Translate this page into:
Plant-based paraben-free moisturizer, Venusia Max Cream, is a nonirritant

*Corresponding author: Dr Monil Yogesh Neena Gala, Department of Medical Affairs, Dr. Reddy’s Laboratories Ltd., Global Generics - India, Ameerpet, Hyderabad, Telangana, India. monil.yogesh@drreddys.com
-
Received: ,
Accepted: ,
How to cite this article: Gala MY, Muchhala SS, Charugulla SN, Rathod R, Mane A, Pandit S, et al. Plant-based paraben-free moisturizer, Venusia Max Cream, is a nonirritant. CosmoDerma 2022;2:25.
Abstract
Objectives
To ensure that the marketed product is irritant-free, extensive premarket clinical testing of cosmetic products is necessary. Therefore, the present study evaluated the skin irritation reactions of the test product Venusia Max Cream-Paraben-free using a patch test.
Material and methods
This single group, blinded, controlled trial was conducted to compare our test product with negative saline and positive SLS control in healthy human subjects aged between 18 and 53 years (mean age of 30.93 years) having Fitzpatrick skin phototype classification III-V. During an initial phase, a patch dipped in test product, negative and positive control were applied under occlusion to the upper arm of participants and removed after 24 hours. Clinical evaluation of skin reactions (erythema, edema, dryness, and scaling wrinkling) in the area of the test product, negative and positive control after 24 hours of patch removal and then were scored based on the Draize scale.
Results
A total of 30 subjects were initiated and completed the study. Scoring for skin irritation (erythema/dryness/wrinkles/edema) of the subjects were evaluated based on Draize’s scale between test product, positive, and negative control. The combined mean score, i.e., of erythema/dryness/wrinkles, and edema was 0.00 in test product and negative control whereas 2.60 in the positive control. No adverse events or intolerances were reported due to the test product.
Conclusion
Venusia Max Cream-Paraben-free was dermatologically tested and found to be nonirritant.
Keywords
Plant-based moisturizer
Paraben-free moisturizer
Skin irritation reactions
Patch test
INTRODUCTION
Millions of people suffer from skin ailments like itching, erythema, scaling, edema, and dryness.[1] Overall, 21-87% of skin disorders have been reported in the general population of developing countries by the World Health Organization (WHO).[2] In India, approximately 32.4% of the total population reported to have “sensitive” or “very sensitive” skin and among them, 27.9% are men and 36.7% are women.[3]
Approximately, 75% of the young population use moisturizers daily[4]; moisturizers can prevent skin irritation[5-7] and some of them have a healing effect when applied on inflamed skin or to a skin rash.[8]
A previous in vivo study demonstrated that glycerin stimulated enzymes were responsible for proper desquamation and thus reduced the irritation potential.[9] Further, dimethicone is an emollient used to soften and moisturize the skin; it reduces itching and flaking and forms a protective layer against irritants.[10] Other than these two main components, shea butter demonstrated its beneficial role in patients with atopic dermatitis or eczema.[11,12] Another study showed that the addition of glycerin and cocoa butter in the moisturizer benefits against skin irritation.[13,14] Likewise, mango butter demonstrated skin irritation potential in a foot care cream.[15] Venusia® Max Cream (Paraben-free) referred to as the test product hereafter is a moisturizing cream that combines these plant kinds of butter like cocoa, shea, mango, and aloe into one single cream. In the present study, we evaluated our test product for its skin irritant potential by patch tests.
MATERIAL AND METHODS
Study design and setting
This was a nonrandomized, single group, blinded, controlled trial conducted in January 2020 in Maharashtra, India. Participants’ authorization forms were obtained before enrollment into the study. The study was approved by Institutional Ethics Committee and conducted in compliance with the protocol and principles of the Declaration of Helsinki,[16] its amendments in conformity with the Good Clinical Practices (GCP) principles, and in compliance with the methods of test for safety evaluation of cosmetics-third revision (IS 4011:2018).[17]
Healthy individuals aged between 18 and 53 years having skin phototypes III to V and willing to avoid ultraviolet (UV) exposure, both natural or artificial, unnecessary water contact (i.e., swimming), or activity that causes sweating (i.e., exercise, sauna) were included in the study. Lactating or pregnant women and participants who have jobs involving water contact or causing perspiration or having scars, tattoos, dermatological infections, or pathology on the area to be studied were excluded. Participants having any hypersensitivity, allergy, and clinically significant systemic/cutaneous disease or medical issues related to either systemic/hormonal or topical (past 3 months) which may interfere with the study protocol were excluded.
