Translate this page into:
Medical use of hyaluronic acid – A 2023 perspective

*Corresponding author: Uwe Wollina, Department of Dermatology and Allergology, Städtisches Klinikum Dresden, Academic Teaching Hospital, Dresden, Germany. uwollina@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Wollina U. Medical use of hyaluronic acid – A 2023 perspective. CosmoDerma 2023;3:86.
Abstract
Hyaluronic acid (HA) is a multifunctional natural biopolymer. It is mainly known for its use in esthetic medicine and cosmesis. Classical medical applications are also known in ophthalmology, orthopedics, dentistry, and dermatology. The medical applications of HA in various fields have been growing potentially in the last decade. In this article, most recent developments are reviewed.
Keywords
Hyaluronic acid
Drug delivery
Wound healing
Tissue regeneration
HYALURONIC ACID (HA)
HA is a highly anionic and unbranched non-sulfated glycosaminoglycan. HA is composed of disaccharides containing glucuronic acid and N-acetylglucosamine [Figure 1]. It is an essential component of extracellular matrix. HA interacts non-covalently with membrane receptor protein CD44 and other receptors (hyaladherins). Its biological activities are dependent on its molecular weight. HA regulates tissue homeostasis, inflammation, and exerts oxygen radical scavenger effects.[1]
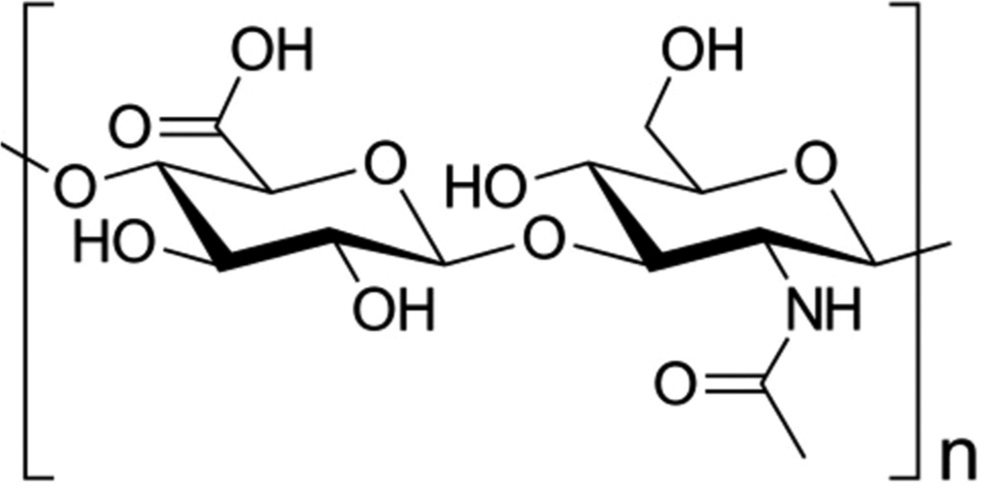
- Skeletal formula of hyaluronic acid (Wikipedia).
HA is widely used in cosmesis and esthetic dermatology, either topical or as injectables.[2] Classical medical indications of HA are symptomatic therapy of dry eyes, osteoarthritis, periodontitis, and scar management.[3-6]
In recent time, however, the possible indications for the medical use of HA have been markedly developed. Here, we provide an overview about most recent approaches to use HA for medical purposes. The article will focus on three topics: Drug delivery, wound healing and tissue engineering, and fibrosis and tissue regeneration [Table 1].
| Medical use as | Examples |
|---|---|
| Drug delivery with HA-based nanosystems | Quercetin in breast cancer p53 and doxorubicin for lung cancer; paclitaxel for targeting of cancer-associated fibroblasts |
| Tissue engineering | Photoluminescent crystals to monitor engineered scaffolds |
| Wound healing | Gels to reduce oxygen radicals in chronic wounds; scaffolds to deliver somatic stem cells; gels with protracted antibacterial activity |
| Fibrosis and tissue regeneration | Olmesartan medoxomil for hepatic cirrhosis; HA sheets to alleviate infrapatellar fat pads; hydrogels containing kartogenin to repair cartilage defects; hydrogels loaded with hydroxy-camptothecin to prevent scar formation after glaucoma surgery |
HA: Hyaluronic acid
DRUG DELIVERY
HA-based systems are used for drug delivery. Of increasing importance are nanosystems such as nanohydrogels, nanoparticles, or self-assembling nanosystems for various indications. In oncology, improvement of drug targeting tumor cells and reduce systemic toxicities is a major goal. For this purpose, drug delivery using HA possesses important qualities, such as targeting CD44 receptors on the surface of tumor cells.
