP | Clinical effects |
|---|---|---|
| Decreased vascularization | Increased VEGF leads to angiogenesis | Improved vascularity of skin |
| Impaired cell replenishment and loss of extracellular matrix | • Fibroblast stimulation increases procollagen 1 • Increased hyaluronic acid production • Increase in TIMP leads to stabilization of ECM |
Increased resistance to tension and stretching Improved skin hydration and firmness |
| Fat atrophy | Significant increase in adipocytes | Volume augmentation |
Abbreviations: VEGF – vascular endothelial growth factor; TIMP – tissue inhibitors of metalloproteinases; ECM – extracellular matri x
PRP has been reported to improve dermal elasticity and skin texture via the stimulation of dermal fibroblasts leading to NC as well as the removal of photodamaged components of the ECM by enhancing the expression of matrix metalloproteinases (MMP) 1 and 3. Furthermore, it accelerates hyaluronic acid production and leads to improvement in skin hydration and volume.[16] Various methods have been employed for the application of PRP in this indication.
Techniques
-
Topical application under occlusion
-
Facial injections
PRP may be injected intradermally or sub-dermally to induce collagen production to improve skin color, texture, and reduce the appearance of fine lines. In general, these treatments are performed at 4- to 6-week intervals for 3–5 months, or until the desired result is achieved.[2,15]
In conjunction with microneedling Microneedling utilizes multiple fine needles which can penetrate up to the papillary and reticular dermis to mechanically induce angiogenesis, elastin as well as collagen production resulting in wound healing and skin remodeling, while preserving the epidermal barrier. PRP may be applied to the skin immediately after MN to improve skin texture and elasticity.[2,15] Microneedling for various areas should be done at different depths, because the skin of the face, neck, and chest varies in its thickness. Each individual, their skin texture, and sebaceous quality should be taken into account, with patients with thicker skin able to have MN performed at deeper depths; Forehead: 1–1.5 mm; Periorbital: 0.5–2.5 mm; Face: 1.5–3 mm; Nasal skin, especially over the upper dorsum: 0.5–1 mm; Neck and de'collete': 0.5–1.5 mm. Microneedling should be performed using multiple passes in various directions to ensure uniform treatment. Microneedling with PRP and injections of PRP can be combined during the same treatment as MN will help the overall skin texture, whereas injections will further help collagen stimulation from the deeper tissues [Figure 7 a,b].
In combination with fractional ablative lasers Following laser therapy, activated PRP serum is applied to skin and it results in accelerated wound healing via augmentation of new collagen formation.[56]
Infraorbital rejuvenation Studies report good results with monthly PRP injections for 3 consecutive months for infra-orbital rejuvenation. Improvement was noted in terms of patient satisfaction, infraorbital wrinkles, decrease in erythema as well as the melanin index.[5] Similar results have been observed in our experience [Figure 8a,b].

- (a) Baseline photograph of a 45-year-old female patient with severe signs of skin ageing including rhytids (arrow). (b) Significant improvement in skin appearance and texture (arrow) after 2 sessions of facial rejuvenation with microneedling radiofrequency (MNRF) and PRP therapy.

- (a) Baseline photograph of a 28-year-old male patient with infraorb ital hyperpigmentation (arrow). (b) Improvement in pigmentation, fine lines, skin texture (arrow) after 2 sessions of periorbital rejuvenation with microneedling radiofrequency (MNRF) and PRP therapy.
II. PRP in trichology
1. Androgenetic alopecia (AGA)
Based on the results of published meta-analyses, PRP significantly improves hair density in AGA patients. PRP may be utilized as monotherapy or may be combined with hair transplant.[61-68]
Mechanism of action
In AGA there is a progressive miniaturization of HF and the anagen phase becomes shorter with each cycle, producing microscopic hairs. GFs released from PRP act on stem cells (SC’s) in the bulge area of the follicles, stimulating the new follicular development and NV. Basic FGF promotes the proliferation of papilla cells in vitro and, therefore, plays a fundamental role in hair shaft elongation. Activated PRP also promotes the proliferation and inhibits the apoptosis of dermal papillary cells (DPCs).[69,70] The various mechanisms have been outlined in [Table 4.]
| Indications | Mechanism of action | Method | Results |
|---|---|---|---|
|
1. Difficult Scars • Atrophic acne scars[4,16,21-25] • Post-burn scars[26-28] • Post-traumatic scars[30-33] • Post-surgical scars |
• Increase in collagen type I via fibroblast stimulation • Increase in elastic fibers • New blood vessel formation • Adipose tissue deposition • Soft tissue augmentation |
1. Microneedling and PRP injections 2. Microneedling and topical PRP 3. Laser and PRP injections 4. Laser and topical PRP |
• Improvement in scar appearance- pigmentation, vascularity, skin texture • Higher patient satisfaction • Decreased post-procedural erythema and edema • Faster recovery, reduced downtime |
|
1. Difficult Scars • Striae distensae[34-39] |
The thermal energy generated by bipolar RF, denatures the elastic fibers and collagen bundles PRP stimulates wound healing, thereby providing synergistic benefits and good results. |
1. Intradermal radiofrequency (RF) device and PRP as a filler through its needle electrode 2. PRP injection monotherapy 3. PRP with ultrasound |
• Improves skin quality • Increase in collagen and elastic fibers • Reduced appearance of striae |
| 2. Facial melanosis[40-44] | • Inhibition of melanogenesis by growth factors such as TGF-β1 • Increase in skin volume resulting from PDGF mediated collagen synthesis • Increase in the ECM components such as hyaluronic acid • Basement membrane healing by laminin, collagen IV, tenascin stimulated by TGF-β |
1. Microneedling 2. Direct intradermal injections 3. As an adjuvant to lasers |
• Enhances the overall appearance • Enhances skin texture • Pigmentary lightening effect |
| 3. Vitiligo surgery[45-47] | • Stimulates melanocyte proliferation and repigmentation in the vitiligo lesions • Enhances hair melanocyte reserves and may reverse leukotrichia. • It contains natural trypsin inhibitors and also increases the viscosity of the suspension, thus preventing its run off. • This results in better melanocyte proliferation, survival, and faster repigmentation. |
