Translate this page into:
Lymphedema after COVID-19 vaccination: Two case reports

*Corresponding author: Uwe Wollina, Department of Dermatology and Allergology, Städtisches Klinikum Dresden, Dresden, Germany. uwollina@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Krönert C, Koch A, Schönlebe J, Unger L, Wollina U. Lymphedema after COVID-19 vaccination: Two case reports. CosmoDerma. 2024;4:75. doi: 10.25259/CSDM_65_2024
Dear Sir,
In response to the COVID-19 pandemic vaccination has been the most effective measure. In addition to classic vaccines, messenger RNA (mRNA) vaccines such as BNT162b2 (BioNTech/Pfizer) and mRNA-1273 (Moderna) were developed and widely used. They contain a modified S-protein of the SARS-CoV-2 virus, of which the S1 subunit binds to angiotensin-converting enzyme-2 of various host cells. The mRNA vaccines are highly immunogenetic inducing a strong antibody response and activating antiviral T-cell reactions.[1] These vaccines have been shown to reduce the risk of symptomatic infection, hospitalization, and mortality of COVID-19 significantly with a favorable general safety profile (<0.1% of adverse events) in controlled trials.[2] Here, we report two patients from Germany who developed lymphedema after booster vaccination with BNT162b2 (BioNTech/Pfizer).
Case 1: A 49-year-old woman was vaccinated with BNT162b2 3 times from December 2020 to November 2021 with injections in the deltoid muscle of her left arm with minor temporary pain at the injection site. Her medical history was positive for severe polytrauma with subsequent amputation of her right leg in 2006 and chronic osteomyelitis. She was negative for COVID-19 disease. Eight weeks after the last booster vaccination, she noticed swellings in both hands with progression to the complete arms. Tension blisters developed. In autumn 2021, she experienced a painless swelling of the left leg, and her GP diagnosed an anemia.
On examination, we observed an impaired arm flexion and impeded complete fist closure. The swelling on the upper extremities and left leg was clinically evident, accompanied by an increased palpable tenderness of the skin with fibrosis and slight pitting on palpation [Figure 1].
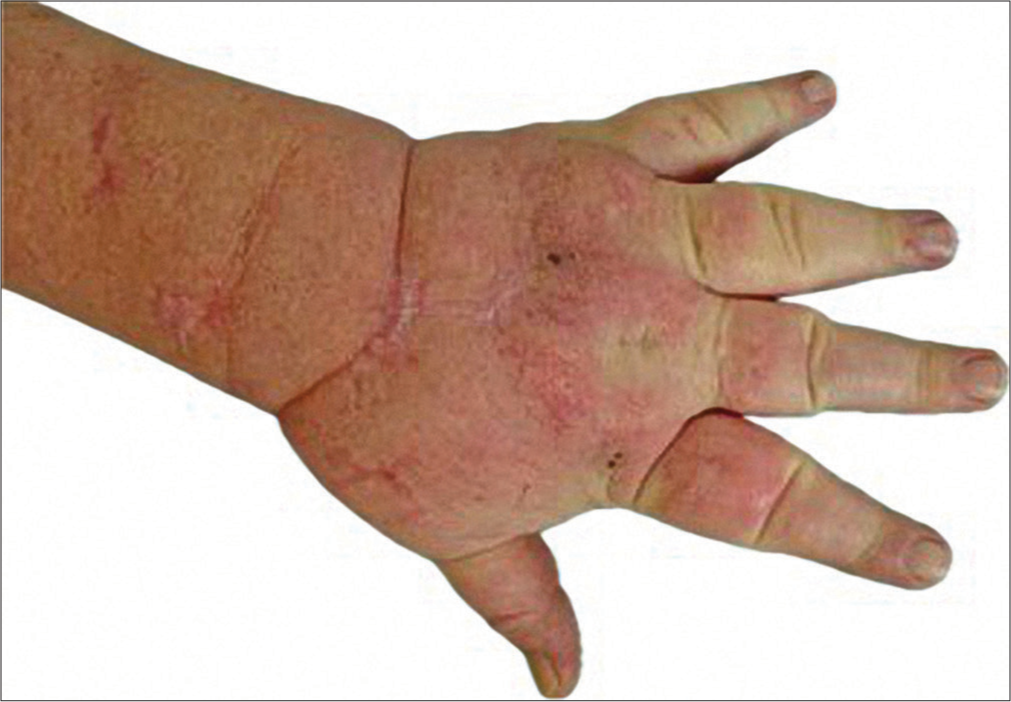
- Lymphedema (Patient 1).
