Translate this page into:
Novel use of chloramphenicol eye ointment for preventing eye injury during trichloroacetic acid cautery of xanthelasma palpebrarum

*Corresponding author: Muhammed Mukhtar, Department of Dermatology, Mukhtar Skin Centre, Katihar, Bihar, India. drmmukhtar20@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shankar V, Mukhtar M. Novel use of chloramphenicol eye ointment for preventing eye injury during trichloroacetic acid cautery of xanthelasma palpebrarum. CosmoDerma. 2025;5:59. doi: 10.25259/CSDM_44_2025
PROBLEM
Xanthelasma palpebrarum (XP) is a cluster of foam cells that carry a high concentration of low-density lipoprotein.[1] XP is a prevalent cosmetic concern in middle-aged women. There are several treatment options for this condition.[2] However, the most common outpatient department method is cautery with 50–100% trichloroacetic acid (TCA) depending on the form/nodularity of the lesions; nonetheless, TCA may trickle or leak down into the surrounding skin particularly of canthus and eye due to eyelid contracting and capillary activity in the patient’s inclined position. Thus, TCA can induce eye harm when used for xanthelasma cautery, particularly on the upper eyelid. To protect from injury, an eye shield and liquid paraffin are employed, but the latter cannot be used in the eye due to concern about its sterility. We created concepts to protect the skin and eyes from TCA during the operation after seeing the effect of an eye ointment in protecting skin injury [Figure 1]. The evidence of chloramphenicol ointment protection from TCA on the cornea is appreciable on chicken eyeballs [Figures 2 and 3, Videos 1 and 2].
Video 1:
Video 1:Application of trichloroacetic acid on cornea of chicken eyeball with no ointment.Video 2:
Video 2:Application of trichloroacetic acid on cornea of the chicken eyeball with ointment.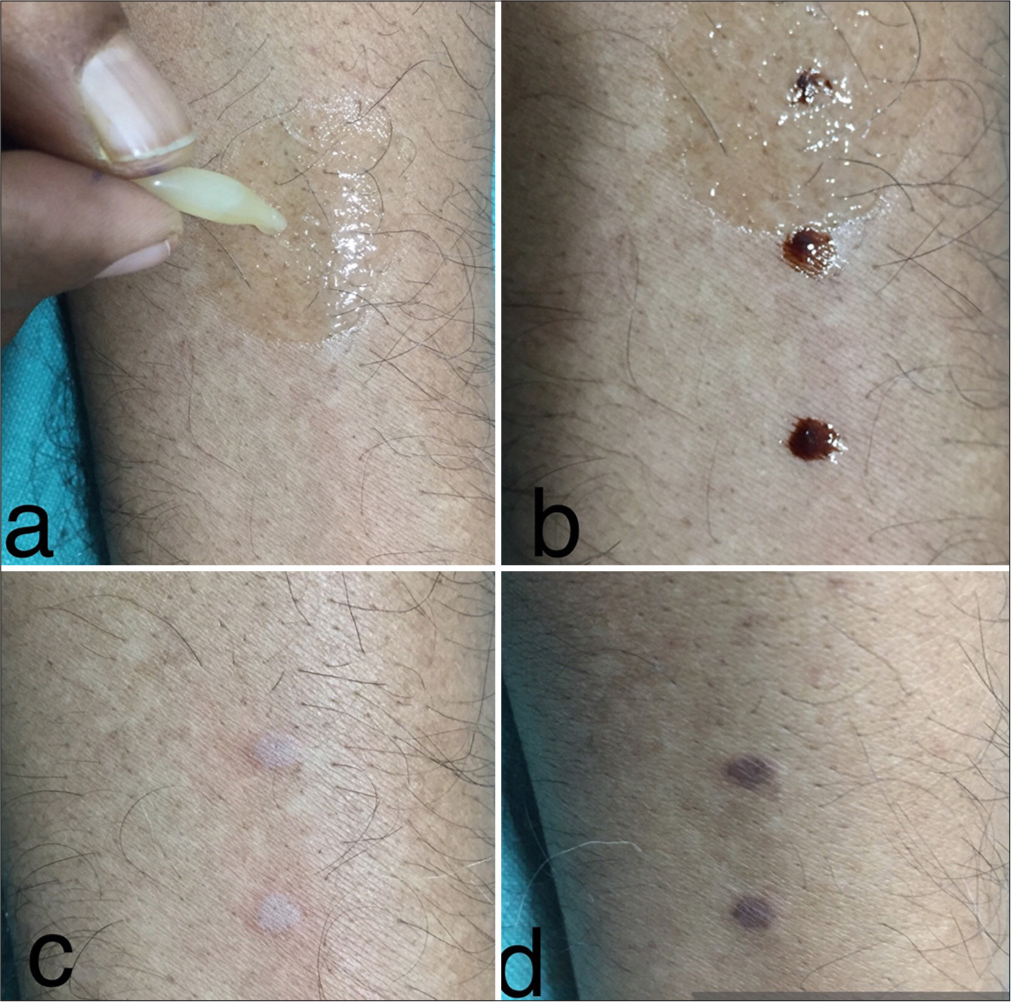
- (a) Chloramphenicol eye ointment is placed on normal skin. (b) Trichloroacetic acid (TCA) is applied on normal skin with and without ointment. (c) The effect of TCA on skin with and without ointment after 5 minutes. (d) The effect of TCA cautery on the skin after one week.
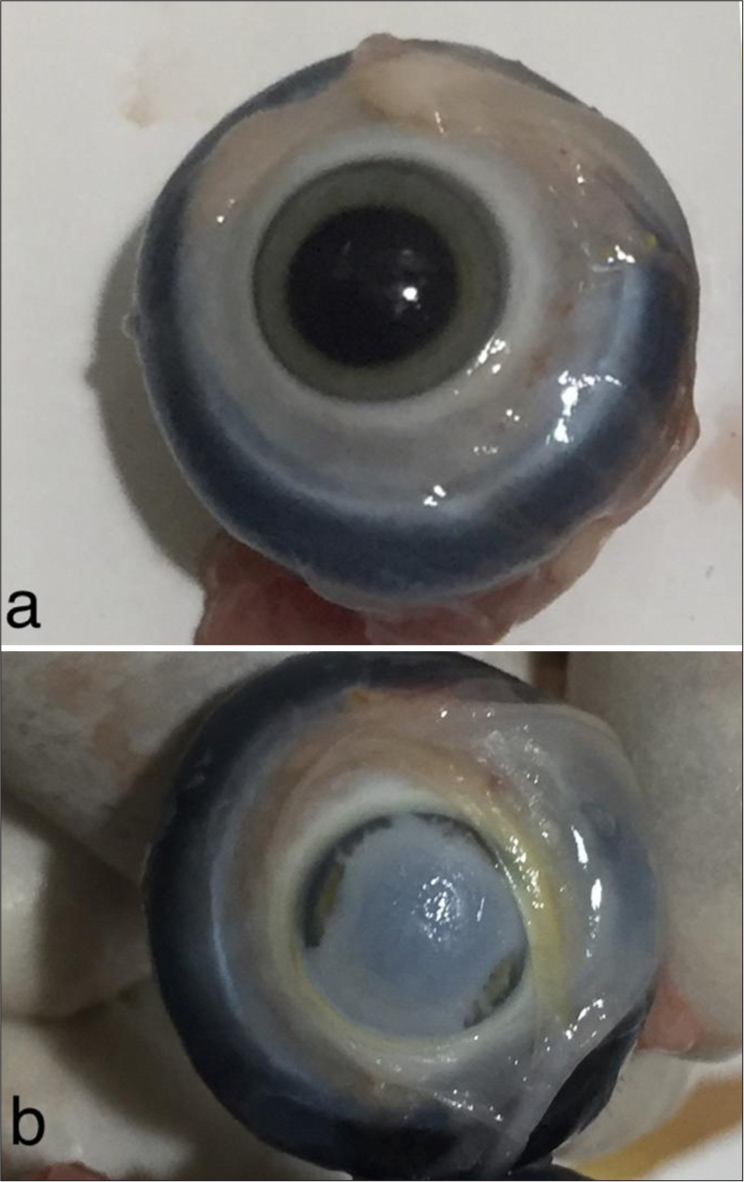
- (a) Clear cornea of the eyeball without ointment before cautery. (b) Frosted cornea after cautery.