1. Non-cultured epidermal cell suspension in PRP, followed by autologous transplantation 2. Combination of PRP with fraction carbon dioxide laser 3. Autologous non-cultured tiny epidermal fragments suspended in PRP grafted on superficially pixel erbium YAG laser-ablated vitiligo lesions |
• Significantly better repigmentation and patient satisfaction • Potential to improve the rate of healing and repigmentation |
|
4. Chronic ulcers[48-53] • Diabetic foot ulcers • Venous leg ulcers • Pressure ulcers • Traumatic ulcers • Vasculitic ulcers |
• Mitogenic, angiogenic, and chemotactic properties • Platelets exert an antimicrobial action • Mesenchymal cell recruitment, proliferation, and extracellular matrix synthesis, thus enhancing wound healing |
1. Intralesional injection 2. PRP gel 3. Platelet-rich fibrin matrix |
• Reduction in area, depth of the ulcer • Complete healing of the ulcer |
| • Lipodermatosclerosus[54] | • Stimulates adipose tissue regeneration, neoangiogenic vascularization, and ulcer re-epithelization. | Intralesional subcutaneous injections of PRP in five sessions (fortnightly) | • Complete re-epithelialization • Improvement in hyperpigmentation and induration |
|
5. Skin rejuvenation[2,16,55-57] • Rhytids • Photodamaged skin • Periorbital hyperpigmentation • Breast augmentation • Vulvovaginal rejuvenation |
• Increased proliferation of human dermal fibroblasts and stimulation of collagen synthesis • Increased expression of MMP enhances removal of photodamaged ECM • Increases expression of G1 cell cycle regulators accelerates wound healing • Accelerates hyaluronic acid production which improves skin hydration |
1. Topical application under occlusion 2. Direct intradermal injections 3. As an adjuvant to lasers or micro needling. |
• Improves skin quality, elasticity, and firmness • Significant improvement in fine wrinkles- nasolabial folds> crow’s feet>transverse forehead lines • Lower erythema index • Reduces transient adverse effects and decrease the downtime associated with lasers • Improves skin volume and turgor |
| 6. Lichen sclerosus of the vulva[58-60] | • Provides growth factors involved in stem cell migration, differentiation and proliferation • Stimulation of fibroblasts and endothelial cells induces new deposition of extracellular matrix and neovascularization |
1. Multilayer PRP injections used along with autologous fat transfer 2. Intradermal PRP, two sessions 6 weeks apart |
• Significant improvement in symptoms and quality of life • Therapy was well-tolerated. • Significantly decreased histopathological inflammation |
Abbreviations: PRP – platelet-rich plasma; ECM – extracellular matrix; RF – radiofrequency, TGF – transforming growth factor; PDGF – platelet derived growth factor; MMP – Matrix metalloproteinases
Technique
To reduce injection pain, topical local anesthetic cream, block anesthesia with lignocaine 2% solution without adrenaline in supraorbital and supratemporal nerve, air-cooling devices and/or vibratory distracting devices may be utilized. However, local anesthesia may affect the pH of PRP and has been reported to have a deleterious effect on efficacy.[82]
Great variation exists in the technique of PRP preparation (kits/procedures/methods), addition of activators (CaCl2, Ca2+; thrombin, calcium gluconate, etc.), method of PRP injection (mechanical and controlled vs. manual), and standardized evaluation methods (trichoscan/phototricograms/magnifying glass). Furthermore, there is a wide variability regarding the number of sessions, time interval between procedures, administration methods, and follow-up period. Currently there is no consensus regarding a standardized treatment protocol for usage of PRP in AGA and available techniques include the following: [Table 5][70,83,84]
| Mechanism | Effector molecule/pathway | Postulated molecular process | Result |
|---|---|---|---|
| Proliferation of hair follicle | FGF-7[71,72] | Promotes differentiation and proliferation of dermal papilla cells and stem cells in the bulge area Angiogenesis stimulator |
Improvement in • Hair count • Terminal hair density • Hair diameter Decreased Hair shedding |
| Wnt-Beta catenin pathway activation[71] | In the DP cells, the activation of Wnt will lead to an accumulation of β-catenin- promotes proliferation, survival, and angiogenesis. | ||
| Reciprocal interactions between DP, ESC, EBM mediated by PDGF.[73,74] | Activates proliferative phase of the hair | ||
| VEGF[75] | Regulates perifollicular angiogenesis | ||
| EGF, HGF, IGF-1, PDGF[76] | Angiogenesis stimulator | ||
| Maintenance of hair follicle | • Activation of the ERK/Akt signal pathway[77] • Increased Bcl-2(anti-apoptotic protein) associated death promoter levels[76] |
Inhibit apoptosis of dermal papillary cells | Improvement in • Hair count • Terminal hair density • Hair diameter Decreased Hair shedding |
| IGF-1[78] | Prevents the HF from developing catagen like status | ||
| • Deposition of nephronectin in basement membrane of HF bulge region[3] |
Attachment between bulge portion of HF containing stem cells, bulge portion of APM and basement membrane comprising the “golden anchorage” for HF survival and integrity[3,64] | ||
| Hair growth, renewal and Hair cycle | Arrector Pilli Muscle[79,80] | Maintains hair follicle integrity-‘Ribbon on a bunch of flowers’ | Improvement in • Hair count • Terminal hair density • Hair diameter Decreased Hair shedding |
| Activation of Wnt and accumulation of β-catenin[81] | Transition from the telogen to the anagen phase is initiated β-Catenin signaling is important for the hair growth cycle. |
||
| VEGF[76] | Increases perifollicular vessel size during the anagen growth phase | ||
| FGF, PGDF, IGF-1[76] | Increases hair growth by inducing the anagen phase of HF Faster transition from telogen to anagen |
Abbreviations: FGF – fibroblast growth factor; DP – dermal papilla, ESC – epidermal stem cells; EBM – epidermal basement membrane; PDGF – platelet derived growth factor; VEGF – vascular endothelial growth factor; EGF – epidermal growth factor, HGF – hepatocyte growth factor; IGF – insulin like growth factor; ERK – extracellular signal-regulated kinases, HF – hair follicle; APM arrector pili muscle.