Study plan and outcome
This study was conducted to evaluate Venusia Max Cream (Paraben-free) a plant-based moisturizing cream test product (Dr. Reddy’s Laboratories Ltd., India) for 48 hours using a patch test under a constant artificial daylight source. Positive (1% Sodium lauryl sulfate; SLS) and negative (0.9% saline) controls were compared to evaluate the skin irritation potential of the test product in the participants. Patch dipped in 0.04 g of the test product and positive and negative controls were applied occlusively on the participants’ upper arm at 0 hours and were removed after 24 hours. Clinical examination and scoring of the test area were conducted after 48 hours. Subjects who had reactions on the positive control site were called for a follow-up after 1 week to confirm the recovery. Any adverse reactions after the test product application were evaluated throughout the study. Grading of skin irritation (erythema, dryness, scaling wrinkles, and edema) was done based on Draize scale rating as presented in Table 1. The Draize scale was used to evaluate and score by observing skin erythema, edema, and crust formation after the application of the test product. The mean score was calculated by adding the score for erythema with the score for edema.
| Score for erythema/dryness/wrinkles | Score for edema |
|---|---|
| 0 = No reaction | 0 = No reaction |
| 1 = Very slight erythema/dryness with shiny appearance | 1 = Very slight edema |
| 2 = Slight erythema/dryness/wrinkles | 2 = Slight edema |
| 3 = Moderate erythema/dryness/wrinkles | 3 = Moderate edema |
| 4 = Severe erythema/wrinkles/scales | 4 = Severe edema |
Statistical methods
The sample size was based on the methods of test for safety evaluation of cosmetics-third revision (IS 4011:2018), which states that at least 24 participants are required for the study. The analysis was based on the combined mean score obtained after 48 hours, which is calculated by the following formula:
Mean score for irritation = Total score [erythema (A) + edema (O)] for each subject
Total number of subjects
No other statistical analysis was carried out. Grading of the mean score for irritation was done based on scale rating as presented in Table 2.
| Mean score | Classification |
|---|---|
| <2.0/8.0 | Non-Irritant |
| 2.0/8.0-4.0/8.0 | Mildly Irritant |
| >4.0/8.0 | Irritant |
RESULTS
A total of 30 participants (15 men and 15 women) were enrolled in the study. The mean ± SD age of the recruited participants was 30.93 ± 11.44 years (range: 18-53 years). All the participants completed the study.
Scoring for irritation of the participants was assessed between test product, positive and negative control where the scoring was based on Draize’s scale [Table 1]. The combined mean score (erythema, dryness, wrinkles + edema) was 0.00 in test product and negative control compared to 2.60 in the positive control [Figure 1].
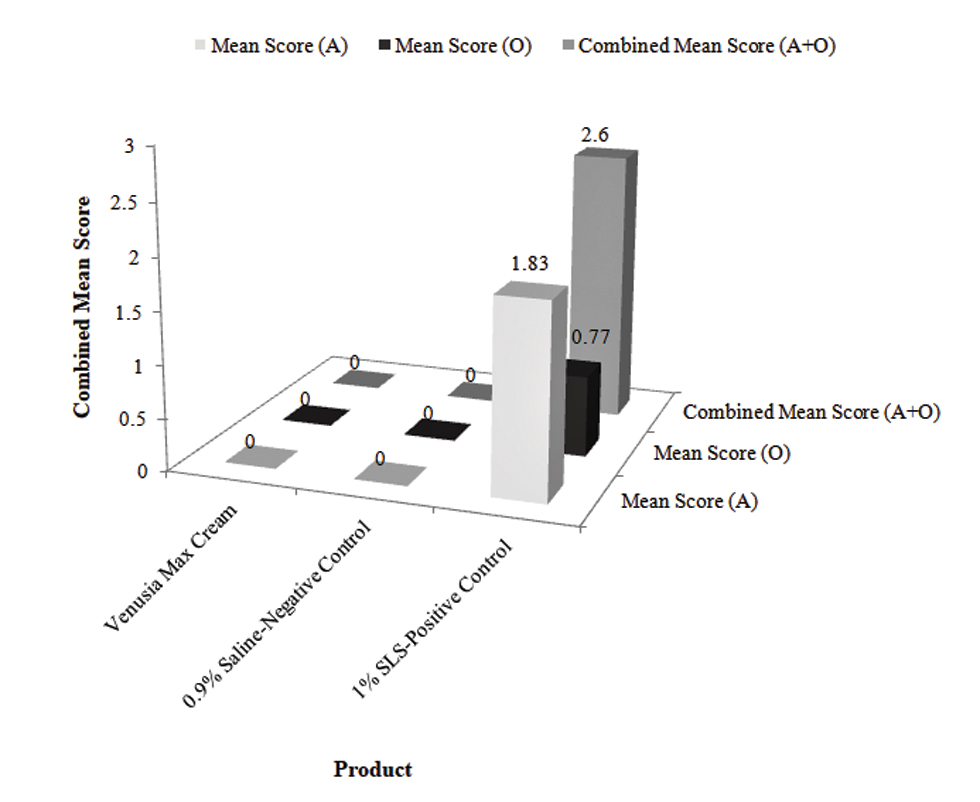
- Combined mean irritation scores of test products, positive, and negative control.
