Translate this page into:
Facial dyschromias: A review of clinical and dermoscopic features

*Corresponding author: Devinder Mohan Thappa, MD, DHA, FRCP (Edin), FRCP (Glasg), FAMS, FIMSA, Dean Research, JIPMER, Professor Senior Scale, Department of Dermatology and STD, JIPMER, Puducherry, India. dmthappa@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kotekar S, Thappa DM. Facial dyschromias: A review of clinical and dermoscopic features. CosmoDerma. 2024;4:130. doi: 10.25259/CSDM_156_2024
Abstract
Facial dyschromias are a common complaint among individuals with skin of color. Until the advent of dermoscopy, clinical examination and histopathology were used to arrive at a definitive diagnosis. Dermoscopy is an emerging tool used to diagnose various pigmentary conditions. It may be used to diagnose various facial dyschromias, including melasma, lichen planus pigmentosus, facial acanthosis nigricans, post-inflammatory pigmentation, maturational dyschromia, vitiligo, and salt and pepper pigmentation, to name a few. Some of these conditions show characteristic dermoscopic features, thereby obviating the need for a skin biopsy for confirmation of diagnosis. Dermoscopy is, therefore, a reliable, non-invasive tool which can be used to diagnose various facial dyschromias.
Keywords
Dermoscopy
Facial dyschromias
Pigmentary disorders
INTRODUCTION
Cutaneous pigmentary disorders may present with a variety of features depending on the skin phenotype. Indian skin has been shown to possess unique properties and, therefore, cannot accurately be described as “skin of color” or “Asian skin.”[1]
The burden of pigmentary disorders in India has been found to be relatively high, with significant psychological and social impact.[2] Indian skin has been found to be more susceptible to various pigmentary disorders, including post-inflammatory hyperpigmentation.[3] Climate, diet, and social factors have been suggested to be contributory factors in the variation in pigmentation within the country.[4]
There are several disorders presenting with facial pigmentation requiring the attention of a dermatologist. The various disorders presenting as facial hyperpigmentation include melasma, post-inflammatory hyperpigmentation, exogenous ochronosis, lichen planus pigmentosus (LPP), seborrheic melanosis, facial acanthosis nigricans, nevus of Ota, freckles, and lentigines, to name a few. Common hypopigmentary disorders include vitiligo, pityriasis alba, pityriasis versicolor, nevus depigmentosus, and idiopathic guttate hypomelanosis.[5]
The above-mentioned conditions may be diagnosed with the aid of dermoscopy. Further, dermoscopy has also been found to be useful in follow-up and treatment response assessment in patients with pigmentary disorders.[6]
FACIAL DYSCHROMIAS
Facial dyschromias or dyspigmentation is a commonly encountered complaint among individuals with skin of color. Pigmentation over the face may occur in isolation or may be part of generalized dermatological disease. There are a wide variety of causes of facial dyschromia, some of which may be challenging to diagnose.[7]
Pigmentary disorders may present as hyperpigmentation, hypopigmentation, mixed pattern, or unusual discoloration. Hypopigmentation refers to a decrease in skin pigmentation due to various causes. Hypomelanosis refers to decreased melanin content, which may be further subdivided into melanopenic hypomelanosis arising due to defective melanocyte function or melanocytopenic hypomelanosis arising due to a decrease in melanocyte number. Similarly, hypermelanosis may be classified into melanotic hypermelanosis (increased melanin production) and melanocytic hypermelanosis (increased melanocyte count).
Pigmentary changes in the skin of color may further be classified into physiological pigmentation and acquired pigmentation. Physiological pigmentary changes include familial periorbital hypermelanosis, pigmentary demarcation lines, and inherited patterns of lentiginosis. Acquired causes of dyspigmentation include post-inflammatory pigmentation, melasma, vitiligo, LPP, acanthosis nigricans, seborrheic melanosis, exogenous ochronosis, and nevus depigmentosus.[8]
There are various ways to classify pigmentary disorders, as described in Table 1. Based on the distribution of pigmentation, disorders of hypo- and hyperpigmentation may be broadly classified as diffuse and circumscribed. Diffuse pigmentation is contiguous without skip areas and may sometimes involve extra facial sites [Table 2]. Circumscribed pigmentation is well-defined and non-contiguous. Circumscribed pigmentary disorders may further be divided into various subtypes [Table 3].[8]
| Color of pigmentation |
|
| Etiology | Physiological
|
| Distribution of pigmentation | Diffuse pigmentation
Circumscribed pigmentation (guttate, linear, patchy, reticulate, and flagellate)
|
| Depth of pigmentation |
|
| Hypopigmentation/Depigmentation | Hyperpigmentation |
|---|---|
| Oculocutaneous albinism | Gray baby syndrome |
| Hermansky–Pudlak syndrome | Universal acquired melanosis |
| Chediak–Higashi syndrome | Vitamin B12 deficiency |
| Programmed death-1 inhibitor-induced pigmentation. (Pembrolizumab) | Folic acid deficiency |
| Type of pigmentation | Description | Examples |
|---|---|---|
| Guttate/Punctate | <1 cm diameter | Lentigines, punctate vitiligo |
| Patchy | ≥1 cm diameter | Melasma, Chemical leukoderma |
| Linear/whorled/segmental | Blaschkoid or zosteriform | Segmental vitiligo |
| Reticulate | Lacy, net-like pattern | Reticular type of lichen planus pigmentosus |
| Flagellate | Parallel linear or curvilinear arrangement | Drug-induced pigmentation, for example, bleomycin |
Hyperpigmentary disorders may also be classified based on the depth of pigmentation into epidermal, dermal, and mixed types. Epidermal pigmentation is well-defined and may be light to dark brown in color. Dermal pigmentation is comparatively ill-defined and has a bluish-grey hue. Wood’s lamp may be used to differentiate between the two, as epidermal pigmentation shows enhancement, whereas dermal pigmentation does not. This classification is useful in assessing the prognosis of pigmentary disorders since dermal lesions are usually more resistant to treatment.[9]
EPIDEMIOLOGY OF FACIAL DYSCHROMIAS
Facial dyschromias are commonly encountered in patients with skin of color and cause significant morbidity. Pigmentary disorders represent one of the top five dermatological complaints in patients with darker skin types. In a 3-month long, cross-sectional study conducted by Dlova et al.[10] in South Africa, it was found that among 3814 patients who visited the dermatology outpatient department, 304 patients (7.97%) presented with facial dyschromias. Vitiligo (118, 38.82%), post-inflammatory hyperpigmentation (60, 19.74%), melasma (24, 7.89%), ashy dermatosis (14.4.6%), and exogenous ochronosis (10, 3.29%) were the most-frequently encountered conditions among the patients with facial dyschromias. In a study conducted by Adil et al.[11] over 1 year in a tertiary healthcare center in North India, it was found that among the 68,345 patients who visited the department of dermatology during the study period, 7124 patients (10.42%) presented with complaints of hyperpigmentation over one or more regions of the body (facial and extra-facial). Melasma (2396, 33.63%), post-inflammatory hyperpigmentation (892, 12.52%), acquired melanocytic nevi (533, 7.48%), LPP (525, 7.37%), ephelides (491, 6.89%), and periorbital melanosis (324, 4.55%) were the most commonly encountered conditions.[11] Although this study recorded the prevalence of hyperpigmentation irrespective of site, conditions such as melasma, periorbital melanosis, and LPP with predominantly facial involvement were found to be more common.[11]
In a 1½-year-long descriptive study conducted by Thoyyib et al.[12] in a tertiary healthcare center in South India, it was found that among 1024 patients presenting with facial hyperpigmentation, post-inflammatory hyperpigmentation (362, 35.3%), melasma (177, 17.2%), periorbital melanosis (161, 15.7%), and facial acanthosis nigricans (60, 5.85%) were among the most common etiologies.[12]
A similar pattern of prevalence has been recorded in a descriptive study conducted by Amatya et al.[13] in a healthcare center in Nepal. The most common causes of facial melanosis in this study include melasma, post-inflammatory pigmentation secondary to acne and other causes, ashy dermatosis, and discoid lupus erythematosus (DLE), among others.[13]
In a study conducted by Mareddy et al.[14] among 123 patients presenting with hypopigmented lesions to a hospital in South India, around 12.2% of the patients had hypopigmentation involving the face. The common causes of hypopigmentation (facial and extra facial) encountered in this study include vitiligo (34, 27.6%), tinea versicolor (26, 21.1%), idiopathic guttate hypomelanosis (12, 9.7%), pityriasis alba (10, 8.12%), and lichen sclerosus et atrophicus (6, 4.9%).[14]
ROLE OF DERMOSCOPY IN DISORDERS OF FACIAL PIGMENTATION
Dermoscopy is also known as surface microscopy or epiluminescence microscopy. It is a rapidly evolving imaging modality in dermatology and has now become a part of routine practice. Although this modality was initially limited to diagnosing cutaneous malignancies, dermoscopy is presently being used to diagnose and manage various conditions, including disorders of pigmentation, vascular conditions, benign and malignant melanocytic lesions, papulosquamous disorders, disorders of hair, and infective conditions.[15]
The dermoscopy of ethnic non-facial skin shows a reticular pattern – due to the pigmentation in the basal layer as seen from above downwards. Dermoscopy of normal ethnic facial skin shows a pseudo-reticular pattern – due to interruption of the pigment network by follicular and eccrine openings.[5]
Dermoscopy may be used to determine the type and source of pigmentation. Melanocytic lesions show the presence of a reticular/pseudoreticular pigment network. This dermoscopic finding histologically corresponds to the presence of melanocytes in the basal layer of epidermal rete ridges, with the pale areas in between the network corresponding to the dermal papillae and the overlying epidermis.[8]
Dermoscopy may also aid in assessing the depth of pigmentation, with epidermal and dermal pigmentation showing varied colors [Table 4].[8]
| Color of pigmentation | Depth of melanin pigment |
|---|---|
| Black | Stratum corneum |
| Brown | Remaining epidermis, till upper dermis |
| Gray | Papillary dermis |
| Blue | Lower papillary and reticular dermis |
Several conditions such as melasma, vitiligo, exogenous ochronosis, acanthosis nigricans, post-inflammatory hyperpigmentation, and nevus depigmentosus may be reliably diagnosed using this modality, as their dermoscopic features are well-described in the literature. The use of dermoscopy in routine diagnosis may, therefore, obviate the need for skin biopsy in these conditions.[8]
ROLE OF HISTOPATHOLOGY IN DISORDERS OF FACIAL PIGMENTATION
The use of histopathology in the diagnosis of facial pigmentation is often limited by its invasive nature, with patients expressing hesitation for skin biopsy from the face due to cosmetic concerns. With the advent of dermoscopic examination, the role of histopathology is limited to ascertaining the diagnosis in doubtful cases.