1. PRP monotherapy: Interfollicular PRP injection
An amount of 0.05–0.2 mL/cm2, is injected using a 30 gauge needle in a retrograde fashion from deep to superficial, at distance of a 1 cm (nappage technique), throughout the affected region.[70] PRP injections (1 PRP session every 4 weeks, 3 sessions in total) significantly improve hair density in AGA patients. Several studies report a PRP-induced improvement in hair count (HC), terminal hair density (HD), hair shedding (HS), and hair diameter (HD), although in a few studies researchers did not find any statistically significant difference in the results obtained between the treated area and baseline. Overall, all the studies in which a minimum of three PRP sessions were conducted displayed an improvement in at least one objective measure.[85] Gentile et al. performed a randomized blinded half-head study evaluating the effects of an inter-follicular injection of PRP (0.1 mL/cm2) with 30-gauge needles amongst 23 males suffering from AGA. Patients were treated with PRP infiltrations three times at intervals of 1 month, performed on half of the scalp, while the other side received saline as a placebo and a statistically significant increase in mean HC, HD, and terminal HD was documented after three months of the last PRP injection compared to saline.[64]
The author studied the efficacy of PRP therapy in 65 male patients with androgenetic alopecia and 50 patients with FPHL, where PRP therapy was given as three sessions repeated every month.
PRP was injected using an insulin needle, starting in the subcutaneous plane (3–4 mm), at an angle of 45 preferably parallel to direction of hair, thus minimizing trauma to hair. Infiltration of PRP was continued, while the needle was gradually pulled outward toward the skin surface until a mild nodular elevation was appreciated. New HG was observed as early as 4–6 weeks and HC increased in both groups. Improvement in HD, perifollicular halo and pigmentation, skin texture, multiplicity of hair in follicular units were also reported.[3] Similar results have been observed in female pattern hair loss [Figure 9a,b].

- (a) Dermoscopic image of a 45-year-old female with patterned hair loss with decrease in hair count, diameter and density. (b) Dermoscopic image showing increase in hair count, diameter and density after 4 sessions of PRP therapy, nutritional supplements, increased protein intake, topical peptides, and minoxidil therapy.
2. PRP mesotherapy
In a study amongst 20 male patients with AGA, PRP was injected over affected area (multiple small injections few mm apart) followed by microneedling (1.5 mm), over a period of 3 months at 3-week intervals. The patients’ satisfaction was more than 75% in 18 patients and an increase in the number of vellus and total hairs, increased hair shaft diameter, dramatic reduction or disappearance of black/yellow dots and reduction in hair pull test were appreciated after 12 weeks of 3 sessions of PRP in all patients.[86]
In an open-labeled pilot study amongst 30 males with AGA, each one received six (4 weekly) topical PRP massage treatments after the scalp was first activated by micro-needling, a significant increase in HT and HD was reported and response was more significant in those with lower-grade hair loss.[87]
| Technique | Method | Relevance | |
|---|---|---|---|
| PRP monotherapy[70,85,64] |
Interfollicular injections using 30 G needle • 0.05–0.2 mL injected using Nappage technique (at a distance of 1 cm) • Retrograde – from deep to superficial |
• Intradermal • Subdermal • Subcutaneous |
Statistically significant improvement in • Hair count • Hair density/terminal hair density • Hair thickness or diameter • Hair texture • Hair regrowth • Hair loss (hair pull test – negative) • Pigmented hair • Anagen hair • Anagen hair: Telogen hair ratio Greater improvement in patients with: • Lower-grade AGA • Short duration disease • No family history of AGA Histopathology – Improvement in: • Epidermal thickness and density of follicles • Peri-follicular neo-angiogenesis • Terminal/miniaturized hair ratio Decreased perivascular inflammatory infiltrates. Immunohistochemistry-Percentage of Ki67+ cells increased in both basal keratinocytes of the epidermis and hair follicle bulge cells |
|
Mesogun Inter-follicular infiltration (0.2 mL/cm2) to AGA-affected regions at a depth of 5 mm utilizing a mechanical and controlled injection outfitted with a 10-mL Luer lock syringe with 30-gauge needle |
Mechanical and controlled infiltration achieved Software capable of scheduling the amount of PRP delivered, depth and inclination of the needle. |
||
|
PRP mesotherapy or PRP with Microneedling[86,87] |
1. Microneedling 2. PRP injections (manual or mesogun 3. Remaining PRP is sprayed on the scalp (left overnight) |
1. PRP injections using nappage technique followed by microneedling (1.5 mm long needles) till pinpoint bleeding appears. 2. The scalp is activated by microneedling following which PRP was massaged on the scalp. |
|
| PRP with hair transplantation[2,83,84] | Follicular grafts are dipped into PRP for about 15 min, before implantation | The follicular units are kept in the platelet growth factor solution for 15 minutes to allow the growth factors to attach to the stem cells located in the bulge area. | • Increase the rate of follicular graft survival following implantation • Patients with less density and thin hair in the donor area • Simple, efficient, low cost and minimal morbidity. |
| PRP is injected into the recipient area of scalp before or just after graft implantation | After harvesting and slitting, 0.2–0.3 mL PRP was injected at 1 cm gap to the depth of dermis and subcutis in freshly done slits. |
Faster density, reducing the catagen loss of transplanted hair, recovering the skin faster and activating dormant follicles in FUE transplant subjects. | |
| PRP in follicular unit transplantation | PRP is injected at and around the donor strip excision line, in follicular unit transplantation | • To decrease bleeding • Stimulation of wound healing • Reduction of scarring |
Abbreviations: PRP – platelet-rich plasma; AGA – androgenetic alopecia; FUE – follicular unit extraction
3. PRP as an adjunct to hair transplantation
According to the results of a study amongst 20 male patients with AGA where on the right side, follicular units embedded with PRP were implanted and left side equal number of untreated follicular units were implanted as controls, there was 15.1 percent more hair yield in follicular units and density in the area treated by PRP.[83] In a single-blind, prospective randomised study on 40 follicular unit extraction (FUE) hair transplant subjects, allocated in two groups, PRP was injected intra-operatively immediately after creating slits over the recipient area in PRP group; and normal saline in non-PRP group. In PRP group, all subjects had > 75% hair regrowth (HRG) at 6 months, density of > 75% grafts was noticed in 12 patients at 4 weeks as compared to the non-PRP group where four patients had > 75% hair regrowth at 6 months and none showed > 75% graft density at 4 weeks.[84]