A skin biopsy from the right lower arm demonstrated septal fibrosis with lipophageous panniculitis and thrombosis of venous and arterial blood vessels. The SARS-CoV-2-spike- subunit 1 was detected in endothelial cells of smaller subcutaneous blood vessels and perivascular macrophages [Figure 2]. Nucleocapsid-SARS-CoV-2 protein could not be detected. The bone marrow biopsy revealed reactive-dysplastic changes and iron deficiency, but excluded amyloidosis, myelodysplastic syndrome, or malignancy.
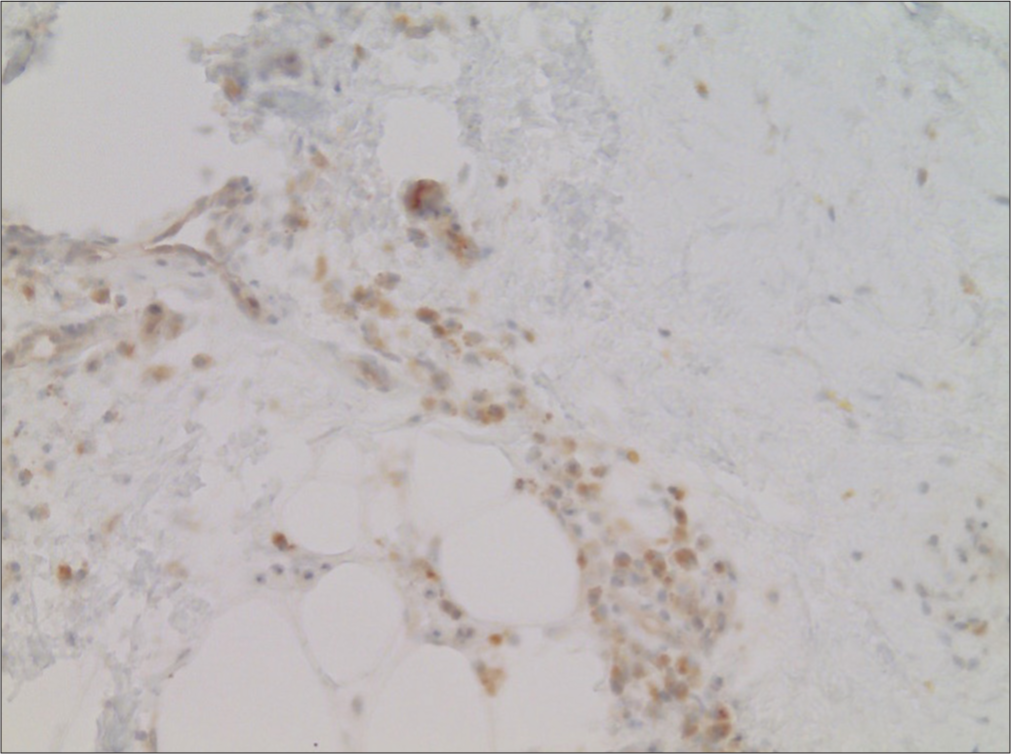
- Detection of SARS-CoV-2 spike subunit 1 in endothelia of small caliber blood vessels in the subcutaneous adipose tissue (Immunoperoxidase, ×20).
Laboratory findings demonstrated normocytic hypochromic anemia (hemoglobin 3.2 mmol/L), diminished iron 4.8 µmol/L, and transferrin saturation 5.6%, mild lymphopenia, but no vasculitis signs and no eosinophilia.
Color duplex, compression sonography, and computer tomography remained unremarkable. There was no filarial dance sign visible.[3]
A secondary lymphedema Stage II–III was confirmed.
Case 2: A 72-year-old man developed a progressive swelling of the left arm and both legs after booster vaccination with BNT162b2 on his left arm in January 2022. His medical history was positive for arterial hypertension and absolute tachyarrhythmia, CHADVASC 3 and HASBLED 3, and peripheral neuropathy. He was treated with apixaban, ramipril, metoprolol, vitamin B1, and torsemide.
The swelling of the legs was regredient after three weeks, but the edema of the left arm persisted.