Quercetin (QT) is an anticancer drug used for treatment of breast cancer. To improve efficacy of QT amphiphilic HA polymers (dHAD) were synthesized using dodecylamine to produce micelles. The dHAD-QT micelles improve CD44 drug targeting resulting in a tumor inhibition rate of 91.8% in a mouse model. QT toxicity to normal tissues was reduced.[7]
For the use in lung cancer, a nanocomposite has been constructed. HA was loaded with CaO2, p53 and doxorubicin (DOX) and combined with mesoporous silica nanoparticles (MSN) with high loading rate. This resulted in nanoparticle complexes-SiO2@CaO2@DOX@P53-HA. The HA-nanoparticles allowed an enrichment of anti-cancer drugs within the tumor cells leading to increased apoptosis and reduction of tumor load in a mouse model with lesser systemic toxicity to normal tissues.[8]
Recent strategy in oncology follows a dual concept – targeting tumor cells and cancer-associated fibroblasts (CAFs). Nano-micelles were loaded with paclitaxel. HA was the major carrier combined a dipeptide Z-glycine-proline binding to fibroblast activating protein on the surface of CAFs was modified on HA to achieve precise targeting of CAFs. This results in higher concentration of anti-tumor drug in liver cancer cells and CAFs and increases the bioavailability of paclitaxel in the neoplastic tissue.[9]
WOUND HEALING AND BIOENGINEERING
Tissue-engineering is a growing field. However, monitoring the products in vivo remains a challenge. Non-invasive visualization and monitoring would be most helpful. Scaffolds based on HA polymers were loaded with β-NaYF4:Yb3+, Er3+ nanocrystals as photoluminescent markers. These scaffolds were implanted on BALB/c mice. Using 975 nm laser excitation, it was demonstrated that the photoluminescent signal decreased along with gradual biodegradation of the scaffold. The non-invasive photoluminescent analysis correlated with histopathology.[10]
Chronic wounds are a huge burden to patients, their families, and the health-care system. Recently, a double-network hydrogel composed of HA methacrylate, pluronic F127 diacrylate, and SS-31-loaded mesoporous polydopamine nanoparticles had been developed for chronic wounds. SS-31 is a synthetic peptide that improves mitochondrial function. This topical hydrogel was capable to diminish reactive oxygen species, promote cell proliferation and migration, and exert anti-bacterial activity. In a rat model for diabetic full-thickness wounds, the gel promoted polarization of M2 macrophages, collagen deposition, neovascularization, and eventually wound healing.[11]
Another attempt used a polysaccharide-based hydrogel scaffold with alginate, pullulan, and HA to deliver adipose tissue-derived stem cells for better wound healing. The scaffold was tested in vitro and in vivo. In full-thickness animal wound models, this HA-hydrogel improved healing and wound closure.[12]
A hybrid gelatin methacrylate (GelMA) gel with antibacterial properties was developed using HA-coated MSN. It was loaded with complexes of chlorhexidine (CHX) and β-cyclodextrins (CHX⊂CD-MSN@HA@GelMA). The construction allows a slow delivery of CHX over time, which makes it attractive for topical wound management.[13]
FIBROSIS AND TISSUE REGENERATION
Olmesartan medoxomil (OLM) is a selective angiotensin II receptor blocker that exerts anti-hypertensive and anti-fibrotic activity.[14] HA had been used in coating of polymeric micelles loaded with OLM for drug therapy of hepatic cirrhosis. In a mouse model, HA-micelles accumulated in high concentrations in the liver, where they exhibited a strong anti-fibrotic effect on hepatic stellate cells.[15]
Osteoarthrosis and surgery are risk factors for fibrosis of the infrapatellar fat pad (IFP). The implantation of HA sheets to alleviate IFP has been investigated in a rat model. It was observed that transplantation of HA sheets reduced pain, inhibited articular cartilage degeneration, and fibrosis with in 10–28 days after injury. This could be a promising tool in orthopedics.[16]
The repair of cartilage defects is of major importance for protection of joint mobility. HA hydrogels modified by arginyl-glycyl-aspartic acid and histidine-alanine-valine peptides containing kartogenin were investigated in cartilage defect in the knee joint of rabbits. The system allowed a sustained release of kartogenin, a heterocyclic, drug-like compound facilitating cartilage repair. In the so treated joints, expression and morphology of collagen Type II were similar to intact cartilage tissue. The HA hydrogel promoted repair of cartilage defects within 1 month in the animal model.[17]
Glaucoma surgery can be compromised by scar formation. HA hydrogels loaded with different dosages of hydroxycamptothecin (HCPT), a plant alkaloid targeting intracellular topoisomerase I, were placed under the scleral flap after filtration surgery in a rabbit model. HA-HCPT hydrogel effectively inhibited the growth of scleral fibroblasts, downregulated collagen Type I/III, and accelerated the degradation of extracellular matrix deposition. Thereby, scar formation was prevented, and the success rate of glaucoma surgery improved.[18]
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019;78-9:1-10.