4. PRP as an adjunct to minoxidil and/or finasteride
In a controlled clinical trial to compare PRP with medical treatments in 20 patients, HG was observed in 6 patients after just 1 week. Evaluation after 3 months, displayed superior results in PRP-treated patients than in the control group and the patients who underwent medical treatments displayed no improvement in the hair pull test or HG.[88] A randomized placebo-controlled, double-blinded, half-head study to assess the efficacy of PRP on the treatment of AGA showed a statistically significant positive effect on HC, HD, terminal HD, anagen hairs (%), and telogen hairs of PRP associated with medication versus baseline, six months after the first treatment. Overall, after 6 months, combination of PRP and minoxidil 5% showed superiority in mean hair count, hair density, anagen and telogen percentages, and mean anagen/ telogen ratio in comparison with the association of PRP and finasteride 1 mg.[89]
2. Alopecia areata (AA)
GFs released from PRP, promote the proliferation and inhibit the apoptosis of dermal papillary cells, stimulating new follicular development and NV. In patients treated with PRP, an increased expression of Ki-67, an indicator of cellular proliferation is seen.[90] Furthermore, in AA, β-catenin and basic FGF act on melanocyte differentiation and melanin synthesis to encourage the growth of pigmented hairs.[91,92] PRP may also be effective in AA through anti-inflammatory mechanisms owing to its ability to suppress MCP-1, a chemokine involved in generating a local inflammatory reaction around the hair bulb.[93,94] Furthermore, PRP stimulates release of TGF-β, creating an anti-inflammatory milieu. TGF-β, an immune modulator is normally released by HFs to create a local “immune privileged” environment and its levels are significantly reduced in patients with AA.[95]
In a double-blinded, placebo and active-controlled triamcinolone acetonide (TAC) injections (2.5 mg/mL), half-head, parallel group study on 45 patients of AA, PRP was found to significantly improve HRG and to decrease hair dystrophy as well burning and pruritus sensation without any side effects.[90] Moreover, 96% of the patients treated with PRP appeared to regrow pigmented hairs from the beginning of hair regrowth compared with 25% of those treated with TAC.
PRP therapy also has the ability to treat steroid-resistant forms of AA such as ophiasis and may be utilized effectively to treat AA patients who develop limiting side effects from intralesional steroids, as elaborated by Donovan et al.[96] A recent comparative study, evaluated the treatment outcome of intralesional PRP and triamcinolone acetonide injections in 40 patients of AA. A similar treatment response was reported in the both the groups at the end of 12 weeks, however, there was a statistically significant in the duration of response at 8 weeks with PRP.[97] PRP has also been found to be more effective in the treatment of AA than topical minoxidil 5% in a study by El Taieb et al. amongst 90 patients treated with PRP.[98]
3. Telogen effluvium (TE)
A paucity of literature exists on the role of PRP in TE. MN is an effective method for treating telogen effluvium whether alone or when combined with PRP and is thought to act by induction of NV. In our experience, significant improvement in HC and HD after three sessions of PRP, offered once a month, were observed in a patient with TE, along with visibly thicker and healthier HFs.[3]
4. Cicatricial alopecia (CA)
The various anti-inflammatory, proangiogenic cytokines present in PRP may help diminish the inflammation, protecting and stimulating the new follicle in CA. In a study by Fakahany et al., one case each of CA and traction alopecia were treated with 20 sessions of automated MN combined with PRP, every two weeks. Marked clinical improvement with variable degrees of patient satisfaction was reported.[99] In a similar study to patients of CA, a case of central centrifugal cicatricial alopecia (CCCA) and second patient was a 70-year-old woman with a diagnosis of lichen planopilaris (LPP), lobal improvement of hair density in both patients with cicatricial alopecia after administration of 3 (1 monthly) sessions of PRP was noted.[100] A recent study also reported an improvement of perifollicular erythema, scaling, and lichenoid papules on the frontotemporal hairline in a case with frontal fibrosing alopecia with five sessions of PRP injected into the frontotemporal hairline and eyebrows of the patient.[101]
CA is associated with poor survival of follicular grafts and PRP induced production of angiogenic GF’s helps improve the cutaneous ischemic conditions via perifollicular NV. In a study by Saxena et al., 1 mL PRP injected intradermally into the recipient area, prior to graft implantation resulted in a successful hair transplant outcome in cicatricial lichen planus of the scalp. PRP with its potential to improve the vascularity and regenerate the scarred tissue, making it more receptible, can serve as an adjuvant to hair transplant in compromised recipient areas as seen in scarring alopecia.[102]
The author performed a combination of punch grafting with 2-mm diameter biopsy punches to transplant around 25 grafts with intraoperative PRP therapy in a patient with stable lichen sclerosus et atrophicus (LSEA) of 4-years duration. Post procedure evaluation at four months revealed that all the grafts were surviving well. Thus, in our opinion, in cases of CA, scarcity of ostia might be associated with a reduced chance of the reversal of damaged HF and the transplant of new follicles through FUE punch grafting can serve as a vital source of SCs, APM, epidermal basement membrane as well as nerve bundle.[3]
Future prospects
With proven efficacy in such as melasma, as well the pigmentation associated with acne or atrophic scars, in the author’s experience, PRP (alone or with microneedling) may also be utilized in lichen planus pigmentosus and post-inflammatory hyperpigmentation where it provides a skin lightening effect, improves the overall skin appearance, and may reduce symptoms such as erythema or itching due to its potent anti-inflammatory action.[40-42,95] The vast regenerative potential of PRP has been extrapolated to terminal nerve fiber regrowth in post-traumatic scars, post-burn scars, and chronic ulcers. Garg et al. have successfully documented significant improvement in sensation in post-traumatic and post-burn scars following PRP therapy and this neuronal regenerative potential remains to explored.[3,103] Recent research also points toward the efficacy of three weekly intramatricial PRP injections in refractory nail disorders such nail lichen striatus and idiopathic trachyonychia, with improvement noted in 3–6 weeks.[104]