Dermoscopic findings in various pigmentary disorders are mirrored in histopathological examination, making it a reliable tool for diagnosis. However, histopathology continues to remain the gold standard for diagnosing conditions such as exogenous ochronosis, tattoo-induced pigmentation, cutaneous amyloidosis, and congenital nevi, to name a few.[16]
The role of skin biopsy is critical in differentiating benign and malignant melanocytic lesions. Dermoscopic algorithms provide clues to differentiate between the two, but biopsy remains essential for confirmation.[17]
DISORDERS OF FACIAL HYPERPIGMENTATION
Melasma
Clinical profile
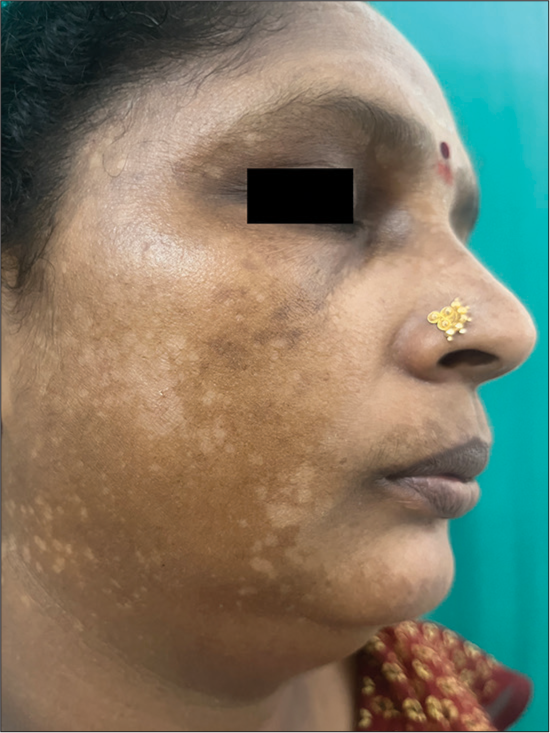
Melasma is a commonly encountered condition characterized by symmetrical hyperpigmented macules with irregular borders commonly seen over the cheeks, forehead, and mandibular region [Figure 1].[18] Although this condition is commonly encountered among females with the skin of color, men have been reported to constitute around one-quarter of the total number of cases, as reported in a study conducted by Sarkar et al.[19]. The risk factors for developing this condition include the presence of a family history of melasma, use of oral contraceptives, hypothyroidism, exposure to ultra-violet light, and certain drugs, including antiepileptics like phenytoin.[20]
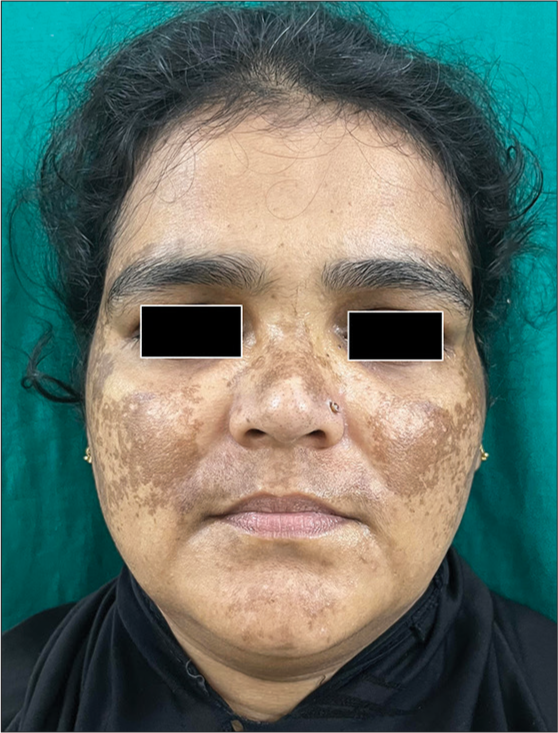
- Clinical image of melasma.
Melasma has been classified as centrofacial, malar and mandibular types based on the distribution of the skin lesions, with centrofacial type being reported as the most common variant. Further, melasma has been classified based on the depth of pigmentation into epidermal, dermal, and mixed types. Epidermal lesions show enhancement when examined under Wood’s lamp, which is not seen in the dermal subtype. Focal areas of enhancement are seen in the mixed subtype.[18]
The differential diagnosis for melasma includes Hori’s nevus, LPP, exogenous ochronosis, and pigmented contact dermatitis, to name a few. Dermoscopic examination is useful in confirming the diagnosis, especially since skin biopsy is not commonly done from the face due to cosmetic concerns.[21]
Dermoscopic profile
Dermoscopic examination of melasma showed an increase in the pseudoreticular pigment network, with sparing of follicular and eccrine openings, arcuate and annular structures, brown globules, and occasional telangiectasias [Figure 2].[21] Nearly 98% of the patients in this study showed sparing of follicular and eccrine openings.[22] Reticuloglobular pigmentation, patchy pigmentation, granular pattern, and telangiectasias were found to show statistically significant association with melasma.[23] The telangiectasias reported in melasma may be due to prior topical steroid abuse, extensive photodamage or due to increased vascular endothelial growth factor, which supposedly plays an important role in melanogenesis.[24]
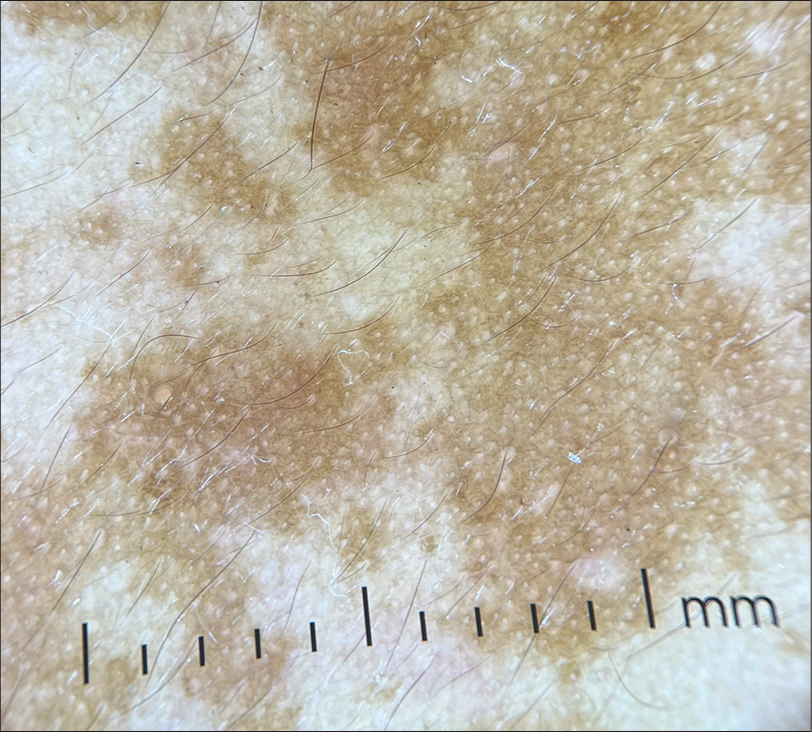
- Dermoscopy of melasma (DermLite DL4, ×10, non-polarized) showing increased pseudoreticular pigment network with sparing of follicular and eccrine openings.
Histopathological profile
Classical histopathology of melasma shows an increase in epidermal melanin content across all layers without any increase in the number of melanocytes. There may also be the presence of dermal melanophages and melanin in the dermal subtype.[25] However, recent studies have reported other dermal changes in melasma, including solar elastosis, basement membrane disruption, an increase in number of mast cells, and increased vascularity of lesions, which may have therapeutic implications.[24]
Further, the melanocytes in melasma are found to be more biologically active, which is evidenced by the increased number of dendritic processes, rough endoplasmic reticulum, and mitochondria. Increased fragmentation of dermal elastic fibers may also be found.[18] Thus, melasma, which was hitherto considered to be a pure pigmentary disorder, is now considered to be a disorder of skin photoaging.[26]
Exogenous ochronosis
Clinical profile
Exogenous ochronosis is a condition characterized by bluish-black hyperpigmentation, usually occurring following the application of hydroquinone-containing creams. This condition may present as speckled pigmentation, papules or nodules over the zygomatic region of the face and can sometimes closely resemble melasma.[27]
Dermoscopic profile
Dermoscopy in exogenous ochronosis shows dark brown globules.[27] Curvilinear and worm-like structures have also been described, which may be arranged in a reticular pattern. These findings help in differentiating this condition from melasma, which usually shows an accentuated pseudoreticular pigment network.[28]
Histopathological profile
Histopathology remains the gold standard in the diagnosis of exogenous ochronosis. Characteristic ochre-colored banana-shaped bodies may be seen in the dermis, along with swelling of collagen bundles. These “banana bodies” may be highlighted using methylene blue stain.[27]
Pigmentary demarcation lines
Clinical profile
Pigmentary demarcation lines are physiological lines characterized by abrupt transition from dark pigmented areas to lighter pigmented skin, which is commonly encountered in females. These lines are present over the face, trunk, and limbs (Groups A–H), out of which groups F, G, and H have been described on the face. Group F presents as a “V”-shaped region of pigmentation over the lateral part of the face, Group G presents as a “W”-shaped region of pigmentation over the lateral face, and Group H presents as a band of pigmentation from the angle of mouth extending to the lateral chin. This condition is often mistaken for melasma and post-inflammatory hyperpigmentation.[29]
Dermoscopic profile
Dermoscopy in pigmentary demarcation lines shows an abrupt transition between the lighter and darker areas of skin in the form of an increased pseudoreticular pigment network. White clods have also been found in the area of increased pigment network.[29]
Lichen planus pigmentosus (LPP)
Clinical profile
LPP is a condition characterized by asymptomatic slate-gray pigmentation, usually seen over the face- and sun-exposed areas. This condition was first described in India by Bhutani et al.[30] in 1974 – who described the clinical and histopathological features in 40 patients. Subsequently, several studies have been conducted describing this condition in various parts of the world.[31]
LPP usually presents during middle age, with a female preponderance. The photo-distributed variant is usually seen in darker Fitzpatrick skin types. LPP is thought to be a delayed hypersensitivity reaction to an unknown antigen, which subsequently leads to basal cell degeneration and melanin incontinence.[31] In India, this condition is thought to be associated with the use of mustard oil, hair dye, gooseberry oil, and cosmetics. Some other associations include co-existing lichen planus, hepatitis C, autoimmune thyroid disease, alopecia areata, and vitiligo.[32]
LPP has an insidious onset, presenting as slate-gray to brown macules, usually over the face and photo-exposed areas [Figure 3]. There is usually no history of preceding erythema or inflammation. The various patterns of pigmentation include diffuse, reticular blotchy, perifollicular, gyrate, and annular. Rare patterns such as linear and segmental pigmentation have also been described.[31]
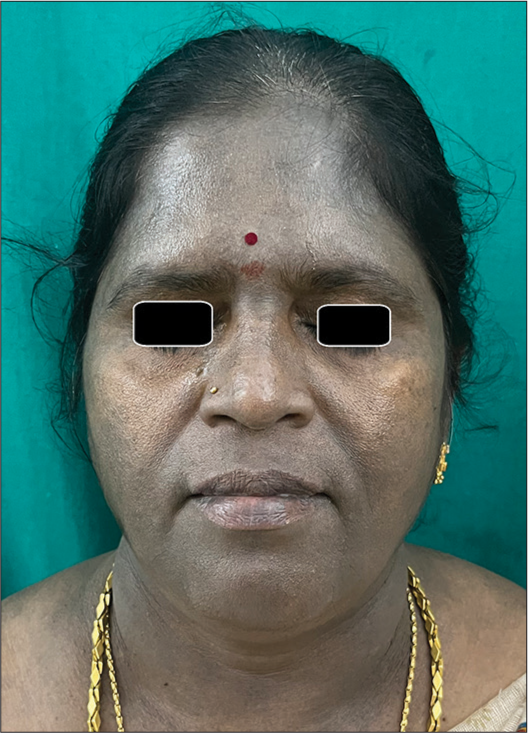
- Clinical image of lichen planus pigmentosus showing diffuse slate-gray pigmentation.