On examination, the complete left arm showed swelling without color changes [Figure 3]. On palpation, the skin was firm. Pitting was positive on palpation of the hand. Laboratory investigation showed a temporary mild increase of D-dimer but no eosinophilia. The color duplex was unremarkable. On ultrasound, there was no filarial dance sign visible.[3]
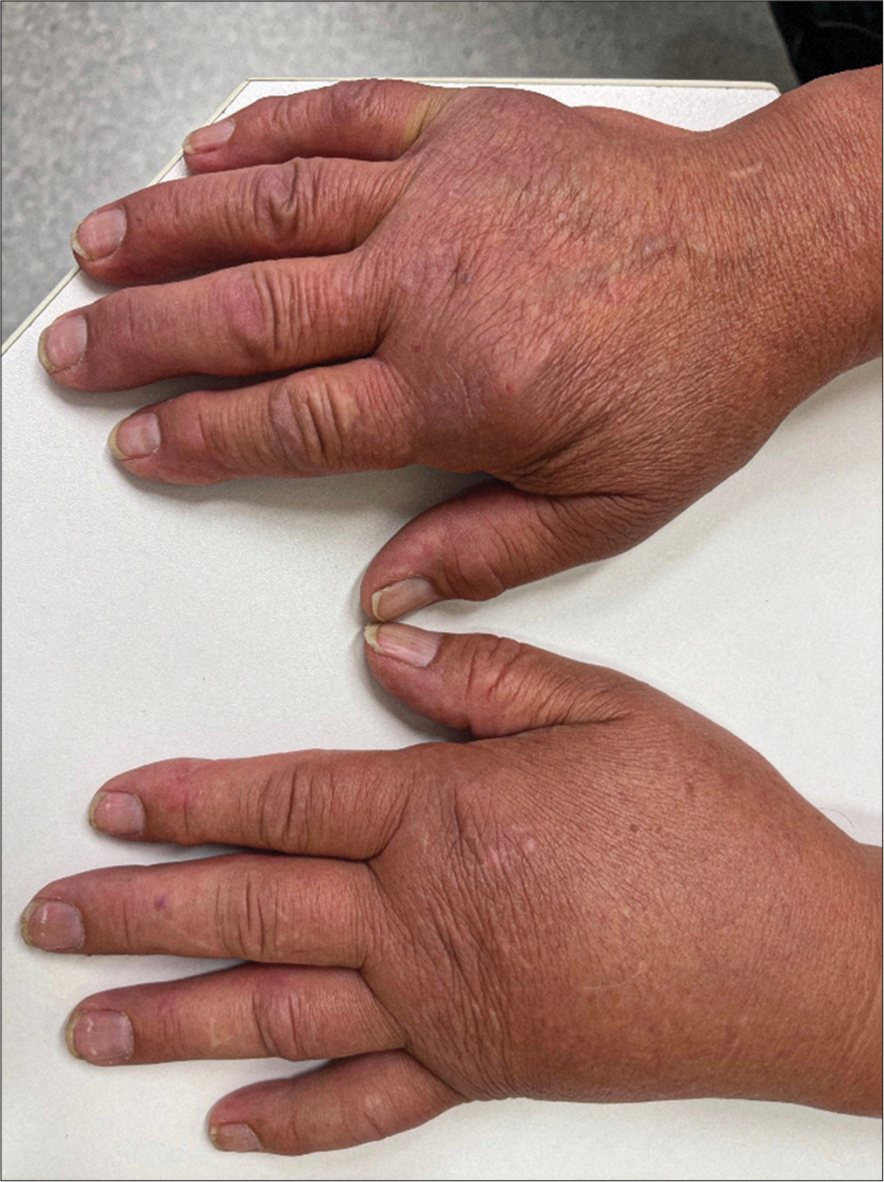
- Lymphedema on the left side (Patient 2).
A secondary lymphedema of the left arm, Stage II, was confirmed.
For both patients, complex decongestion therapy was recommended.
In contrast to temporary lymphadenopathy, lymphedema is a rare complication of COVID-19 vaccination. The mRNA vaccine, both patients had received, is encapsulated by lipid nanoparticles of about 100 nm. Therefore, it becomes mainly distributed by the lymphatic system. After contact with the vaccine, dendritic cells in the draining lymph nodes develop an interferon type I immune response. The lipid nanoparticle formulation has an intrinsic adjuvant activity, which induces the production of inflammatory cytokines by the innate immune system, including interleukin (IL)-6.[4]
Lymphedema is a chronic disorder of the lymphatic system characterized by impaired lymphatic drainage, local immune dysfunction, adipose deposition, and chronic inflammation. Primary lymphedema is a rare condition in contrast to acquired lymphedema. Germany, in contrast to tropical countries like India, is not an endemic area for parasitic lymphedema. In a review on non-endemic lymphatic filariasis, no cases from Central Europe were observed.[5] The major causes of secondary lymphedema in Germany are tumor surgery and/or radiotherapy and repeated erysipelas.
We report two patients with acquired (secondary) lymphedema after vaccination with an mRNA vaccine against COVID-19 disease. Both patients had never visited countries outside Europe.