Current data indicates that human hair follicles contain multipotent stem cells (MSCs) other than epithelial and melanocytic stem cells. These cells are located in the bulge area and demonstrate plasticity in ex-vivo and in-vitro conditions, help in reversal of the pathological mechanisms underlying hair loss in AGA and result in regeneration of complete HF from bulge SCs, a process known as neogenesis.[105-107] This has been utilized in the management of AGA where follicular suspension (containing SCs) alone or in combination with PRP has produced favorable results. In a recent study by Gentile et al., the effect of autologous hair follicle stem cell suspension (AHFSC) was compared to placebo in 11 patients with AGA and a 29 ± 5% increase in the HD in the treatment group was reported as compared to < 1% in the placebo group.[107] Kadry et al. compared autologous adipose tissue derived SC and PRP therapy in 60 patients of AGA, who were randomized to 2 groups to receive 3 monthly sessions of either of the therapy. Results confirmed the overall increase in hair density in both the groups as compared to baseline however an increase in both the terminal and intermediate hair count was reported with adipose tissue derived stem cells while only the terminal hair count was higher in PRP therapy group with no significant improvement in intermediate hair count.[108]
As per the author’s experience, combination of both the techniques produces longer lasting results with more sustained effect on entire follicular unit and terminal hair growth. Follicular suspension is prepared using 50 follicular unit grafts which are extracted from the occipital area of scalp (known to be resistant in AGA) and mixed with 5 mL of trypsin EDTA solution. The solution containing grafts is incubated at a temperature of 40°C for 2 hours with frequent shaking in between. Subsequently, it is mechanically agitated, filtered, and centrifuged at 3200 rpm for 10 min.
The supernatant is discarded, the pellet formed is mixed with PPP and subjected to a second centrifugation at 3,200 rpm for 10 min. After removal of the supernatant, lower portion of the solution along with pellet is mixed with PRP to produce a uniform suspension which is then injected at a distance of 1 cm apart in the affected area. As per the author’s experience, patients treated with 5 sessions of PRP followed by a gap of 6 months and then a single session of autologous HF suspension comprising of SCs along with ECM admixed with PRP for AGA resulted in noticeable improvement in the texture of hair along with a filling effect in the frontotemporal angles, and relative increase in the proportion of coarse terminal hair after PRP therapy.[109] The author recommends the combined use of intraoperative follicular suspension with hair transplant (follicular unit extraction) and PRP for management of AGA [Figure 10 a–c].

- (a) A 32-year-old male with androgenetic alopecia and severe frontotemporal hair loss. (b) Four months after follicular unit extraction (FUE) hair transplant + PRP + follicular suspension, there was noticeable improvement in the texture of hair and relative increase in the proportion of coarse terminal hair. (c) A filling effect in the frontotemporal angles was seen 1 year after surgery (no oral finasteride).
CONCLUSION
With its proven regenerative and regrowth potential in a plethora of conditions, PRP has been deemed as the “futuristic elixir.” Current evidence suggests that PRP effectively stimulates angiogenesis, collagen as well as elastin regeneration and is a safe, easy to prepare, minimally invasive technique with limited downtime, and negligible risk of allergic/ hypersensitivity reactions owing to its autologous nature. It has shown excellent results when utilized as monotherapy or in combination with microneedling or ablative lasers in acne scars, post-burn or post-traumatic scars, melasma, striae distensae, chronic ulcers, and lichen sclerosus. PRP injections or PRP combined with microneedling are increasingly being utilized for skin rejuvenation and recently have been utilized to provide non-invasive face lifts, offering a safer alternative to Botox and fillers. However, this must be taken with a pinch of salt, as in a recently reported case, a 49-year-old woman developed acute visual loss in the right eye following bilateral cosmetic PRP injections to rhytids in the glabellar region, performed by an unlicensed practitioner.[110]
A novel technique combining non-cultured epidermal cell suspension suspended in PRP results in superior repigmentation outcomes in case of vitiligo. Use of PRP alone or in combination with HF transplant in AGA is another well-researched indication with data suggesting greater improvement with PRP as compared to traditional therapies such as minoxidil or finasteride. With its proven stimulation of hair follicles, dermal papilla cells, stem cells, and melanocytes, its use has been successfully extrapolated to indications such as alopecia areata, chronic telogen effluvium, and cicatricial alopecia. A variation in the efficacy of results has often been discussed. Authors would like to emphasize the importance of correcting underlying nutritional deficiencies for best results, as PRP which comprises of growth factors generates a positive cascade of events, which may go partially or completely wasted, in case of underlying deficiencies.
In spite of its established efficacy in such a vast number of indications, PRP should be used with utmost caution. It may be considered to be our own living and breathing “‘dragon warrior with fire,” as unlike inert medications, PRP utilizes living cells which release millions of growth factors. These growth mediators exert their own endocrine, paracrine, and enzymatic effects, the complete influence of which still remains a mystery and only years of experience, in the times to come will unravel the absolute power of our “mighty dragon warrior.”
ACKNOWLEDGEMENTS
Nil.
Declaration of patient consent
Patient's consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
References
- Autologous platelet-rich plasma preparations: influence of nonsteroidal anti-inflammatory drugs on platelet function. Orthop J Sports Med. 2015;3:2325967115588896.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma in dermatology: boon or a bane? Indian J Dermatol Venereol Leprol. 2014;80:5-14.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma: an 'Elixir' for treatment of alopecia: personal experience on 117 patients with review of literature. Stem Cell Investig. 2017;4:64-4.