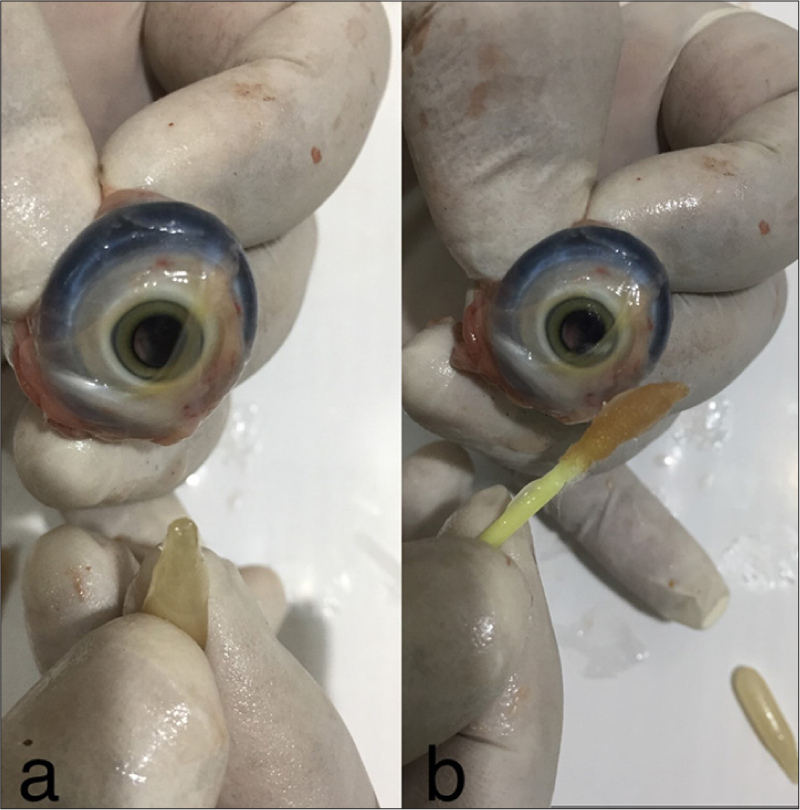
- (a) Clear cornea after ointment application. (b) Clear cornea with ointment after trichloroacetic acid treatment, demonstrating no effect of cautery.
SOLUTION
First, spirit or acetone is used to degrease the xanthelasma and surrounding areas. After that, chloramphenicol eye ointment (Rs 3–4) is applied to the eye and XP border [Video 3]. Following that, TCA (50–100%), depending on the depth of the lesion, is applied for 1–2 min using a cotton bud-stuffed toothpick until the lesion’s surface is frosted [Figure 4, Video 4]. After cauterization, cover the lesion with the same ointment. There is no TCA leakage from the lesions to the normal skin of the eyelid. Thus, eye ointment offers a novel and cost-effective antibacterial solution for protecting the eye and skin against TCA damage during XP cautery.