According to the International Society of Lymphology lymphedema, Stages 0–III have been defined as:
Stage 0 – Preclinical disease with impaired lymphatic drainage but absent edema,
Stage I – A dynamic interstitial fluid accumulation that is relieved by elevation,
Stage II – Worsening of fluid accumulation leading to tissue fibrosis, nor relieved by elevation, Stage III – Irreversible non-pitting edema, increased adiposity, fibrosis, papillomatosis cutis, and Elephantiasis nostras.[6]
Our patients presented with Stage II to III disease. In the more advanced stages, the upregulation of Th2 activity leads to an overexpression of proinflammatory cytokines such as IL-4, IL-5, IL-6, IL-13, and transforming growth factor-beta 1.[7] mRNA COVID-19 vaccines induce the production of interferon-γ that promotes the differentiation of CD4+ and CD8+ effector T-cells. Subsequently, T-cells respond by the production and release of proinflammatory and cytotoxic mediators.[8] We suppose that the vaccine-induced inflammation is involved in the initiation of a process leading to lymphedema. The rareness of this adverse event is in favor of a pre-disposition of affected patients, which might have been polytrauma in patient #1 and polyneuropathy in patient #2. Further, investigations are necessary to elucidate the underlying pathological mechanisms.
An overview of published cases of lymphedema after COVID-19 vaccination is provided in Table 1.[9-15] One patient developed lymphedema in association with cellulitis. We observed no cellulitis in our patients. Treatment of choice is complex decongestive therapy, which consists of manual lymph drainage, flat-knitted compression garment, and skin care.[16]
| Authors | Age and Sex | Vaccine | Remarks |
|---|---|---|---|
| Breckwoldt et al. 2024 | 40 y, f | mRNA-1273 mRNA | After 1st vaccination lymphedema of the left arm |
| Watabe and Amano, 2023 | 93 y, f | NT162b2 mRNA | After 2nd vaccination unilateral lymphedema of the left arm |
| Tang and Chua, 2023 | 84 y, m | BNT162b2 mRNA | After booster unilateral lymphedema of the left arm |
| Chung et al. 2021 | 79 y, f | BNT162b2 mRNA | After 2nd vaccination temporary lymphedema of both legs |
| Hosseinzadeh et al. 2022 | 68 y, m | BBIBP-CorV inactivated virus | After 2nd vaccination unilateral lymphedema of the leg, followed by cellulitis |
| Aimo et al. 2022 | 24 y, f | ChAdO×1nCov-19 replication deficient adenoviral vector, containing spike protein gene | After 1st vaccination lymphedema of the left arm |
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int J Pharm. 2021;601:120586.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev. 2022;12:CD015477.
- [CrossRef] [PubMed] [Google Scholar]
- Review of dancing parasites in lymphatic filariasis. Ultrasound Int Open. 2019;5:E65-74.
- [CrossRef] [PubMed] [Google Scholar]
- The lymphatic system and COVID-19 vaccines. Front Immunol. 2022;13:1041025.
- [CrossRef] [PubMed] [Google Scholar]
- Non-endemic cases of lymphatic filariasis. Trop Med Int Health. 2014;19:1377-83.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnosis and treatment of peripheral lymphedema: 2016 Consensus Document of the International Society of Lymphology. Lymphology. 2016;49:170-84.
- [Google Scholar]
- The role of inflammation in lymphedema: A narrative review of pathogenesis and opportunities for therapeutic intervention. Int J Mol Sci. 2024;25:3907.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 vaccines: Modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195-7.
- [CrossRef] [PubMed] [Google Scholar]
- Arm lymphedema after vascularized lymph node harvest following Covid-19 vaccination. Case Reports Plast Surg Hand Surg 2024. ;. ;11:2342332.
- [CrossRef] [PubMed] [Google Scholar]
- Unilateral lymphoedema after a second dose of BNT162b2 COVID-19 mRNA vaccine. Eur J Dermatol. 2023;33:312-4.
- [CrossRef] [PubMed] [Google Scholar]
- Ipsilateral upper-limb lymphedema after COVID-19 vaccine booster. Clin Nucl Med. 2023;48:264-5.
- [CrossRef] [PubMed] [Google Scholar]
- Transient lower extremity lymphedema following COVID-19 vaccination: A case report. Medicine (Baltimore). 2021;100:e28092.
- [CrossRef] [PubMed] [Google Scholar]
- Lower limb lymphedema and cellulitis as a complication of COVID-19 vaccine: A case report. Clin Case Rep. 2022;10:e6317.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphedema of the arm after COVID-19 vaccination in a patient with hidden breast cancer and paraneoplastic dermatomyositis. Vaccines (Basel). 2022;10:1219.
- [CrossRef] [PubMed] [Google Scholar]
- Frequent onsets of cellulitis in lower limbs with lymphedema following COVID-19 mRNA vaccination. Vaccines (Basel). 2022;10:517.
- [CrossRef] [PubMed] [Google Scholar]