Safety/adverse events
There were no product-related adverse events or intolerances reported during the study period.
DISCUSSION
An exposure to ingredients of beauty products can lead to several skin reactions, mainly allergy and irritation responses.[18] The current study evaluated the combined mean score for irritation (erythema, dryness, wrinkles + edema) of the test product, positive, and negative controls. This mean score was based on the classification where <2.0 was nonirritant, 2.0-4.0 was mildly irritant and >4.0 was irritant [Table 2]. The combined mean score for irritation was 0.00, which was in the range of non-irritant in the case of both test product and negative control whereas 2.60 in the case of positive control demonstrating that the tested product is nonirritant to the human skin.
Further, the test product is paraben-free, which is the second most common allergen found in moisturizers and is known to permeate and accumulate in the skin causing paraben-related sensitization and allergy.[16,19] In addition, some moisturizers may have possible adverse effects like ICD, ACD, subjective irritation, cosmetic acne, occlusive folliculitis, contact urticaria, poisoning in burn patients, intoxication, photosensitive eruptions, and photo melanosis.[17] However, none of these adverse effects were observed after the application of the test product.
Though, there are few limitations associated with the present study, including the small sample size, short duration of the study, and individuals with skin phototype III-V making the generalize ability of the results difficult for the individuals with deep skin pigmentation; however, the study provides real-world evidence of non-irritant plant-based paraben-free moisturizer in Indian population.
CONCLUSION
Based on our study findings, Venusia® Max Cream (paraben-free) appears to be a well-tolerated, nonirritant moisturizer, suitable for topical use in individuals with Fitzpatrick skin phototype III-V.
Venusia® Max Cream is a plant-based paraben-free moisturizer providing long-lasting skin hydration and is suitable for individuals with dry skin.
Acknowledgement
The author acknowledges Knowledge Isotopes Pvt. Ltd. for medical writing assistance.
ETHICAL APPROVAL
The study was approved by Institutional Ethics Committee and conducted in compliance with the protocol and principles of the Declaration of Helsinki.
Declaration of patient consent
Participants’ authorization forms were obtained before enrollment into the study.
Financial support and sponsorship
The study was funded by Dr. Reddy’s Laboratories Pvt. Ltd., Hyderabad, India.
Conflicts of interest
All the contributing authors are employees of Dr. Reddy’s Laboratories.
References
- Ability of moisturizers to reduce dry skin and irritation and to prevent their return. Int J Cosmet Sci. 2006;28:232.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and demographic profile of skin disorders in school-going children of urban and rural Jaipur. Int J Contemp Med Res. 2019;6:G6-G10.
- [CrossRef] [Google Scholar]
- Sensitive skin in the Indian population: An epidemiological approach. Front Med. 2019;6
- [CrossRef] [PubMed] [Google Scholar]
- So moisturizers may cause trouble! Int J Dermatol. 2001;40:12-3.
- [CrossRef] [PubMed] [Google Scholar]
- Moisturizers: Reality and the skin benefits. Dermatol Ther. 2012;25:229-33.
- [CrossRef] [Google Scholar]
- Treatments improving skin barrier function. Skin Barrier Function. 2016;49:112-22.
- [CrossRef] [PubMed] [Google Scholar]
- Preventive and therapeutic effects of a moisturizer. An experimental study of human skin. Acta Derm Venereol. 1997;77:335-7.
- [CrossRef] [Google Scholar]
- The effect of glycerol and humidity on desmosome degradation in stratum corneum. Arch Dermatol Res. 1995;287:457-64.
- [CrossRef] [Google Scholar]
- Irritant contact dermatitis: Mechanisms to repair. Clin Exp Dermatol. 2014;5:2.
- [CrossRef] [Google Scholar]
- Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-25.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study investigating the efficacy of botanical anti-inflammatory agents in an OTC eczema therapy. J Cosmet Dermatol. 2016;15:117-9.
- [CrossRef] [Google Scholar]
- Cocoa bioactive compounds: Significance and potential for the maintenance of skin health. Nutrients. 2014;6:3202-13.
- [CrossRef] [PubMed] [Google Scholar]
- Formulation and evaluation of exotic fat based cosmeceuticals for skin repair. Indian J Pharm Sci. 2008;70:539-42.
- [CrossRef] [PubMed] [Google Scholar]
- Consumer preferences, product characteristics, and potentially allergenic ingredients in best-selling moisturizers. JAMA Dermatol. 2017;153:1099-105.
- [CrossRef] [PubMed] [Google Scholar]
- The role of moisturizers in addressing various kinds of dermatitis: A review. Clin Med Res. 2017;15:75-87.
- [CrossRef] [PubMed] [Google Scholar]
- Specific barrier response profiles after experimentally induced skin irritation in vivo. Contact Derm. 2018;79:59-66.
- [CrossRef] [PubMed] [Google Scholar]
- Moisturizer allergy: Diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38.
- [PubMed] [Google Scholar]