- [CrossRef] [Google Scholar]
- Platelet rich plasma in dermatology and aesthetic medicine. Our Dermatol Online. 2015;6:207-11.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225-8.
- [CrossRef] [Google Scholar]
- Identification of an optimal concentration of platelet gel for promoting 24 angiogenesis in human endothelial cells. Transfusion. 2009;49:771-8.
- [CrossRef] [Google Scholar]
- Principles and methods of preparation of platelet-rich plasma: a review and author's perspective. J Cutan Aesthet Surg. 2014;7:189-97.
- [CrossRef] [Google Scholar]
- The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: clinical and histomorphometric evaluation. Biomed Res Int. 2014;2014:760709.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638-46.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-96.
- [CrossRef] [Google Scholar]
- Classification of platelet concentrates (Platelet-Rich 25. Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4:3-9.
- [CrossRef] [Google Scholar]
- Principles and methods of preparation of platelet-rich plasma: a review and author's perspective. J Cutan Aesthet Surg. 2014;7:189.
- [CrossRef] [Google Scholar]
- Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater. 2008;84:415-21.
- [CrossRef] [Google Scholar]
- To compare different methods of preparation of platelet rich plasma (PRP) and to analyse the correlation between initial CBC and final yield of PRP. J Dermatol Cosmetol. 2019;3:89-92.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma for skin rejuvenation: facts, fiction, and pearls for practice. Facial Plast Surg Clin North Am. 2019;27:405-11.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma and its utility in the treatment of acne scars: a systematic review. J Am Acad Dermatol. 2019;80:1730-45.
- [CrossRef] [Google Scholar]
- Combined autologous platelet-rich plasma with microneedling verses microneedling with distilled water in the treatment of atrophic acne scars: a concurrent split-face study. J Cosmet Dermatol. 2016;15:434-43.
- [CrossRef] [Google Scholar]
- Therapeutic effect of microneedling and autologous platelet-rich plasma in the treatment of atrophic scars: a randomized study. J Cosmetol Dermatol. 2017;16:388-99.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma versus CROSS technique with 100% trichloroacetic acid versus combined skin needling and platelet rich plasma in the treatment of atrophic acne scars: a comparative study. Dermatol Surg. 2014;40:864-73.
- [Google Scholar]
- Split face comparative study of microneedling with PRP versus microneedling with vitamin C in treating atrophic post acne scars. J Cutan Aesthet Surg. 2014;7:209-12.
- [CrossRef] [Google Scholar]
- The efficacy of autologous platelet rich plasma combined with ablative carbon dioxide fractional resurfacing for acne scars: a simultaneous split-face trial. Dermatol Surg. 2011;37:931-8.
- [CrossRef] [Google Scholar]
- Autologous platelet rich plasma: topical versus intradermal after fractional ablative carbon dioxide laser treatment of atrophic acne scars. Dermatol Surg. 2014;40:152-61.
- [CrossRef] [Google Scholar]
- Fractional CO2 laser vs. fractional CO2 with topical platelet-rich plasma in the treatment of acne scars: a split-face comparison trial. J Cutan Aesthet Surg. 2017;10:136-44.
- [CrossRef] [Google Scholar]
- Activated platelet-rich plasma improves fat graft survival in nude mice: a pilot study. Dermatol Surg. 2011;37:619-25.
- [CrossRef] [Google Scholar]
- Application of platelet-rich plasma to fat grafting during plastic surgical procedures: Clinical and in vitro evaluation. Tissue Eng Part C Methods. 2009;15:625-34.
- [CrossRef] [Google Scholar]
- Photodynamic photorejuvenation of the face with a combination of microneedling, red light, and broadband pulsed light. Lasers Surg Med. 2010;42:150-9.
- [CrossRef] [Google Scholar]
- Evaluation of the effect of platelet-rich plasma on post-burn scars. Open Access J Surg. 2017;5:555-660.
- [CrossRef] [Google Scholar]
- Fat graft, laser CO2 and platelet-rich plasma synergy in scars treatment. J Med Life. 2013;6:430-3.
- [Google Scholar]
- A pilot study of making new skin and appendages for post burn scars through a unique cocktail of platelet rich plasma, ablative pixel erbium: Yag laser and microneedling radiofrequency. SCRI [Internet] 2018:2. [cited 2021 Mar 17]
- [CrossRef] [Google Scholar]
- Treatment of traumatic scars using fat grafts mixed with platelet-rich plasma, and resurfacing of skin with the 1540 nm nonablative laser. Clin Exp Dermatol. 2012;37:55-61.
- [CrossRef] [Google Scholar]
- Correction of scars by autologous fat graft and platelet rich plasma (PRP) Acta Med Mediterr. 2012;28:99-100.
- [Google Scholar]
- Application of autologous platelet-rich plasma (PRP) on wound healing after cesarean section in high-risk patients. Iran Red Crescent Med J. 2016;18:e34449.
- [CrossRef] [Google Scholar]
- Post traumatic scar with repeated trophic ulcers treated with a cocktail of ablative pixel Erbium YAG laser, PRP therapy and autologous fat transplant-a novel case report of terminal nerve ending regeneration through regenerative surgery. J Mol Genet Med [Internet] 2017:11. [cited 2021 Mar 17]
- [CrossRef] [Google Scholar]
- Utility of platelet-rich plasma for treatment of striae distensae: a current exploration. J Cosmet Dermatol. 2021;20:437-41.
- [CrossRef] [Google Scholar]
- Comparison between the efficacy and safety of platelet-rich plasma vs. microdermabrasion in the treatment of striae distensae: Clinical and histopathological study. J Cosmet Dermatol. 2015;14:336-46.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma versus tretinoin in treatment of striae distensae: a comparative study. Dermatol Surg. 2018;44:697-704.