Acquired dermal macular hyperpigmentation is a recently coined umbrella term used to describe conditions with dermal pigment incontinence, with or without active interface dermatitis. This umbrella term includes LPP, ashy dermatosis, and Riehl’s melanosis (pigmented contact dermatitis). The above-mentioned conditions show considerable clinical and histological overlaps with very subtle differentiating features [Table 5].[32]
| Lichen planus pigmentosus | Ashy dermatosis | Riehl’s melanosis | |
|---|---|---|---|
| Geographic distribution | Reported commonly in India | Reported commonly in Mexico, South American countries | Commonly reported in Japan |
| Etiology | Mustard oil, hair dye, cosmetics | Ammonium nitrite, radiocontrast media, whipworm infections, Cobalt allergy | Cosmetics, textiles, para-phenylenediamine in hair dyes |
| Morphology | Slate-gray to brown macules over face, neck, and outer arms | Ash colored macules predominantly over trunk, early lesions may show erythematous elevated borders | Diffuse or patchy brown pigmentation, ill-defined borders, common over forehead, temple regions |
ADMH: Acquired dermal macular hyperpigmentation
Patients with pigmented contact dermatitis usually complain of mild itching, and the pigmentation may be accompanied by fine whitish scaling. In patients with contact dermatitis to paraphenylene diamine (PPD)-containing hair dyes, the pigmentation may involve the helix, lobule of the ear and upper back. Patients with contact dermatitis to textile materials may show pigmentation over the axilla, which characteristically spares the vault. A detailed cosmetic history is, therefore, essential to differentiate this condition from LPP.[32]
Patch testing for common allergens may be positive even in the general population. Therefore, some dermatologists consider LPP to have spectral overlap with pigmented contact dermatitis rather than being a separate condition.[33]
Ashy dermatosis was first described in El Salvador[34] Subsequently, it was also termed “Erythema dyschromicum perstans” in view of the characteristic erythematous, raised border seen in early lesions. In a global consensus statement on acquired dermal pigmentary disorders,[35] it was decided that the presence of an erythematous border (current or past) surrounding the areas of pigmentation was essential to making a diagnosis of erythema dyschromicum perstans.[35]
Dermoscopic profile
Sharma et al.[36] performed dermoscopy and patch testing in 50 patients with LPP. The most common dermoscopic finding described included brown to gray dots and globules with varied patterns of arrangement [Table 6 and Figure 4].
| Study | Dermoscopic finding | Frequency (n=50) (%) |
|---|---|---|
| Sharma et al.[19] | Dots | 17 (34) |
| Globules | 13 (26) | |
| Dots + Globules | 13 (26) | |
| Arrangement of dots and globules | ||
| Incomplete reticular | 17 (39.5) | |
| Hem-like | 9 (20.9) | |
| Arcuate | 8 (18.6) | |
| Complete reticular | 6 (14) | |
| Not otherwise specified | 3 (7) | |
| Other dermoscopic features | ||
| Exaggerated pseudoreticular pattern | 21 (42) | |
| Pigmentation around the hair follicle | 9 (18) | |
| Targetoid appearance | 7 (14) | |
| Obliteration of reticular pattern | 2 (4) |
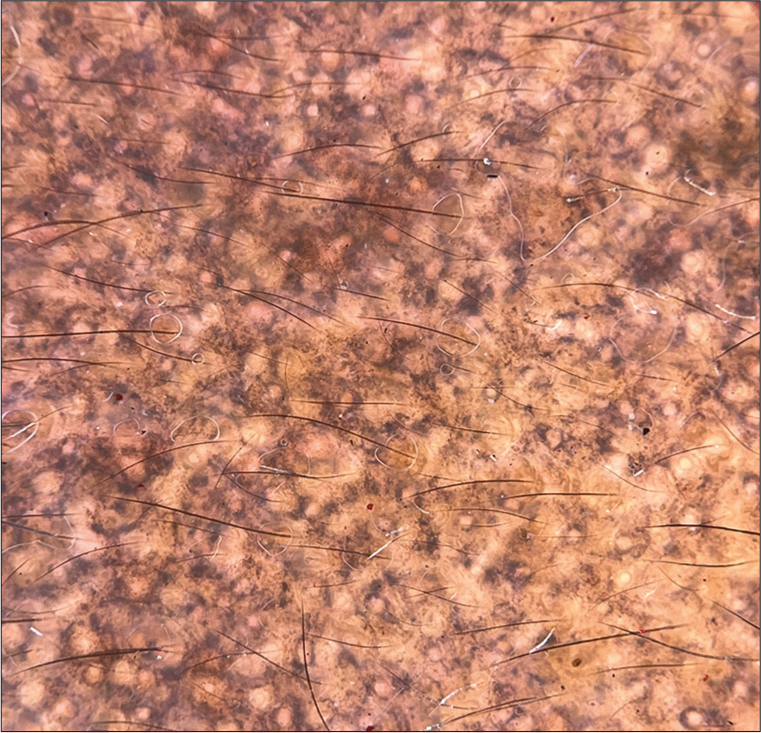
- Dermoscopy of lichen planus pigmentosus (DermLite DL4, ×10, polarized) showing blue dots, gray dots.
Additional dermoscopic findings include dots and globules arranged in a “Chinese letter pattern,” as described by Devanda et al.[37] in a cross-sectional study on 27 patients with LPP.[37] Although Riehl’s melanosis shows significant overlap with LPP in terms of clinical and dermoscopic features, Wang and Xu[38] described the presence of “flour-like” scales in the former, which may be a differentiating feature. Follicular plugging and perifollicular halo have also been described in Riehl’s melanosis.[38]
However, some authors do not consider any dermoscopic feature to be specific enough to differentiate between the various acquired dermal macular hyperpigmentary disorders. A dermoscopic grading system has been devised for these disorders based on the number and arrangement of dots and globules [Table 7].[32]
| Grade | Dermoscopy findings |
|---|---|
| Grade 1 | Discretely arranged dots, no pattern |
| Grade 2 | Dots and globules coalescing in a broken-net/Chinese letter pattern |
| Grade 3 | Dots and globules form a net-like pattern |
| Grade 4 | Extensive dots and globules, only sparing eccrine and sebaceous openings |
ADMH: Acquired dermal macular hyperpigmentation
Histopathological profile
The histopathological profile of LPP varies with disease duration. Early lesions show extensive interface dermatitis, which later results in pigment incontinence [Figure 5]. Older lesions show dermal melanophages without active inflammation at the dermoepidermal junction. Immune deposits have been reported in around 15% of patients with this condition, which may include immunoglobulin M C3, deposits in a linear pattern or the colloid bodies.[31]
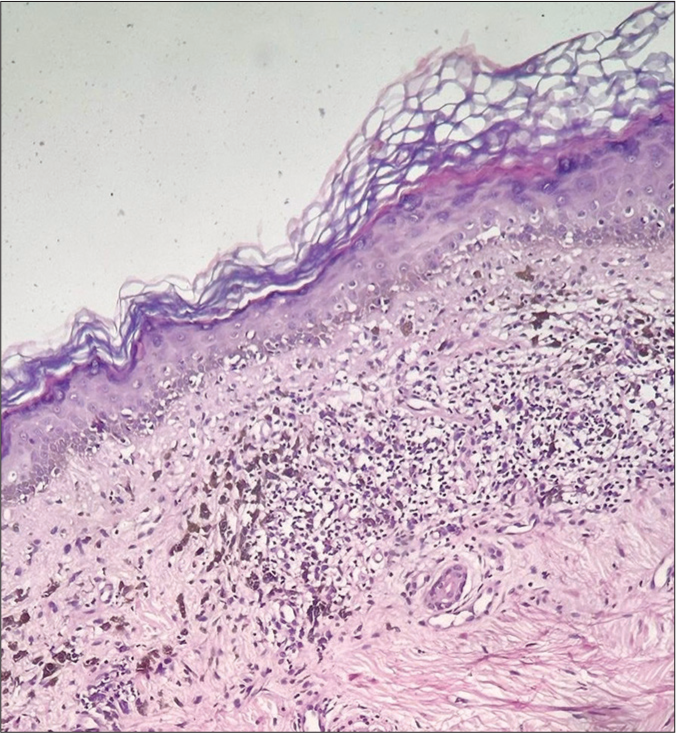
- Histopathology in lichen planus pigmentosus (H&E, ×200) showing basal vacuolar degeneration and pigment incontinence in the dermis. H&E: Hematoxylin and eosin.
