Translate this page into:
Pemphigus vulgaris post-coronavirus disease-19 infection: A case report and review of literature

*Corresponding author: Deepthi Konda, Department of Dermatology, All India Institute of Medical Sciences, Aushapur, Telangana, India. knddeepthi@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Konda D, Nayak VT, Palo S. Pemphigus vulgaris post-coronavirus disease-19 infection: A case report and review of literature. CosmoDerma. 2025;5:23. doi: 10.25259/CSDM_217_2024
Abstract
Pemphigus vulgaris is an autoimmune blistering disorder caused by autoantibodies directed against desmogleins. Severe acute respiratory syndrome coronavirus 2 is known to trigger autoimmune skin reactions. We report a case of pemphigus vulgaris developing in a patient, around 35 days after the coronavirus disease-19 infection.
Keywords
Coronavirus disease
Infection
Pemphigus
INTRODUCTION
Pemphigus vulgaris is an autoimmune blistering disorder characterized by painful blisters and/or erosions on skin and mucosae. It is caused by autoantibodies directed against desmogleins. Various environmental factors including viruses such as herpes simplex virus and herpes zoster virus are known to induce the autoimmune process and cause pemphigus in genetically susceptible individuals.[1] Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that mainly affects the respiratory system is also known to trigger autoimmune musculoskeletal and skin reactions. There are many reports showing the development of pemphigus vulgaris after coronavirus disease-19 (COVID-19) vaccination. However, there are only a few cases of pemphigus vulgaris after COVID infection reported so far in the literature.[1-5] Hence, we report this case of pemphigus vulgaris developing in a patient, around 35 days after onset of symptoms of COVID-19 infection.
CASE REPORT
A 45-year-old otherwise healthy male patient attended our Dermatology outpatient department with painful oral erosions of a two-month duration and skin erosions of a one-month duration. The oral erosions were initially few in number, which later spread to involve the entire buccal mucosa, palate, gingival, and labial mucosa, leading to extreme difficulty in eating, swallowing, and even speech. Later, he developed fluid-filled blisters over the abdomen which ruptured to form painful non-healing erosions [Figure 1]. The lesions gradually spread to involve the entire trunk, back, upper and lower limbs, and scalp covering around 70% of the body surface area. The patient had no history of any drug intake before the onset of lesions. After the development of these lesions, he was started on oral prednisolone 50 mg and oral cyclophosphamide 50 mg for three weeks with not much improvement before he visited our hospital. About a month before the development of oral erosions, the patient had a history of fever with sore throat which was diagnosed to be COVID-19 infection by reverse transcriptase polymerase chain reaction. It was categorized as mild COVID infection and the patient was treated at home with antipyretics and supportive treatment. Cutaneous examination showed very few intact blisters. Multiple erosions, most of them with overlying crusting, were seen covering the entire chest, abdomen, back, thighs, and arms, and part of the face and genitalia. Scalp showed thick crusted erosions. Nikolsky and peri Nikolsky’s sign were positive. Oral examination showed multiple erosions over bilateral buccal mucosa, tongue, palate, and gingival mucosa with mild crusting over the lips. His vitals were stable and baseline blood investigations were within normal limits. An intact blister was taken for biopsy and histopathology showed suprabasal cleft with intra-epidermal vesicle formation and tonsil-shaped basal layer characteristic of pemphigus vulgaris [Figure 2]. There was patchy perivascular and peri-adnexal infiltration of lymphocytes in the upper dermis. The cavity of the vesicle showed eosinophils and acantholytic keratinocytes. The patient did not give consent for direct immunofluorescence due to financial constraints. The patient was started on the first cycle of dexamethasone cyclophosphamide pulse (DCP) therapy (dexamethasone 100 mg in 5% dextrose given intravenously over 3 h on three consecutive days with injection cyclophosphamide 500 mg added to it on the second day). After three days, he was started on oral prednisolone 40 mg and oral cyclophosphamide 50 mg along with proper oral care measures (gentle cleaning with soft bristles baby brush, gargling before and after food). Two weeks later, lesions started re-epithelization and most of the erosions healed with post-inflammatory hyperpigmentation, and only a few small erosions were active [Figure 3]. Hence, the patient was discharged and advised to continue the same treatment. The patient was reviewed after one month but despite proper counseling, he refused to take the next cycle of DCP pulse as his lesions healed completely. Hence, he was maintained only on oral prednisolone and cyclophosphamide. Oral prednisolone was slowly tapered and stopped over a period of six months. Oral cyclophosphamide 50 mg was continued for seven months with no exacerbation of lesions. We lost the follow-up after a period of seven months.