- [CrossRef] [Google Scholar]
- Clinical and immunohistochemical comparative study of the efficacy of carboxytherapy vs. platelet-rich plasma in treatment of stretch marks. J Cosmet Dermatol. 2018;17:1008-015.
- [CrossRef] [Google Scholar]
- Efficacy of intradermal radiofrequency combined with autologous platelet-rich plasma in striae distensae: a pilot study. Int J Dermatol. 2012;51:1253-8.
- [CrossRef] [Google Scholar]
- Treatment of striae distensae combined enhanced penetration platelet-rich plasma and ultrasound after plasma fractional radiofrequency. J Cosmet Laser Ther. 2012;14:272-6.
- [CrossRef] [Google Scholar]
- Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. Int J Biochem Cell Biol. 2004;36:1482-91.
- [CrossRef] [Google Scholar]
- Regression of melasma with platelet-rich plasma treatment. Ann Dermatol. 2014;26:401-2.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma is a useful therapeutic option in melasma. J Dermatol Treat. 2019;30:396-401.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma: the journey so far! Indian Dermatol Online J. 2020;11:685-92.
- [Google Scholar]
- Response to intradermal autologous platelet rich plasma injection in refractory dermal melasma: report of two cases. J Health Translat Med. 2015;18
- [Google Scholar]
- Use of platelet-rich plasma to suspend noncultured epidermal cell suspension improves repigmentation after autologous transplantation in stable vitiligo: a double-blind randomized controlled trial. Int J Dermatol. 2019;58:472-6.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma versus combined fractional carbon dioxide laser with platelet-rich plasma in the treatment of vitiligo: a comparative study. Clin Cosmet Investig Dermatol. 2018;11:551-9.
- [CrossRef] [Google Scholar]
- Laser ablation of the recipient area with platelet-rich plasma-enriched epidermal suspension transplant in vitiligo aurgery: a pilot study. Dermatol Surg. 2019;45:83-9.
- [CrossRef] [Google Scholar]
- Intralesional autologous platelet rich plasma therapy in chronic nonhealing cutaneous ulcers: an interventional study from a tertiary care centre in North Kerala. Int J Res Dermatol. 2019;5:116.
- [CrossRef] [Google Scholar]
- Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002;18:27-34.
- [CrossRef] [Google Scholar]
- Use of platelet rich plasma gel on wound healing: a systematic review and meta-analysis. Eplasty. 2011;11:e38.
- [Google Scholar]
- Efficacy of autologous platelet-rich plasma in the treatment of chronic nonhealing leg ulcers. J Plastic Aesthetic Res. 2014;1:65-9.
- [CrossRef] [Google Scholar]
- Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci. 2017;24:16.
- [CrossRef] [Google Scholar]
- Autologous platelet-rich fibrin matrix in non-healing trophic ulcers in patients with Hansen's disease. J Cutan Aesthet Surg. 2017;10:3-7.
- [CrossRef] [Google Scholar]
- Refractory lipodermatosclerosis treated with intralesional platelet-rich plasma. J Am Acad Dermatol. 2011;65:e157-8.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma: applications in dermatology In: Actas Dermo-Sifiliográficas (English Edition). Vol 106. 2015. p. :104-11.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma combined with fractional laser therapy for skin rejuvenation. Dermatol Surg. 2012;38:623-30.
- [CrossRef] [Google Scholar]
- Infraorbital rejuvenation using PRP (platelet-rich plasma): a prospective, randomized, split-face trial. J Am Acad Dermatol. 2013;68:AB24.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma (PRP) for the treatment of vulvar lichen sclerosus in a premenopausal woman: a case report. Case Rep Womens Health. 2018;18:e00062.
- [CrossRef] [Google Scholar]
- New surgical approach to lichen sclerosus of the vulva: the role of adipose-derived mesenchymal cells and platelet-rich plasma in tissue regeneration. Plast Reconstr Surg. 2010;126:210e-1e.
- [CrossRef] [Google Scholar]
- Intradermal injection of autologous platelet-rich plasma for the treatment of vulvar lichen sclerosus. J Am Acad Dermatol. 2017;76:158-60.
- [CrossRef] [Google Scholar]
- Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42:1243-7.
- [CrossRef] [Google Scholar]
- The effect of autologous activated platelet-rich plasma injection on female pattern hair loss: A randomized placebo-controlled study. J Cosmet Dermatol. 2018;17:47-53.
- [CrossRef] [Google Scholar]
- Evaluation of not-activated and activated PRP in hair loss treatment: role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci. 2017;18:408.
- [CrossRef] [Google Scholar]
- The effect of platelet-rich plasma in hair regrowth: a randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4:1317-23.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma on female androgenetic alopecia: tested on 10 patients. J Cosmet Dermatol. 2019;18:59-64.
- [CrossRef] [Google Scholar]
- Autologous platelet rich plasma as a treatment of male androgenetic alopecia: study of 14 cases. J Clin Exp Dermatol Res. 2015;6:292.
- [Google Scholar]
- The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43:658-670.
- [CrossRef] [Google Scholar]
- Mechanical and controlled PRP injections in patients affected by androgenetic alopecia. J Vis Exp 2018:56406.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma in androgenic alopecia: myth or an effective tool. J Cutan Aesthet Surg. 2014;7:107-10.
- [CrossRef] [Google Scholar]
- The wonder tool platelet rich plasma in cosmetic dermatology, trichology and hair transplant. In: Vereecken P, ed. Dermatologic Surgery and Procedures; In Tech. 2018. p. :194-206.
- [Google Scholar]
- Fibroblast growth factors stimu-late hair growth through ß-catenin and Shh expression in C57BL/6 mice. Biomed Res Int. 2015;2015:73017.
- [CrossRef] [Google Scholar]
- Fibroblast growth factor and epi-dermal growth factor in hair development. J Invest Dermatol. 1993;101:106S-13S.
- [CrossRef] [Google Scholar]
- The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577-89.
- [CrossRef] [Google Scholar]
- Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg. 2011;37:1721-9.