Although Riehl’s melanosis occurs due to contact dermatitis to various agents, spongiosis is not a commonly described finding in this condition.[38] Patch testing is therefore considered essential to arrive at a diagnosis of pigmented contact dermatitis.[31]
Ashy dermatosis obtains its characteristic color from melanophages in the upper dermis. Interface dermatitis is a less frequently encountered finding and is therefore not essential to diagnose this condition.[35]
Since there is considerable overlap between the various acquired dermal macular hyperpigmentary conditions, a detailed drug history needs to be elicited, and patch testing may be considered in select cases to arrive at a possible diagnosis.[32]
Chronic cutaneous lupus erythematosus (LE)
Clinical profile
Chronic cutaneous LE is of varied clinical types, with s being the most commonly encountered variant.[39] This condition presents as well-defined erythematous scaly plaques, usually over the head and neck region, but may also be disseminated in other areas [Figure 6]. Well-established lesions may show atrophy, scarring, telangiectasia, and pigmentary alterations.[40]
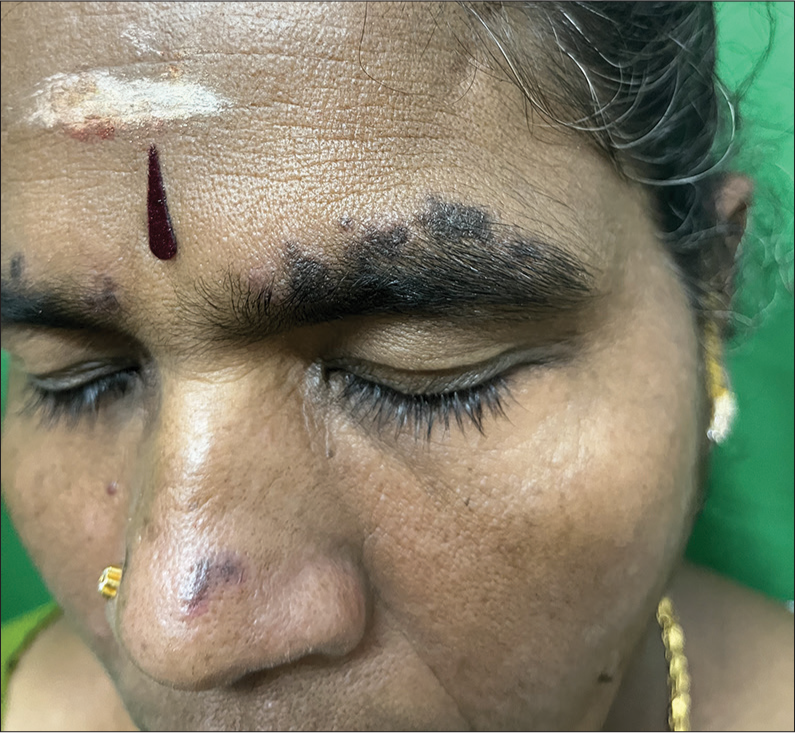
- Clinical image of discoid lupus erythematosus.
A newer variant of chronic cutaneous LE presenting as hyperpigmented macules over the head and neck region has been described. Atrophy and scarring were conspicuously absent in these lesions, making the diagnosis challenging. However, histopathology and direct immunofluorescence were consistent with the diagnosis of chronic cutaneous LE.[41]
Another variant of chronic cutaneous LE presenting as reticulate pigmentation over the face has been reported. The pigmentation had a slate-grey to lichenoid hue, with few areas of depigmentation interspersed in between. Histopathology, direct immunofluorescence and antinuclear antibodies (ANA) titers were employed to clinch the final diagnosis of cutaneous LE.[39]
Dermoscopic profile
DLE has been described to have various dermoscopic findings based on the site of the lesion and the duration of the disease.[42]
The dermoscopic features in classical DLE include follicular plugging, patchy scaling, perifollicular halo, structureless white areas, rosettes, starburst pattern, and speckled pigmentation. Vascular patterns include red dots, linear blood vessels, arborizing vessels, and perifollicular erythema [Figure 7].[43]
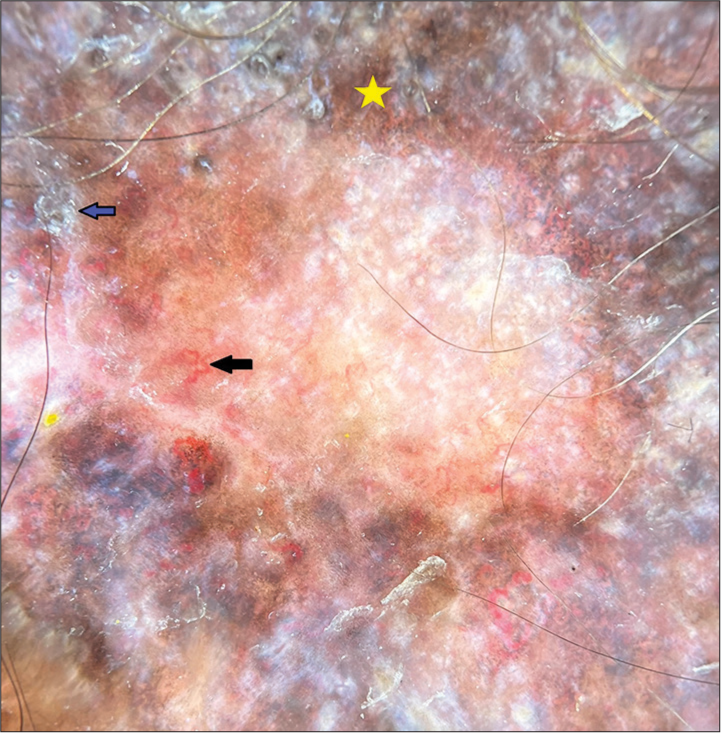
- Dermoscopy of discoid lupus erythematosus (DermLite DL4, ×10, polarized) showing peripheral hyperpigmentation (yellow star), telangiectasia (black arrow), and scaling (blue arrow).
The dermoscopic features of a pigmented macular variant of chronic cutaneous LE have not been extensively described in the previous literature as it is an under-recognized entity.
Histopathological profile
Classical DLE shows ortho/hyperkeratosis, epidermal atrophy, basal vacuolar degeneration, periadnexal lymphocytic infiltration, basement membrane thickening, and dermal deposition of mucin.[44] Histopathology from the pigmented macular variant shows epidermal atrophy, basal vacuolar degeneration, and melanin incontinence. Direct immunofluorescence shows linear deposition of immunoreactants along the basement membrane zone.[39]
Facial acanthosis nigricans
Clinical profile
Facial acanthosis nigricans has an appearance similar to that of acanthosis nigricans at other sites and commonly involves the temporal regions, cheeks, chin, periorbital, and perioral regions.[45,46] The patient may show evidence of acanthosis nigricans at other characteristic sites, and there may be overlying acrochordons.[47] Accurately diagnosing this condition is important as it is a clue to underlying insulin resistance and metabolic complications.[48]
Dermoscopic profile
In a clinico-investigative study conducted on 40 patients diagnosed with facial acanthosis nigricans, it was found that all cases showed cristae cutis, sulci cutis, and hyperpigmented dots within the cristae. Other patterns commonly observed include brown dots, globules, and papillary projections.[48] It has been observed that non-polarized dermoscopy images highlight the cristae and sulci clearly, while polarized dermoscopy highlights pigmented dots.[49] Further, the increase in severity of acanthosis nigricans corresponds to increased cristae and sulci on dermoscopy.[50]
Dermoscopy may also be used to assess the severity of facial acanthosis nigricans – with mild cases showing only sulci, which can progressively become interconnected with folding of cristae and hyperpigmentation in moderate-to-severe facial acanthosis nigricans. Chronic cases may show additional findings such as perifollicular hyperpigmentation and large pigmented globules.[48]
Histopathological profile
Histology of facial acanthosis nigricans shows hyperkeratosis, increased basal melanization, acanthosis, and papillomatosis. The cristae cutis on dermoscopy represents the epidermis, which is elevated by the underlying papillomatosis in the dermis. The sulci correspond to the surrounding epidermis, with the white color being due to the basket-weave cornified layer in the downwardly depressed epidermis. The papillary projections seen on dermoscopy are thought to be due to extreme papillomatosis.[48]
Maturational hyperpigmentation
Clinical profile
Maturational hyperpigmentation is a newly described facial melanosis – which is thought to be a cutaneous marker of metabolic syndrome, similar to acanthosis nigricans. It clinically presents as asymptomatic hyperpigmentation over the cheek or zygomatic region with a soft, granular surface without well-defined margins [Figure 8]. Dermoscopy and histopathology are distinct from other causes of facial melanosis.[51]
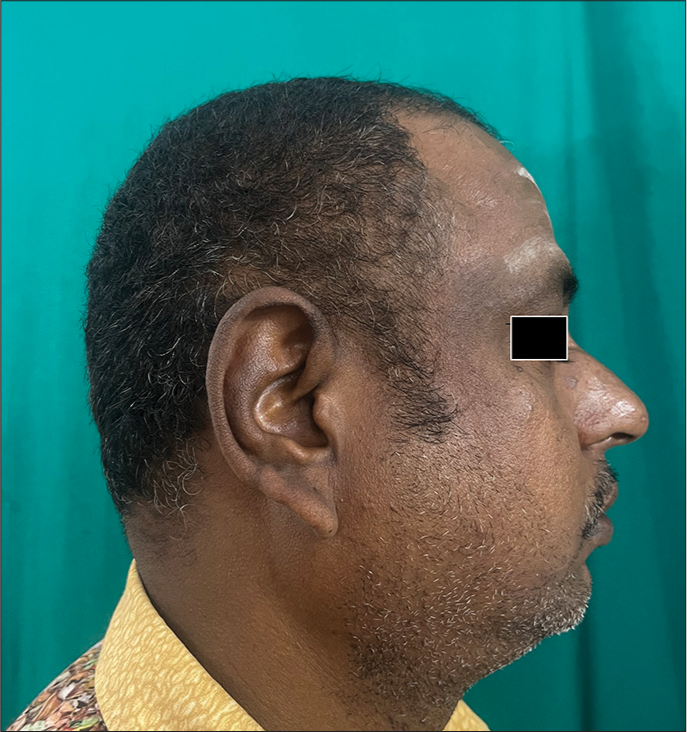
- Clinical image of maturational dyschromia.