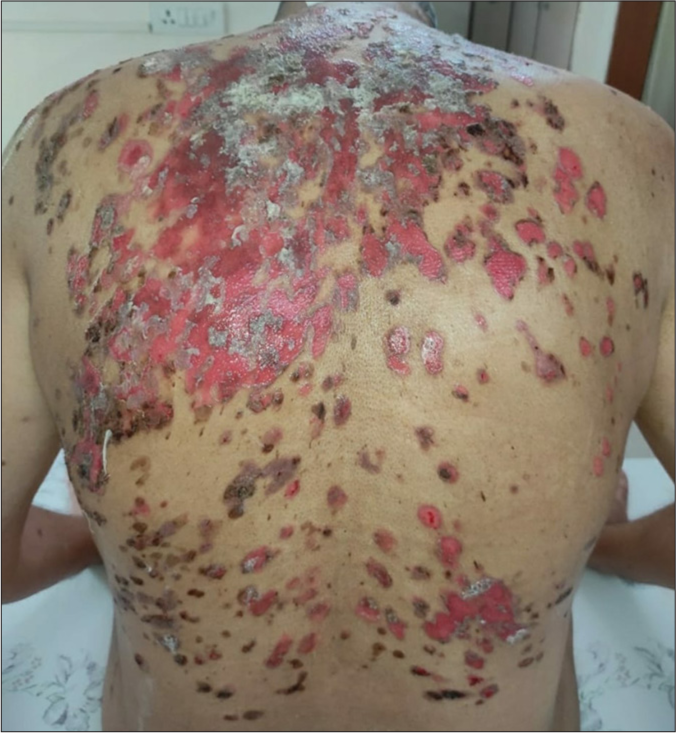
- Multiple erosions with erythematous base and few crusted lesions over the back.
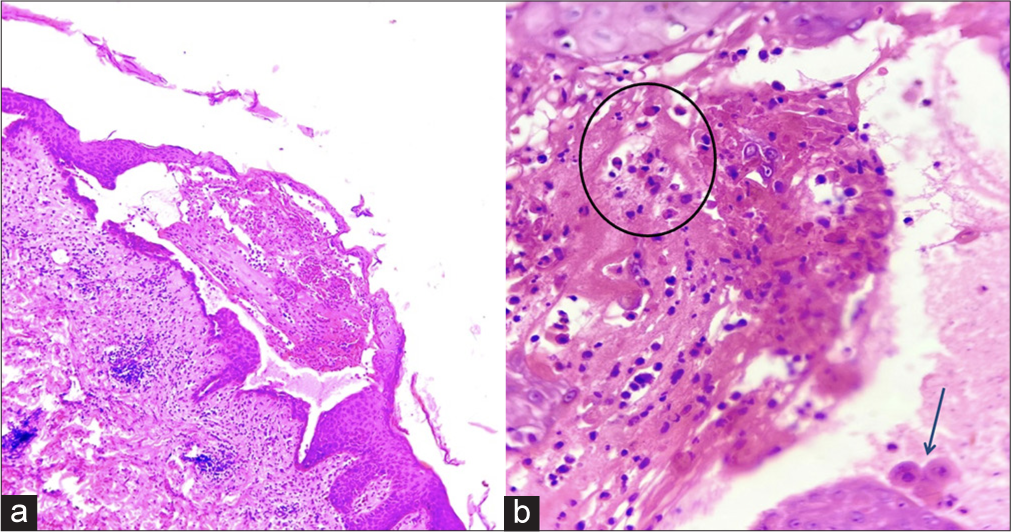
- (a) Skin biopsy showing characteristic supra-basal split with intra-epidermal vesicle formation. Superficial dermis shows moderate degree of lymphocytic infiltrate (Hematoxylin and eosin; ×100), (b) The vesicle content shows eosinophils (circled) and few acantholytic keratinocytes (arrow) (Hematoxylin and eosin; ×400).
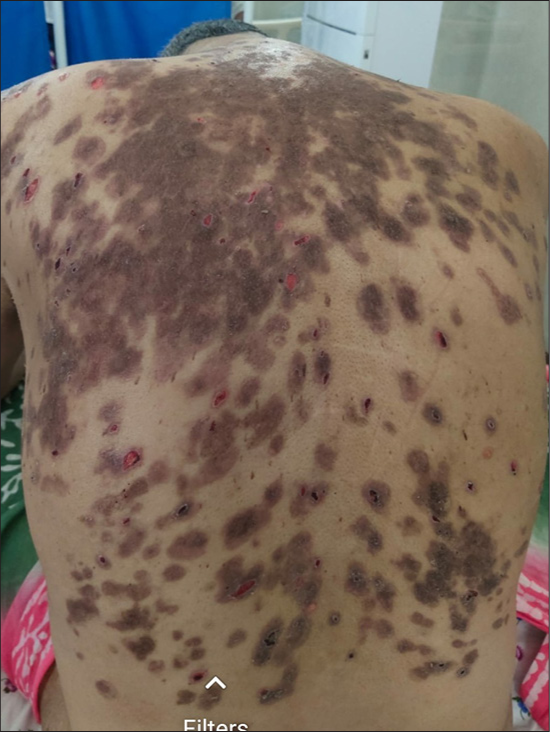
- Two-week post-treatment, healed erosions with post-inflammatory hyperpigmentation and few small active erosions over the back.