- [CrossRef] [Google Scholar]
- Paus R: Do we need hair follicle stem cells, hair follicle neogenesis to cure common hair loss disorders? Hair Transplant Forum Int. 2008;18:89-90.
- [CrossRef] [Google Scholar]
- A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. 2018;4:18-24.
- [CrossRef] [Google Scholar]
- Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38:1040-6.
- [CrossRef] [Google Scholar]
- Effects of insulin and insulin-like growth factors on cultured human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J Invest Dermatol. 1994;102:857-61.
- [CrossRef] [Google Scholar]
- Beyond goosebumps: Does the arrector pili muscle have a role in hair loss? Int J Trichology. 2014;6:88-94.
- [CrossRef] [Google Scholar]
- The arrector pili muscle and the follicular unit of the scalp: a microscopic anatomy study. Dermatol Surg. 2002;28:800-3.
- [CrossRef] [Google Scholar]
- A mechanistic model of platelet-rich plasma treatment for androgenetic alopecia. Dermatol Surg. 2016;42:1335-9.
- [CrossRef] [Google Scholar]
- Progress in the use of platelet-rich plasma in aesthetic and medical dermatology. J Clin Aesthet Dermatol. 2020;13:28-35.
- [Google Scholar]
- The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconst Surg. 2006;118:1458-66.
- [CrossRef] [Google Scholar]
- Outcome of intra-operative injected platelet-rich plasma therapy during follicular unit extraction hair transplant: a prospective randomised study in forty patients. J Cutan Aesthet Surg. 2016;9:157-64.
- [CrossRef] [Google Scholar]
- Systematic review of platelet-rich plasma use in androgenetic alopecia compared with minoxidil®, finasteride®, and adult stem cell-based therapy. Int J Mol Sci. 2020;21:2702.
- [CrossRef] [Google Scholar]
- Original article: Platelet-rich plasma with microneedling in androgenetic alopecia along with dermoscopic pre-and post-treatment evaluation. J Cosmet Dermatol. 2018;17:313-8.
- [CrossRef] [Google Scholar]
- A study of the efficacy of platelet-rich plasma in the treatment of androgenetic alopecia in males. Indian J Dermatol Venereol Leprol. 2017;83:412.
- [CrossRef] [Google Scholar]
- Efficacy of platelet-rich plasma in treatment of androgenic alopecia. Asian J Transfus Sci. 2015;9:159-62.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma in combination with 5% minoxidil topical solution and 1 mg oral finasteride for the treatment of androgenetic alopecia: a randomized placebo-controlled, double-blind, half-head study. Dermatol Surg. 2018;44:126-30.
- [CrossRef] [Google Scholar]
- A randomized, double-blind, placebo-and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br J Dermatol. 2013;169:690e4.
- [CrossRef] [Google Scholar]
- Basic fibroblast growth factor promotes melanin synthesis by melanocytes. Arch Dermatol Res. 1996;288:633e5.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma in the treatment of alopecia areata: a review. J Invest Dermatol Symp Proc. 2020;20:S45-9.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:67.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma: growth factors and pro-and anti-inflammatory properties. J Periodontol. 2007;78:661e9.
- [CrossRef] [Google Scholar]
- Selective expression of chemokine monokine induced by interferon-gamma in alopecia areata. J Invest Dermatol. 2003;121:933e5.
- [CrossRef] [Google Scholar]
- Successful treatment of corticosteroid-resistant ophiasis-type alopecia areata (AA) with platelet-rich plasma (PRP) JAAD Case Rep. 2015;1:305-7.
- [CrossRef] [Google Scholar]
- A comparative study of therapeutic response to intralesional injections of platelet-rich plasma versus triamcinolone acetonide in alopecia areata. Indian Dermatol Online J. 2020;11:920-4.
- [Google Scholar]
- Platelets rich plasma versus minoxidil 5% in treatment of alopecia areata: a trichoscopic evaluation. Dermatol Ther. 2017;30 Epub 2016 Oct 28
- [CrossRef] [Google Scholar]
- Using automated microneedling with platelet rich plasma for treating cicatricialalopecia, recalcitrant alopecia areata and traction alopecia, casereport. J Am Acad Dermatol. 2016;74;5:140.
- [CrossRef] [Google Scholar]
- Use of platelet-rich plasma in cicatricial alopecia. Dermatol Surg. 2019;45:979-81.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma for treatment resistant frontal fibrosing alopecia: a case report. Dermatol Ther. 2019;23:e13072.
- [CrossRef] [Google Scholar]
- Successful hair transplant outcome in cicatricial lichen planus of the scalp by combining scalp and beard hair along with platelet rich plasma. J Cutan Aesthet Surg. 2016;9:51-5.
- [CrossRef] [Google Scholar]
- Follicular unit extraction (FUE) hair transplantation in combination with platelet rich plasma for the treatment of scarring alopecia: a case series. Arch Clin Med Case Rep. 2019;3:299-308.
- [CrossRef] [Google Scholar]
- Intramatricial platelet rich plasma therapy: a novel treatment modality in refractory nail disorders. Dermatol Ther. 2019;32:e12831.
- [CrossRef] [Google Scholar]
- Hair organ regeneration via the bioengineered hair follicular unit transplantation. Sci Rep. 2012;2:424.
- [CrossRef] [Google Scholar]
- Epidermal stem cells and skin tissue engineering in hair follicle regeneration. World J Stem Cells. 2015;7:711-27.
- [CrossRef] [Google Scholar]
- Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- [CrossRef] [Google Scholar]
- Autologous adipose derived stem cell versus platelet rich plasma injection in the treatment of androgentic alopecia: efficacy, side effects and safety. J Clin Exp Dermatol Res. 2018;9:1-7.
- [Google Scholar]
- Use of stem cells in intervention dermatology and trichology: a hope. J Stem Cell Res Dev Ther 2019:S1004.
- [CrossRef] [Google Scholar]
- Irreversible blindness following periocular autologous platelet-rich plasma skin rejuvenation treatment. Ophthalmic Plast Reconstr Surg. 2017;33:S12-6.
- [CrossRef] [Google Scholar]