Dermoscopic and histopathological profile
Dermoscopy may show an brown exaggerated pseudoreticular pigment network, brown dots and globules, and characteristic “ring-like structures” which are brown oval or round rings showing perifollicular accentuation [Figure 9]. Histopathology shows increased epidermal and basal cell melanization with minimal papillomatosis.[52]
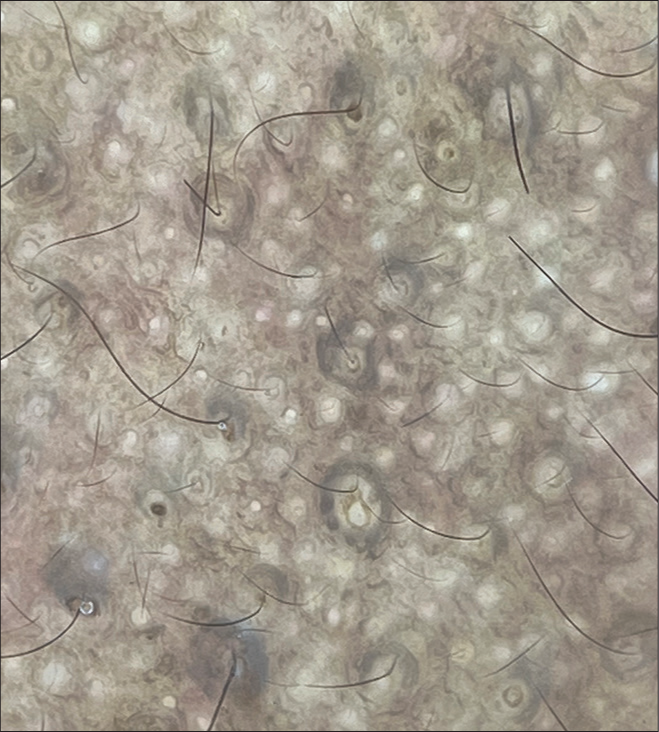
- Dermoscopy in maturational dyschromia (DermLite DL4, ×10, non-polarized) showing characteristic perifollicular ring structures
Maturational hyperpigmentation is an under-recognized facial melanosis which has been described in African and Indian populations. Studies conducted on Indian patients have shown fasting hyperglycemia and hyperinsulinemia in patients with maturational hyperpigmentation.[51]
Post-inflammatory hyperpigmentation
Clinical profile
Post-inflammatory hyperpigmentation is a condition commonly encountered in individuals with skin of color, which may last for months to years and can be a cause of significant distress to patients. This condition may arise following several dermatological conditions, including acne vulgaris, atopic dermatitis, lichen planus, contact dermatitis, cutaneous infections, vesicobullous disorders, and chronic cutaneous LE. This condition may also arise following mechanical injury to the skin or following dermatological procedures such as lasers and chemical peels.[53]
Post-inflammatory hyperpigmentation presents as macules over the region of initial inflammation. The color of the lesion may vary depending on the depth of the melanin pigment.[54] The pigmentation is more intense in higher Fitzpatrick skin types and may also worsen with ultraviolet ray exposure or recurrent inflammation.[53]
Dermoscopic profile
Jurairattanaporn et al. [54] found that the common dermoscopic patterns of post-inflammatory hyperpigmentation over the face include increased pseudoreticular pigment network, clustered aggregated lobules, homogenous pigmentation, and arcuate-annular structures. Vascular patterns were also commonly observed, including ill-defined erythema, red dots, straight lines, glomerular vessels, and arborizing vessels.[54]
Histopathological profile
Epidermal post-inflammatory hyperpigmentation has been found to have increased basal layer pigmentation. Dermal post-inflammatory hyperpigmentation has been described as melanophages in the upper dermis, which may be accompanied by lymphoplasmacytic infiltrate and basal layer vacuolation.[55]
Seborrheic melanosis
Clinical profile
Seborrheic melanosis is a hitherto ill-defined clinical entity, largely described by dermatologists in India. The condition is said to affect the alar grooves, angles of the mouth, and labiomental crease – which are considered to be seborrheic areas of the face. Individuals with Fitzpatrick skin types IV and above are thought to be primarily affected by this condition.[56]
Some dermatologists hypothesize that seborrheic melanosis is a form of post-inflammatory hyperpigmentation to seborrheic dermatitis of the face.[57] Some patients may give a history of preceding flakiness or redness of skin before developing hyperpigmentation. Clinical diagnosis may be aided by some supportive findings such as oily skin, plugging of follicles with inspissated sebum, or concomitant seborrheic capitis.[56]
Dermoscopic profile
Verma et al.[56] described the dermoscopic features of seborrheic melanosis in 12 patients with skin of Fitzpatrick types III, IV, and V. The dermoscopic findings were divided into – vascular, pigmented, and mixed patterns. The vascular pattern showed a light pink background and arborizing vessels which were out of focus. The pigmented type of presentation was found in darker skin types – with prominent brown pigmented pseudonetwork and annular granular structures not occluding the orifices. Grey dots and clods were conspicuously found to be absent. The finding seen most commonly among patients with seborrheic melanosis include white excrescences of sebum coating the vellus hair shafts and prominence of follicular openings.[56] Dermoscopy from alar grooves of healthy individuals may occasionally show erythema, brown globules, and vessels.[58]
Periorbital pigmentation
Clinical profile
Periorbital pigmentation, commonly known as “dark circles,” is a commonly encountered yet clinically ill-defined condition arising due to various causes. Genetic causes, post-inflammatory pigmentation, atopic diathesis, increased vasculature, tear trough deformity, and shadowing due to skin laxity have all been hypothesized to be some of the causative factors.[59] Further, habits such as lack of sleep, frequent rubbing of eyes, cosmetic use, and refractive errors have also been implicated.[60]
Periorbital pigmentation is described as bilateral homogenous hyperpigmentation predominantly involving the lower eyelid but may also extend to the upper eyelid, eyebrow region, temporal region, malar region, and the root of the nose.[60] Periorbital pigmentation has been graded from 0 to 4 based on the extent of pigmentation[61] [Table 8]. Periorbital pigmentation has been classified into pigmented, vascular, structural, and mixed types based on the color and pattern of pigmentation [Table 9].[62]
| Grade | Extent of periorbital melanosis |
|---|---|
| 0 | Skin comparable to other facial skin areas |
| 1 | Faint pigmentation of infraorbital fold |
| 2 | Pigmentation is more pronounced that in grade 1 |
| 3 | Deep dark color involving all lids |
| 4 | Pigmentation spreading beyond the infraorbital fold. |
| Type of pigmentation | Clinical description | Effect on stretching eyelid |
|---|---|---|
| Pigmented | Brown hue | No effect |
| Vascular | Red/purple/blue hue | Increased pigmentation |
| Structural | Loss of fat, blepharoptosis, and tear trough deformity may be associated | Pigmentation due to tear trough deformity improves on stretching |
| Mixed | Combination of two or three of above appearances | Variable |
Dermoscopic profile
Dermoscopic patterns described in periorbital pigmentation include scattered brown dots, blotches, and increased pigment network, predominantly in the pigmented type. Vascular type of periorbital pigmentation was found to have dilated veins, telangiectasia, along with brown dots. An additional feature seen in the structural type of periorbital pigmentation is an increase in skin markings.[63]
Histopathological profile
Nayak et al.[64] in their study on 50 patients with periorbital pigmentation, it was found that 90% of the patients had increased melanin in the basal and spinous layers. Dermal melanin incontinence, melanophages in the upper dermis, and lymphoplasmacytic perivascular infiltrate were uniformly found in all the patients studied. Increased pigmentation of the basal layer of vellus hair follicles in the infraorbital region has also been reported. Perl’s stain did not demonstrate hemosiderin in any of the cases.[64]
Nevus of Ota
Clinical profile
Nevus of Ota or oculodermal melanocytosis is a condition characterized by pigmentation of the skin and mucus membranes along the distribution of the first two branches of the trigeminal nerve. The onset is usually at birth or in early childhood, presenting as a bluish-gray macule which is usually unilateral, with irregular borders. The ipsilateral sclera and palate may also be involved. The diagnosis is usually made clinically, with histopathology being reserved for doubtful cases.[65]
This condition is thought to be due to the defective migration of melanocytes from the neural crest to the epidermis, resulting in melanocytes being trapped within the dermis. Production of melanin within the dermis produces a bluish hue due to the Tyndall effect.[66]
Dermoscopic profile
Commonly encountered dermoscopic findings include gray-brown structureless areas, brown-gray dots, and white clods in a “four-clod dots” arrangement. Characteristically, the reticular pigment network is usually not seen over the nevus.[67] The presence of terminal hair with perifollicular hypopigmentation has also been described.[65]
Histopathological profile
Histology reveals pigmented dendritic melanocytes within the reticular dermis, with dissection of dermal collagen bundles by melanophages.[67]
A close clinical and histological differential for nevus of Ota is Hori’s nevus, which is a dermal melanocytosis usually encountered among Asian patients.[22] However, this condition commonly presents in the third or fourth decade as symmetrical slate gray macules over the face without mucosal involvement.[65]
Differentiating dermal melanocytosis from melasma is important to formulate an appropriate treatment approach, with depigmenting agents and chemical peels being used for the former and pigment-specific lasers being preferred for the latter.[22] Dermoscopy may aid in accurate diagnosis, thereby obviating the need for skin biopsy in patients who have cosmetic concerns.[65]
Pigmented transverse nasal band
Clinical profile
Transverse nasal line is an embryological defect first described by Cornbleet in 1951, who also coined the synonyms transverse nasal groove, stria nasi transversa, and transverse nasal strip. This entity is thought to be due to an embryological defect during the fusion of the triangular and alar cartilages of the nose.[68]
A pigmented transverse nasal band is a band-like eruption measuring about 2 mm in width, spanning the entire nasal bridge.[69] It is an asymptomatic condition with an incidence of about 0.3% in the Indian population. Due to the faulty embryological line, retention cysts may occasionally arise over the transverse nasal band in the form of milia or pseudocomedones.[70]
Differential diagnoses for this condition include allergic salute and wrinkles due to aging, which may be distinguished by certain clinical clues [Table 10].