DISCUSSION
COVID-19 is a multisystemic disease affecting almost all the organs of the body including the skin. SARS-CoV-2 virus is known to cause various cutaneous manifestations such as urticarial lesions, chilblain-like lesions, vesicular eruptions, maculopapular rash, and livedo. Many autoimmune reactions have been described post-COVID infection of which the most widely studied are Guillain–Barre syndrome and Kawasaki disease.[6] Skin autoimmune phenomena related to SARS-CoV-2 have also been studied. IgG-mediated autoimmunity is known to play a role in its pathogenesis. The human proteome and SARS-CoV-2 viral elements are believed to have structural homology resulting in cross-reactivity and thus production of autoantibodies. SARSCoV-2 also has a higher potential to trigger autoimmune disorders due to its hyperstimulation of the immune system and excess production of pro-inflammatory cytokines.[7] De Medeiros et al.[1] for the first time in 2021 reported a case of pemphigus vulgaris as an autoimmune phenomenon developing after COVID-19 infection. Since then, only a few cases of pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid have been reported after COVID-19 infection.[1-5] Coronavirus like any other virus can trigger an autoimmune process leading to the development of autoimmune blistering disorders.[8] Three mechanisms were proposed by De Medeiros et al.[1] by which viruses can induce an autoimmune process and thus develop the lesions of pemphigus vulgaris: First, molecular mimicry – structural similarity between viral and host antigens leads to autoantigen identification. Second, bystander activation – cytolysis process due to virus leads to exposure of self-antigens to the immune system, and third, epitope spreading – release of self-antigens due to virus, in turn, leads to exposure of endogenous epitopes which can result in the development of autoimmune response. Coronavirus can induce pemphigus lesions by any one of the above mechanisms or a combination of them.[8-10] However, the possibility of pemphigus vulgaris developing coincidentally after COVID-19 infection without any relation to the virus cannot be ruled out.
Pastukhova and Ghazawi[7] reported a case of pemphigus vulgaris two weeks after testing positive for COVID-19 infection by rapid antigen testing indicating that viruses can initiate the autoimmune process and thus lead to the development of autoimmune blistering disorders like pemphigus vulgaris. However, in our case, lesions of pemphigus developed 35 days after the onset of symptoms of COVID-19 infection. This delay in the development of lesions can be due to the need for strong permanent autoimmunity to develop after an initial inflammatory response by the coronavirus. As in our case, De Medeiros et al.[1] also reported the development of pemphigus vulgaris lesions 40 days after the coronavirus infection. Certain case reports also show the development of pemphigus lesions after COVID-19 vaccination.[4,5,9] The messenger ribonucleic acid of the vaccine upregulates the production of interleukins (ILs) IL-4, IL-17, IL-21, and tumor necrosis factor that mediates the autoimmune process and thus results in pemphigus vulgaris.
CONCLUSION
Coronavirus can trigger the autoimmune response by molecular mimicry or bystander activation or epitope spreading or combination, leading to the development of lesions of pemphigus vulgaris. Moreover, we report one such case of pemphigus vulgaris developing after the COVID infection thus adding to the early basket of such reports.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Pemphigus vulgaris after COVID-19: A case of induced autoimmunity. SN Compr Clin Med. 2021;3:1768-72.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus vulgaris possibly associated with COVID-19 infection. Cureus. 2023;15:e33897.
- [CrossRef] [Google Scholar]
- Pemphigus vulgaris after COVID-19 infection and vaccination. J Am Acad Dermatol. 2022;87:709-10.
- [CrossRef] [PubMed] [Google Scholar]
- New-onset pemphigus vulgaris and pemphigus foliaceus following COVID-19 infection and vaccination, systematic review of case reports and a causal hypothesis. J Eur Acad Dermatol Venereol. 2023;37:e1256-60.
- [CrossRef] [PubMed] [Google Scholar]
- De novo pemphigus vulgaris and pemphigus foliaceus development following COVID-19 infection and vaccination: Matched case-control study. J Eur Acad Dermatol Venereol. 2024;38:e374-6.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review on mucocutaneous presentations after COVID-19 vaccination and expert recommendations about vaccination of important immune-mediated dermatologic disorders. Dermatol Ther. 2022;35:e15461.
- [CrossRef] [Google Scholar]
- New-onset of pemphigus following COVID-19 infection: A case report. SAGE Open Med Case Rep. 2024;12
- [CrossRef] [PubMed] [Google Scholar]
- Impact of COVID-19 on autoimmune blistering diseases. Clin Dermatol. 2021;39:359-68.
- [CrossRef] [PubMed] [Google Scholar]
- Pemphigus vulgaris after SARS-CoV-2 vaccination: A case with new-onset and two cases with severe aggravation. Dermatol Ther. 2022;35:e15396.
- [CrossRef] [Google Scholar]
- Clinical markers of herpes simplex virus infection in patients with pemphigus vulgaris. J Am Acad Dermatol. 2023;88:587-92.
- [CrossRef] [PubMed] [Google Scholar]