- [CrossRef] [PubMed] [Google Scholar]
- Hyaluronic acid dermal fillers: Safety and efficacy for the treatment of wrinkles, aging skin, body sculpturing and medical conditions. Clin Med Rev Ther. 2011;3:107-21.
- [CrossRef] [Google Scholar]
- A comparison between hyaluronic acid and other single ingredient eye drops for dry eye, a review. Acta Ophthalmol 2023 doi: 10.1111/aos.15675
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of intra-articular cross-linked sodium hyaluronate for the treatment of knee osteoarthritis: A prospective, active-controlled, randomized, parallel-group, double-blind, multicenter study. J Clin Med. 2023;12:2982.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of hyaluronic acid gel on periodontal parameters, pro-inflammatory cytokines and biochemical markers in periodontitis patients. Gels. 2023;9:325.
- [CrossRef] [PubMed] [Google Scholar]
- Fillers for the improvement in acne scars. Clin Cosmet Investig Dermatol. 2015;8:493-9.
- [CrossRef] [PubMed] [Google Scholar]
- Delivery of quercetin for breast cancer and targeting potentiation via hyaluronic nano micelle. Int J Biol Macromol. 2023;242:124736.
- [CrossRef] [PubMed] [Google Scholar]
- SiO2/hyaluronic acid nanoparticles carry CaO2, DOX and p53 plasmid to effectively achieve ion interference/chemical/gene multimodal therapy of lung cancer. Biomater Sci 2023 doi: 10.1039/d2bm02075k
- [CrossRef] [PubMed] [Google Scholar]
- Novel dual CAFs and tumour cell targeting pH and ROS dual sensitive micelles for targeting delivery of paclitaxel to liver cancer. Artif Cells Nanomed Biotechnol. 2023;51:170-9.
- [CrossRef] [PubMed] [Google Scholar]
- Photoluminescent scaffolds based on natural and synthetic biodegradable polymers for bioimaging and tissue engineering. Life (Basel). 2023;13:870.
- [CrossRef] [PubMed] [Google Scholar]
- Double-network hydrogel enhanced by SS31-loaded mesoporous polydopamine nanoparticles: Symphonic collaboration of near-infrared photothermal antibacterial effect and mitochondrial maintenance for full-thickness wound healing in diabetes mellitus. Bioact Mater. 2023;27:409-28.
- [CrossRef] [PubMed] [Google Scholar]
- Design and characterization of adipose-derived mesenchymal stem cell loaded alginate/ pullulan/hyaluronic acid hydrogel scaffold for wound healing applications. Int J Biol Macromol. 2023;241:124556.
- [CrossRef] [PubMed] [Google Scholar]
- Hyaluronic acid sheet transplantation attenuates infrapatellar fat pad fibrosis and pain in a rat arthritis model. J Orthop Res 2023 doi: 10.1002/jor.25580
- [CrossRef] [PubMed] [Google Scholar]
- Role of the renin-angiotensin system in the pathogenesis of peritoneal fibrosis. Perit Dial Int. 2008;28(Suppl 3):S83-7.
- [CrossRef] [Google Scholar]
- Drug delivery with hyaluronic acid-coated polymeric micelles in liver fibrosis therapy. ACS Biomater Sci Eng 2023 doi: 10.1021/acsbiomaterials.3c00327
- [CrossRef] [PubMed] [Google Scholar]
- Hybrid hydrogel loaded with chlorhexidine⊂β-CD-MSN composites as wound dressing. Int J Nanomedicine. 2023;18:1725-40.
- [CrossRef] [PubMed] [Google Scholar]
- Application of functionalized hyaluronic acid hydrogel with the activity of regulating the behaviors of stem cells in repairing rabbit knee articular cartilage. J Biomater Appl. 2023;37:1617-25.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxycamptothecin regulates scar formation of the filtration channel under scleral flap by inhibiting the proliferation of scleral fibroblasts. PLoS One. 2023;18:e0284618.
- [CrossRef] [PubMed] [Google Scholar]