| Pigmented transverse nasal band | Allergic salute | Wrinkles due to aging | |
|---|---|---|---|
| Number of lesions | Solitary | May be multiple | May be multiple |
| Effect on stretching | No change | Disappears | Flattens |
| Width of lesion | Full width of nose | Does not extend to the full width of the nose | Does not extend to the full width of the nose |
| Associations | Seborrheic dermatitis, acne, atopic dermatitis, dermatosis papulose nigra | Allergic rhinitis | Other signs of photoaging including senile comedones, dyspigmentation |
Dermoscopic profile
Dermoscopic features of this condition include microcomedones appearing as brown dots and milia overlying white transverse streaks. These transverse streaks are thought to arise from adhesions and fibrosis in the nasal cartilage. Histopathology and surgical modalities are avoided in this condition due to the potential damage that can be produced to the nasal cartilage and the transverse nasal vein.[70]
Congenital melanocytic nevus
Clinical profile
Congenital melanocytic nevi (CMN) are neural crest-derived hamartomas that present at birth or shortly thereafter. This condition is prevalent in about 1–6% of newborns. These nevi are classified based on their size into small, medium, and large – measuring <1.5 cm, 1.5–20 cm, and >20 cm, respectively.[71] Larger lesions are associated with an increased risk of developing melanoma. The risk of melanoma varies greatly based on the clinical characteristics of the lesion.[72]
Dermoscopic profile
Dermoscopy is a useful tool for the diagnosis, follow-up, and management of benign melanocytic lesions, including CMN.[72] It is also useful for differentiating benign and malignant melanocytic lesions.[73]
Various dermoscopic patterns may be seen in CMN including reticular pattern, globular pattern, homogenous pattern, and cobblestone pattern. A combination of patterns may also be seen, including a reticuloglobular pattern, peripheral reticular pattern with central homogenous pigmentation, and patchy reticular pattern. Some lesions may also show additional features such as radial streaks, hypertrichosis, focal areas of hypopigmentation, milia-like cysts, and regression structures.[71]
Histopathological profile
On histopathological examination, CMN may show nevus cells in both the epidermis and dermis. They may form orderly clusters, sheets, cords, or nests of cells. Nevus cells are found to aggregate around appendages such as hair follicles sebaceous glands, and also around neurovascular structures. Deeper extension of nevomelanocytes and periappendigeal distribution of cells are some of the features that help in differentiating this condition from acquired melanocytic nevi.[74]
Follicular Becker’s nevus
Clinical profile
Becker’s nevus was first described in 1949 by Becker as a localized hyperpigmented macule with overlying hypertrichosis, classically occurring over the shoulder region during adolescence.[75]
Follicular Becker’s nevus is a newly described variant, presenting as hyperpigmented follicle-based macules. These lesions present unilaterally over the chest or scapular region, but may also occur over other sites.[76] There may or may not be hypertrichosis/thick hair within the center of each follicular macule.[77] The presence of folliculocentric macules might suggest a role of follicular epithelium in the pathogenesis of this condition.[78]
Dermoscopic profile
The dermoscopy from folliculocentric macules shows perifollicular hypopigmentation surrounded by a network of increased pigmentation.[78] There are areas of normal pigment network interspersed in between. In another study, the presence of thick hair has been described in the center of these hyperpigmented macules.[77]
Histopathological profile
Hyperkeratosis, acanthosis, increased basal cell pigmentation, and, occasionally, the presence of melanophages in the papillary dermis have been reported. There is an increase in melanin content but no increased melanocyte count.[79]
Dowling–Degos disease
Clinical profile
Dowling–Degos disease is an autosomal dominant genodermatosis, with onset usually during early adulthood. This condition is characterized by reticulate pigmentation over the flexures. Additional features, such as perioral pits and follicular lesions, may be seen.[80] Variants of this condition include generalized type, follicular variant, and acantholytic variant (Galli-Galli disease), to name a few. Overlap with other reticulate pigmentary disorders has also been described.[81]
Dermoscopic profile
Follicular Dowling–Degos presents with reticulate pigmentation, follicle-based papules, pitted acneiform scars, and comedones predominantly over the face, upper trunk, and flexures. Dermoscopy shows a characteristic “Chinese-letter pattern” of linear pigmentation, which may occur over a background of normal or hyperpigmented skin. Follicular inclusion cysts may also be seen.[82]
Histopathological profile
The histopathology of Dowling–Degos disease shows elongated hyperpigmented rete ridges, forming an “antler-like pattern.” Suprapapillary thinning and increased melanin at the tips of rete ridges are also seen.[83] In the follicular variant, these changes are observed to be confined to the follicular infundibulum.[82]
DISORDERS OF FACIAL HYPOPIGMENTATION
Vitiligo
Clinical profile
Vitiligo is a commonly encountered acquired pigmentary skin disease associated with significant social stigma in some communities.[84] This condition may sometimes mimic other disorders of hypopigmentation, like idiopathic guttate hypomelanosis. This condition presents as depigmented macules and may also affect the hair and mucosa.[85]
Based on the distribution of lesions, vitiligo may be divided into segmental and non-segmental types. Non-segmental vitiligo may be further divided into acrofacial, generalized, mucosal, mixed, and other rare types.[85] The clinical markers of the active disease include a trichrome sign, Koebnerization, inflammatory margins, and confetti-like depigmentation.[86]
Decisions regarding the management of this condition are made based on the stability of the lesions. The stability of vitiligo lesions may be assessed by both clinical examination and dermoscopy.[87]
Dermoscopic profile
Nirmal et al.[87] performed dermoscopy on 85 patients clinically diagnosed with vitiligo. Dermoscopy was found to be a reliable tool for assessing the stability of vitiligo lesions, thereby obviating the need for skin biopsy [Table 11].[87]
| Stablevitiligo | Unstablevitiligo | Re-pigmenting lesions |
|---|---|---|
|
|
|
Other dermoscopic findings in progressive vitiligo include starburst appearance, comet-tail appearance, and sago grain appearance. Intralesional and perilesional erythema have been found to be indicators of stable and re-pigmenting lesions. Most of the dermoscopic clues regarding disease activity are located in the perifollicular region.[84]
Histopathological profile
Vitiligo is characterized by basal hypopigmentation – which is due to an absent or significantly decreased number of melanocytes. Mild dermal inflammation may also accompany the epidermal findings. Immunohistochemistry for melanin may be performed to confirm the above-mentioned findings.[88]
Nevus depigmentosus
Clinical profile
Nevus depigmentosus is a relatively common condition, presenting as a hypopigmented macule with no significant change in size or distribution throughout life. Although most cases are thought to be congenital, there have been reports of cases presenting later in life.[89]
The original diagnostic criteria suggested by Coupe in 1976 include the presence of leukoderma at birth, without alteration of distribution, texture or sensations over the lesion, and an absence of hyperpigmented border around the achromic area.[90]
This condition presents as a well-defined hypopigmented macule, usually having serrated borders.[91] Different clinical variants, such as isolated, segmental, and whorled, have been described based on the distribution of the lesion. Wood’s lamp usually shows an off-white accentuation of the macule.[89]
Although the diagnosis of this condition is usually clinical, dermoscopy may aid in differentiating it from vitiligo and ash leaf macules.[92]
Dermoscopic profile
Dermoscopy reveals the presence of structureless white areas, with few areas showing a faint pigment network. There may be peripheral projections known as pseudopods, which correspond to the serrated borders of the lesion. Total loss of pigment network is usually not a feature of nevus depigmentosus, which aids in differentiating these lesions from vitiligo.[91] Further, the hair within these lesions is of normal color, as opposed to leukotrichia, which may be found in vitiligo.[92]
Nevus anemics is a close differential, presenting as a hypopigmented macule since birth. Diascopy of the lesion classically shows the blending of the borders of the lesion with the surrounding skin. The same test can be replicated using contact dermoscopy. Further, non-contact dermoscopy reveals decreased vasculature in the lesional skin, with a compensatory increase in blood vessels in the surrounding skin.[93]
Histopathological profile
Nevus depigmentosus is thought to be caused by defective melanosome synthesis and transfer, as can be demonstrated by decreased staining compared to normal skin using the Fontana–Masson stain. Ultrastructural studies have demonstrated a normal number of melanocytes with a reduction in melanosomes.[89] These findings co-relate with the dermoscopic features of a faint pigment network, as opposed to the complete absence or reversal of pigment network, which is seen in some lesions of vitiligo.[92]
Indeterminate leprosy
Clinical profile
An indeterminate leprosy is an early form of leprosy presenting as an ill-defined hypopigmented patch, which usually has a minimal sensory impairment and a negative slit skin smear examination. This form of leprosy may progress to tuberculoid and then lepromatous forms if left untreated. The common sites affected by this condition include the face, outer thighs, and extensor aspect of limbs.[94]
Dermoscopic profile
Dermoscopic findings in indeterminate Hansen include patchy areas of decreased pigment network with decreased appendageal structures in comparison to normal skin. These findings are more pronounced in borderline tuberculous leprosy but when present in indeterminate leprosy, may be a useful tool to arrive at an early diagnosis.[95]
Histopathological profile
Histology shows predominantly perineural infiltrate, with very scant or absent bacilli. Small numbers of epithelioid histiocytes may also be found in the perineural region.[96]
Pityriasis alba
Clinical profile
Pityriasis alba is a commonly-encountered benign skin condition characterized by hypopigmented scaly macules, usually in atopic children with darker skin types. This condition may often be mistaken for leprosy or vitiligo – both of which are associated with significant social stigma. The lesions may begin as mildly erythematous macules, which may progressively become hypopigmented and scaly. This may be followed by a chronic stage not associated with scaling.[97]
Dermoscopic profile
Pityriasis alba shows ill-defined areas of decreased pigment network with fine scaling and pigmented overlying hair.[97] The scaling in pityriasis alba is uniformly distributed throughout the lesion, which helps in differentiating this condition from pityriasis versicolor, where the scaling may accumulate within the skin creases.[5]
Histopathological profile
Histopathology in pityriasis alba is usually non-specific, showing a mild chronic inflammatory infiltrate and decreased basal melanin pigmentation. Non-specific features such as acanthosis, hyperkeratosis, spongiosis, and perivascular infiltrates may also be found.[97]
Tinea versicolor
Clinical profile
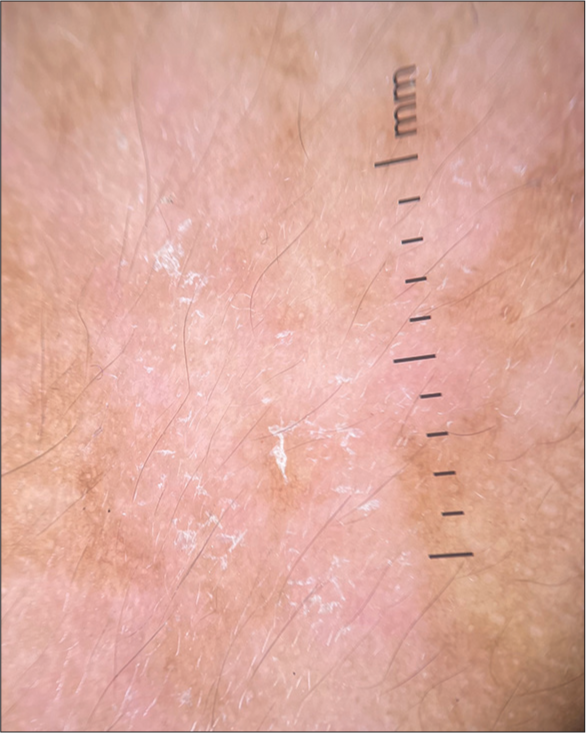
Tinea versicolor is a condition caused by lipophilic yeasts belonging to the genus Malassezia. It is characterized by macules with fine branny scales usually distributed over the trunk and upper back but may also be seen in other seborrheic regions, including the face [Figure 10].[98]These scaly macules may show varied pigmentation (both hypopigmentation and hyperpigmentation) and can coalesce to form larger macules.[99]
- Clinical image of hypopigmented tinea versicolor.