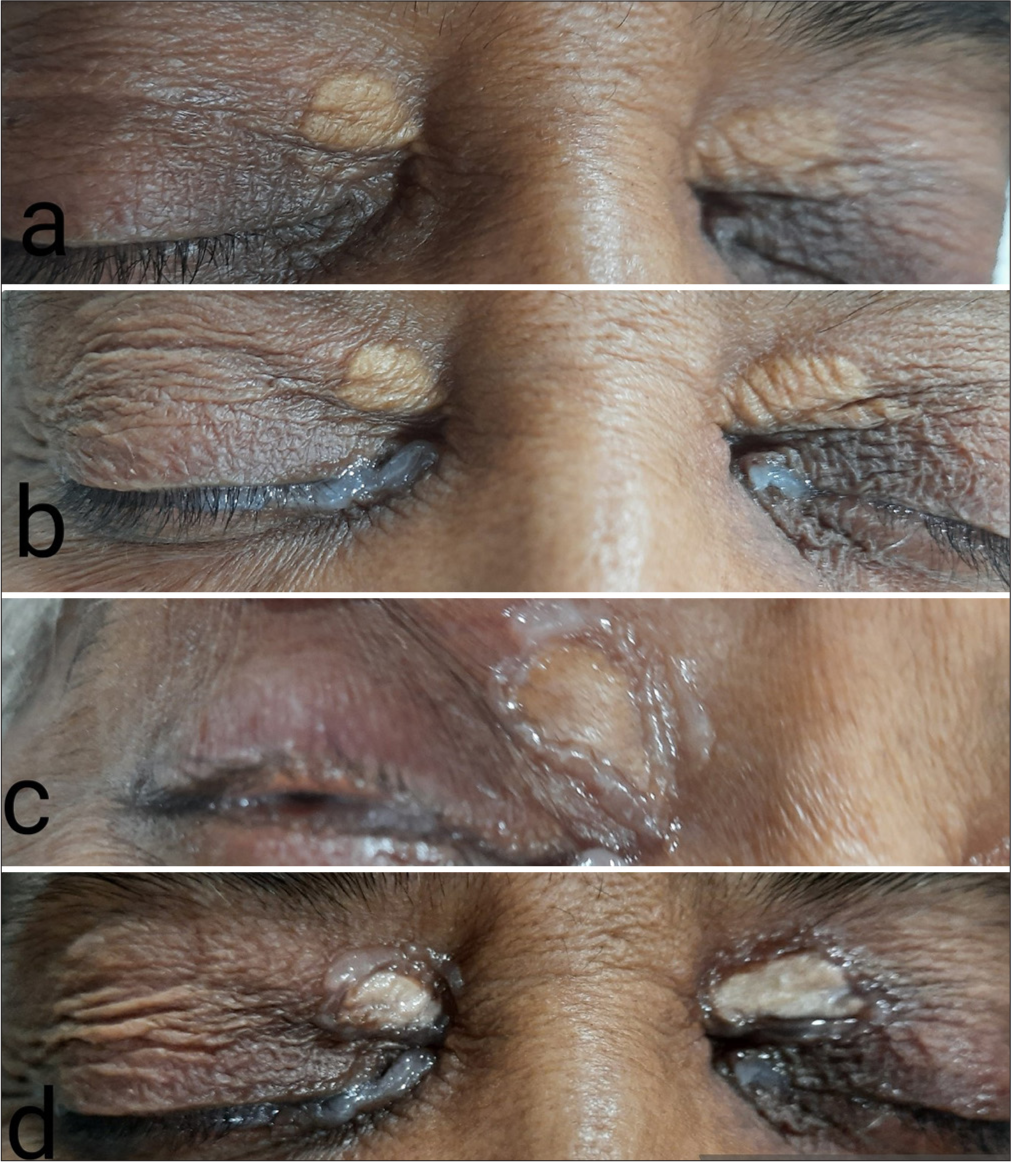
- (a) Xanthelasmas without ointment. (b) Application of the ointment to the eyeball before cautery. (c) Application of the ointment on the lesion’s margin. (d) Trichloroacetic acid cauterized xanthelasma lesions.
Video 3:
Video 3:The ointment chloramphenicol is being applied on the eyeball.Video 4:
Video 4:Xanthelasma palpebrarum is being cauterized with trichloroacetic acid.Ethical approval:
The Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Xanthelasma: An update on treatment modalities. J Cutan Aesthet Surg. 2018;11:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Examining treatment strategies for xanthelasma palpebrarum: A comprehensive literature review of contemporary modalities. Arch Dermatol Res. 2024;316:149.
- [CrossRef] [PubMed] [Google Scholar]