This condition is usually diagnosed based on clinical examination and KOH mount, but dermoscopy has been found to be a useful ancillary diagnostic tool.[100]
Dermoscopic profile
Non-uniform pigmentation within the lesion was found to be a common dermoscopic finding in both hypopigmented and hyperpigmented variants. Other patterns of pigmentation include-perilesional hyperpigmentation, clear demarcation of lesional border, and inconspicuous ridges and furrows.[101] Increased and decreased reticular pigment network have been described in the hyperpigmented and hypopigmented variants of tinea versicolor, respectively.[99]
Scaling is a hallmark feature of tinea versicolor, which has been shown to occur in various patterns on dermoscopy. Patchy scaling was commonly found in hypopigmented lesions, and scaling within the furrows was commonly found in hyperpigmented lesions [Figure 11].[101] Fine scaling along the natural skin creases has also been reported as a common finding.[102] Other patterns of scaling include diffuse, peripheral, and perifollicular scaling.[101]
- Dermoscopy in tinea versicolor (DermLite DL4, ×10, polarized) showing areas of decreased pigment network with overlying patchy scales.
Vascular patterns within the lesion were found uncommonly in a hypopigmented variant of tinea versicolor. This has been postulated to be due to ongoing inflammatory activity within the lesion.[101] Hypopigmentation of hair follicles within the lesion has also been reported, which may possibly be due to the invasion of hair follicles by the yeast.
A unique dermoscopic feature reported in tinea versicolor is the “contrast halo sign” - characterized by a rim of increased pigment network around hypopigmented lesions and vice versa.[99]
Seborrheic dermatitis
Clinical profile
Seborrheic dermatitis is a commonly encountered inflammatory condition, usually affecting the scalp, eyebrows, nasolabial folds, retroauricular region, and upper trunk. This condition affects around 1–3% of adults, with a male preponderance and is often more severe during winter.
Seborrheic dermatitis over the face may present as mildly hypopigmented to erythematous macules or plaques with varying degrees of scaling. The scales are typically described as yellowish and greasy.[103] This condition may often resemble rosacea – but the presence of a darker erythematous background, fine whitish scales, and association with demodicosis is more characteristic of the latter.[104]
Dermoscopic profile
The dermoscopic findings of seborrheic dermatitis over the scalp have been well-described, and dermoscopy is a useful tool in differentiating the former from scalp psoriasis.[105] The dermoscopic features of seborrheic dermatitis over the face include dotted vessels, curved vessels, and polymorphous vessels, which may be superimposed on a normal or light pink background. The distribution of these vessels may be patchy or in a network. Yellowish scaling may also be found on dermoscopy.[104]
Histopathological profile
Early lesions of seborrheic dermatitis may show spongiosis and the formation of a scale-crust. There is mild papillary dermal edema and dilated dermal blood vessels, which may correspond to the dermoscopic finding of red dots.[104] In subacute and chronic stages, there is psoriasiform hyperplasia with scale crusts in a folliculocentric distribution.[106]
Salt and pepper pigmentation
Clinical profile
Systemic sclerosis is a multisystem disorder characterized by skin thickening and induration with internal organ involvement.[107] This disorder may be accompanied by various forms of dyspigmentation, including salt and pepper pigmentation, Addisonian pigmentation, and pigmentary changes in the areas of sclerosis.[108]
Salt and pepper pigmentation clinically appears as well-defined depigmented lesions, with perifollicular sparing of pigmentation. Perifollicular sparing possibly occurs due to the rich vascular supply to this region, which preserves melanogenesis.[109]
Dermoscopic profile
Dermoscopy of salt and pepper pigmentation shows white homogenous areas, with preservation of pigmentation in the perifollicular areas. Regularly arranged brown dots at the periphery of new lesions have also been described, and this finding is thought to be a marker of disease progression.[110]
The presence of an off-white perifollicular halo has also been demonstrated, which may be useful in differentiating perifollicular pigment sparing in systemic sclerosis from repigmenting lesions in vitiligo.[111]
Histopathological profile
Histopathology shows areas of absent pigment in the basal keratinocytes, alternating with areas of normal pigmentation. Dermal melanophages may also be seen underlying the region of normal pigmentation.[110]
CONCLUSION
The increasing prevalence of facial dyschromias in the skin of color indicates the rising physical and psychological morbidity caused by this group of conditions. Large-scale studies on the prevalence of facial dyschromias, with clinical and dermoscopic co-relation, may aid in the diagnosis and management of the same.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Redefining the colour of Indian skin. J Eur Acad Dermatol Venereol. 2008;22:1263-4.
- [CrossRef] [Google Scholar]
- Role of cultural factors in the biopsychosocial model of psychosomatic skin diseases: An Indian perspective. Clin Dermatol. 2013;31:62-5.
- [CrossRef] [Google Scholar]
- Skin hyperpigmentation in Indian population: Insights and best practice. Indian J Dermatol. 2016;61:487-95.
- [CrossRef] [Google Scholar]
- Skin complexion and pigmentary disorders in the facial skin of 1204 women in 4 Indian cities. Indian J Dermatol Venereol Leprol. 2014;80:395-401.
- [CrossRef] [Google Scholar]
- Dermatoscopic features of pigmentary diseases in ethnic skin. Indian Dermatol Online J. 2021;12:24-33.
- [CrossRef] [Google Scholar]
- Dermoscopy of pigmentary disorders in brown skin. Dermatol Clin. 2018;36:473-85.
- [CrossRef] [Google Scholar]
- Facial dyschromias with unresolved boundaries in the skin of color. CSDM. 2022;2:1.
- [CrossRef] [Google Scholar]
- Clinical classification of pigmentary disorders In: Kumarasinghe P, ed. Pigmentary skin disorders. Cham: Springer International Publishing; 2018. p. :1-26. Available From: https://link.springer.com/10.1007/978-3-319-70419-7_1 [Last accessed on 2024 Apr 10]
- [CrossRef] [Google Scholar]
- Hyperpigmentation: Types, diagnostics and targeted treatment options. J Eur Acad Dermatol Venereol. 2013;27(Suppl 1):2-4.
- [CrossRef] [Google Scholar]
- Prevalence of pigmentary disorders: A cross-sectional study in public hospitals in Durban, South Africa. Int J Womens Dermatol. 2019;5:345-8.
- [CrossRef] [Google Scholar]
- Hyperpigmented skin conditions: A study of pattern and prevalence from a tertiary care hospital of North India. Int J Curr Adv Res. 2017;6:3562-5.
- [CrossRef] [Google Scholar]
- Clinical spectrum of facial hypermelanosis: A descriptive study from a tertiary care centre. Int J Res Dermatol. 2020;6:212.
- [CrossRef] [Google Scholar]
- Frequency of different types of facial melanoses referring to the Department of Dermatology and Venereology, Nepal Medical College and Teaching Hospital in 2019, and assessment of their effect on health-related quality of life. BMC Dermatol. 2020;20:4.
- [CrossRef] [Google Scholar]
- Clinical and dermoscopic assessment of patients with hypopigmented skin lesions-a cross-sectional study. Turk Arch Dermatol Venereol. 2023;57:138-44.
- [CrossRef] [Google Scholar]
- Dermoscopy in general dermatology: A practical overview. Dermatol Ther (Heidelb). 2016;6:471-507.
- [CrossRef] [Google Scholar]
- Differentiating malignant melanoma from other lesions using dermoscopy. Can Fam Physician. 2019;65:412-4.
- [Google Scholar]
- Melasma: A comprehensive update: Part I. J Am Acad Dermatol. 2011;65:689-97.
- [CrossRef] [Google Scholar]
- Clinical and epidemiologic features of melasma: A multicentric cross-sectional study from India. Int J Dermatol. 2019;58:1305-10.
- [CrossRef] [Google Scholar]
- A comparative study of dermatoscopic features of melasma and Hori's nevus in Asian patients. J Clin Aesthet Dermatol. 2022;15:16-20.
- [Google Scholar]
- Dermoscopic characteristics of melasma in Indians: A cross-sectional study. Int J Dermoscop. 2017;1:6-10.
- [CrossRef] [Google Scholar]
- Heterogeneous pathology of melasma and its clinical implications. Int J Mol Sci. 2016;17:824.
- [CrossRef] [Google Scholar]
- Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- [CrossRef] [Google Scholar]
- Dermoscopic criteria for differentiating exogenous ochronosis from melasma. Indian J Dermatol Venereol Leprol. 2013;79:819-21.
- [CrossRef] [Google Scholar]
- Facial pigmentary demarcation lines-a new dermoscopic finding. Indian Dermatol Online J. 2024;15:329-30.
- [CrossRef] [Google Scholar]
- Acquired dermal macular hyperpigmentation: An update. Indian Dermatol Online J. 2021;12:663-73.
- [CrossRef] [Google Scholar]
- Pigmented contact dermatitis vs lichen planus pigmentosus. Int J Dermatol. 1977;16:860-2.
- [CrossRef] [Google Scholar]
- A global consensus statement on ashy dermatosis, erythema dyschromicum perstans, lichen planus pigmentosus, idiopathic eruptive macular pigmentation, and Riehl's melanosis. Int J Dermatol. 2019;58:263-72.
- [CrossRef] [Google Scholar]
- Dermoscopy and patch testing in patients with lichen planus pigmentosus on face: A cross-sectional observational study in fifty Indian patients. Indian J Dermatol Venereol Leprol. 2017;83:656-62.
- [CrossRef] [Google Scholar]
- Dermoscopy of lichen planus pigmentosus: A case series. J Am Acad Dermatol. 2022;86:225-6.
- [CrossRef] [Google Scholar]
- Four views of Riehl's melanosis: Clinical appearance, dermoscopy, confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2014;28:1199-206.
- [CrossRef] [Google Scholar]
- Pigmented macular variant of chronic cutaneous lupus erythematosus: An under-recognized subset in dark skin. Clin Exp Dermatol. 2017;42:793-5.
- [CrossRef] [Google Scholar]
- Pigmented macular variant of chronic cutaneous lupus erythematosus: Clinico-dermoscopic features of three patients with skin of color. Dermatol Pract Concept. 2023;13:e2023044.
- [CrossRef] [Google Scholar]
- Localized chronic cutaneous lupus erythematosus masquerading as pigmented lesions: A new clinical subset? Lupus. 2006;15:292-5.
- [CrossRef] [Google Scholar]
- Dermoscopic criteria of discoid lupus erythematosus: An observational cross-sectional study of 28 patients. Indian J Dermatol Venereol Leprol. 2022;88:360-6.
- [CrossRef] [Google Scholar]
- Implications of dermoscopy and histopathological correlation in discoid lupus erythematosus in skin of color. Indian J Dermatol. 2022;67:5-11.
- [CrossRef] [Google Scholar]
- Histopathologic comparison of the subsets of lupus erythematosus. Arch Dermatol. 1990;126:52-5.
- [CrossRef] [Google Scholar]
- Cutaneous features of Crouzon syndrome with acanthosis nigricans. JAMA Dermatol. 2013;149:737-41.
- [CrossRef] [Google Scholar]
- Acanthosis Nigricans: Pointer of endocrine entities. Diagnostics (Basel). 2022;12:2519.
- [CrossRef] [Google Scholar]
- A study of pathogenesis of acanthosis nigricans and its clinical implications. Indian J Dermatol. 2011;56:678-83.
- [CrossRef] [Google Scholar]
- Clinico-investigative study of facial acanthosis Nigricans. Indian Dermatol Online J. 2022;13:221-8.
- [CrossRef] [Google Scholar]
- Dermatoscopy image characteristics and differences among commonly used standard dermatoscopes. Indian Dermatol Online J. 2017;8:233-4.
- [CrossRef] [Google Scholar]
- Therapeutic implications of dermoscopic findings in acanthosis Nigricans: A clinical and histopathological study. Dermatol Ther. 2020;33:e14521.
- [CrossRef] [Google Scholar]
- Maturational hyperpigmentation: Cutaneous marker of metabolic syndrome. Dermatol Pract Concept. 2020;10:e2020046.
- [CrossRef] [Google Scholar]
- Maturational hyperpigmentation: Clinico-dermoscopic and histopathological profile of a new cutaneous marker of metabolic syndrome. Pigment Int. 2018;5:54.
- [CrossRef] [Google Scholar]
- Postinflammatory hyperpigmentation: A review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- [Google Scholar]
- A comparative study of dermatoscopic features of acne-related postinflammatory hyperpigmentation in facial and nonfacial areas in Asian patients. J Clin Aesthet Dermatol. 2022;15:16-21.
- [Google Scholar]
- Two histopathological patterns of postinflammatory hyperpigmentation: Epidermal and dermal. J Cutan Pathol. 2017;44:118-24.
- [CrossRef] [Google Scholar]
- Seborrheic melanosis: An entity worthy of mention in dermatological literature. Indian J Dermatol Venereol Leprol. 2017;83:285-9.
- [CrossRef] [Google Scholar]
- Seborrheic melanosis: A unique under-recognized entity in ethnic skin. J Clin Aesthet Dermatol. 2022;15:15-6.
- [Google Scholar]
- What causes dark circles under the eyes? J Cosmet Dermatol. 2007;6:211-5.
- [CrossRef] [Google Scholar]
- Periorbital hyperpigmentation: A study of its prevalence, common causative factors and its association with personal habits and other disorders. Indian J Dermatol. 2014;59:151-7.
- [CrossRef] [Google Scholar]
- Clinical and dermoscopic evaluation of periorbital melanosis. J Cutan Aesthet Surg. 2022;15:154-60.
- [CrossRef] [Google Scholar]
- Periorbital hyperpigmentation: A comprehensive review. J Clin Aesthet Dermatol. 2016;9:49-55.
- [Google Scholar]
- A cross-sectional study on clinico-dermoscopic features of periorbital melanosis in a tertiary care hospital. J Cosmet Dermatol. 2021;20:2917-23.
- [CrossRef] [Google Scholar]
- A study of clinicopathological correlation of periorbital hyperpigmentation. Indian Dermatol Online J. 2018;9:245-9.
- [CrossRef] [Google Scholar]
- Adult onset nevus of Ota: Dermoscopic characterization of a rare entity-a case report. Apollo Med. 2023;20:269-71.
- [CrossRef] [Google Scholar]
- Nevus of Ota: A series of 15 cases. Indian J Dermatol Venereol Leprol. 2008;74:125-7.
- [CrossRef] [Google Scholar]
- Transverse nasal stripe at puberty (Stria Nasi transversa) AMA Arch Derm Syphilol. 1951;63:70-2.
- [CrossRef] [Google Scholar]
- Pigmented transverse nasal band: A distinct presentation. J Cosmet Dermatol. 2019;18:301-2.
- [CrossRef] [Google Scholar]
- Pigmented transverse nasal band: A review. Indian J Dermatol Venereol Leprol. 2022;88:144-7.
- [CrossRef] [Google Scholar]
- Dermoscopic features of small, medium, and large-sized congenital melanocytic nevi. Ann Dermatol. 2017;29:26-32.
- [CrossRef] [Google Scholar]
- Dermoscopic characteristics of congenital melanocytic nevi in a cohort study in southern Brazil. An Bras Dermatol. 2022;97:660-5.
- [CrossRef] [Google Scholar]
- A descriptive observational study on clinical and dermoscopic features of benign melanocytic neoplasms. Indian J Dermatol Venereol Leprol. 2020;86:251-61.
- [CrossRef] [Google Scholar]
- Congenital melanocytic nevi: Clinical and histopathologic features, risk of melanoma, and clinical management. J Am Acad Dermatol. 2005;52:197-203.
- [CrossRef] [Google Scholar]
- Concurrent melanosis and hypertrichosis in distribution of nevus unius lateris. Arch Derm Syphilol. 1949;60:155-60.
- [CrossRef] [Google Scholar]
- Follicular Becker's nevus: A relatively new variant. Indian Dermatol Online J. 2023;14:146-7.
- [CrossRef] [Google Scholar]
- Follicular Becker nevus: An unusual clinical and dermoscopic manifestation. Dermatol Pract Concept. 2022;12:e2022074.
- [CrossRef] [Google Scholar]
- Clinical and dermoscopic features of follicular Becker's nevus: A rare variant. Indian Dermatol Online J. 2021;12:750-1.
- [CrossRef] [Google Scholar]
- Follicular Becker's nevus: A new clinical variant. Indian J Dermatol. 2020;65:130-2.
- [CrossRef] [Google Scholar]
- Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78:510-9.
- [CrossRef] [Google Scholar]
- Presentation of reticulate acropigmentation of Kitamura and Dowling-Degos disease overlap. J Clin Aesthet Dermatol. 2012;5:41-3.
- [Google Scholar]
- Dermoscopy of follicular Dowling-Degos Disease. Indian J Dermatol. 2020;65:290-4.
- [CrossRef] [Google Scholar]
- Dowling-Degos disease: Classic clinical and histopathological presentation. An Bras Dermatol. 2011;86:979-82.
- [CrossRef] [Google Scholar]
- Dermoscopy in vitiligo: Diagnosis and beyond. Int J Dermatol. 2018;57:50-4.
- [CrossRef] [Google Scholar]
- Dermatoscopic patterns in vitiligo. Dermatol Pract Concept. 2023;13:e2023197.
- [CrossRef] [Google Scholar]
- Clinicopathological correlation of acquired hypopigmentary disorders. Indian J Dermatol Venereol Leprol. 2013;79:376-82.
- [CrossRef] [Google Scholar]
- Cross-sectional study of dermatoscopic findings in relation to activity in vitiligo: BPLeFoSK criteria for stability. J Cutan Aesthet Surg. 2019;12:36-41.
- [CrossRef] [Google Scholar]
- Histopathologic features in vitiligo. Am J Dermatopathol. 2008;30:112-6.
- [CrossRef] [Google Scholar]
- Nevus depigmentosus: Clinical features and histopathologic characteristics in 67 patients. J Am Acad Dermatol. 1999;40:21-6.
- [CrossRef] [Google Scholar]
- Dermoscopy is a new diagnostic tool in diagnosis of common hypopigmented macular disease: A descriptive study. Dermatol Reports. 2019;11:7916.
- [CrossRef] [Google Scholar]
- Dermatoscopy of nevus anemicus. Indian Dermatol Online J. 2022;13:822-3.
- [CrossRef] [Google Scholar]
- Dermatoscopic evaluation of leprosy: A multi-centre cross-sectional study. Indian J Dermatol Venereol Leprol. 2024;90:486-93.
- [CrossRef] [Google Scholar]
- Dermoscopic, clinical, and histopathological aspects of leprosy and lepra reaction cases: An observational cross-sectional study. Indian J Dermatopathol Diagn Dermatol. 2023;10:1.
- [CrossRef] [Google Scholar]
- Usage of dermoscopy as an effective diagnostic tool in Pityriasis alba: A prospective observational study among children in a suburban hospital in South India. Cureus. 2023;11:e40271.
- [CrossRef] [Google Scholar]
- Dermoscopy of pityrosporum folliculitis. J Am Acad Dermatol. 2019;80:e43-4.
- [CrossRef] [Google Scholar]
- Dermoscopy in the evaluation of pityriasis versicolor: A cross sectional study. Indian Dermatol Online J. 2019;10:682-5.
- [CrossRef] [Google Scholar]
- Dermoscopy as an ancillary tool for the diagnosis of pityriasis versicolor. J Am Acad Dermatol. 2015;73:e205-6.
- [CrossRef] [Google Scholar]
- Dermoscopic pattern of pityriasis versicolor. Clin Cosmet Investig Dermatol. 2019;12:303-9.
- [CrossRef] [Google Scholar]
- Dermoscopy: An easy way to solve the diagnostic puzzle in pityriasis versicolor. Indian J Dermatol Venereol Leprol. 2019;85:664-5.
- [CrossRef] [Google Scholar]
- Useful dermoscopic findings for differentiating rosacea from seborrheic dermatitis. Indian J Dermatol. 2020;65:316-8.
- [CrossRef] [Google Scholar]
- Dermoscopy can be useful in differentiating scalp psoriasis from seborrhoeic dermatitis. Br J Dermatol. 2011;164:652-6.
- [CrossRef] [Google Scholar]
- Histopathological differential diagnosis of psoriasis and seborrheic dermatitis of the scalp. Ann Dermatol. 2016;28:427-32.
- [CrossRef] [Google Scholar]
- Vitiligolike macules in systemic scleroderma. Arch Dermatol. 1983;119:129-33.
- [CrossRef] [Google Scholar]
- Dermoscopy of salt and pepper pigmentation. Indian Dermatol Online J. 2022;13:548-50.
- [CrossRef] [Google Scholar]
- Off-white perifollicular halo around the salt and pepper sign in the dermoscopic diagnosis of systemic sclerosis and interstitial lung disease. Actas Dermosifiliogr. 2022;113:970-2.
- [CrossRef] [Google Scholar]







