Translate this page into:
Evaluation of comedogenic potential of a paraben-free plant-based butter moisturizing cream: A double-blind, comparative study

*Corresponding author: Monil Yogesh Neena Gala, MBBS, MD (Pharmacology), Department of Medical Affairs, Dr. Reddy’s Laboratories Ltd., Global Generics- India, 7-1-27, Ameerpet, Hyderabad - 500016, Telangana, India. monil.yogesh@drreddys.com
-
Received: ,
Accepted: ,
How to cite this article: Gala MY, Muchhala SS, Charugulla SN, Rathod R, Mane A, Pandit S, et al. Evaluation of comedogenic potential of a paraben-free plant-based butter moisturizing cream: A double-blind, comparative study. CosmoDerma 2021;1:52.
Abstract
Objectives:
Comedogenicity is a critical factor in making of cosmetics and skin care products. The term “acne cosmetica” was coined to link the relationship between female acne to the use of cosmetic formulations, stating that the ingredients used in the cosmetic formulations have the potential to evoke a comedogenic response or produce comedones. Therefore, it is important that a skin care product is non-comedogenic and efficacious at the same time. The main objective of this study is to evaluate the comedogenic potential of the test product (Venusia Max Cream – paraben free) when applied topically under occluded patch to the skin.
Material and Methods:
This was a randomized, double-blinded, comparative study conducted in 24 healthy female participants, with prominent follicular orifices on the upper back region. Comedogenic potential of the test product (Venusia Max Cream – paraben free) was evaluated in comparison to positive (coconut oil) and negative (glycerin) controls in women. Each participant received topical application of test and control products under occluded patch to the skin on the upper aspect of the back, 3 times weekly for 4 weeks. Cyanoacrylate biopsies were performed before and after treatment to determine the microcomedones histologically. Microcomedones were graded using light microscopy and results were analyzed based on scale rating (0–3).
Results:
The mean comedone grading was assessed between positive versus negative control, and positive control versus test product. The mean comedone grades were significantly less in test product 0.41 ± 0.50 and negative control 0.82 ± 0.73 in comparison to positive control 2.09 ± 0.68. The test product was least comedogenic in this study. Furthermore, no adverse events were reported during the study period.
Conclusion:
Based on the histological evidence, Venusia Max Cream (paraben free) is a non-comedogenic, plant-based intense moisturizing cream and its use in regular skin care routine can be beneficial, particularly for acne prone and dry skin as it improves the skin hydration levels.
Keywords
Comedones
Microcomedones
Comedogenicity
Moisturizer
Glycerine
dimethicone
Cyanoacrylate biopsy
Acne
Coconut oil
INTRODUCTION
Moisturizers are essential for daily skin care and their importance lies in maintaining epidermal skin barrier and skin hydration. Efficacy of a moisturizer depends on factors such as selecting a correct moisturizer based on the skin type and its regular application.[1] Apart from their daily use for normal skin, moisturizers are also used in treating dermatitis, atopic disorders, and psoriasis.[1,2] Physicians recommend the use of moisturizers as an adjunct in the treatment of acne as they may contribute to improve the signs and symptoms of the disease. According to a literature review, glycerine and dimethicone are the most common ingredients found in moisturizing products. Glycerine is the most effective humectant to increase the stratum corneum hydration. Dimethicone has both occlusive and emollient properties and could be suitable for acne sensitive patients, as it is non-comedogenic and hypoallergenic.[3]
Ingredients in skin care products may remain on the skin surface or get absorbed into the skin[4] because they have the ability to penetrate the skin, establishing that the skin care product’s comedogenicity is critical.[5] Comedogenesis refers to the abnormal differentiation of the follicular epithelium resulting in the formation of microcomedones.[6] Few cosmetics may contain comedogenic ingredients such as lanolin, isopropyl myristate, oleic acid, and stearyl alcohol, which may precipitate or aggravate acne.[7] Similarly, application of oils such as coconut oil has been reported to be comedogenic and their application is not advised in acne prone skin.[8] Therefore, it is important that a skin care product is non-comedogenic and efficacious at the same time. Assessing the non-comedogenic potential of a skin care product and determining the number of microcomedones with the use of cyanoacrylate biopsies are an age-old method and have proven beneficial.[9] The aim of this study was to evaluate the comedogenic potential of Venusia Max Cream (paraben free), a plant-based moisturizer, containing dimethicone and glycerin as one of their active ingredients.
MATERIAL AND METHODS
Study design and setting
This was a randomized, double-blinded, comparative study conducted during February and March 2020. The participants were enrolled after obtaining signed patient authorization forms. The study was approved by the Institutional Ethics Committee and conducted in compliance with the protocol and principles of the Declaration of Helsinki, Indian regulatory guidelines (Indian Council of Medical Research [ICMR] and Indian GCP guidelines), and Schedule Y guidelines.[10-12]
Participants were enrolled only after fulfilling the eligibility criteria which included healthy females (18–55 years), displaying prominent follicular orifices on the upper back and willing to avoid ultraviolet exposure (sun or artificial), water contact (swimming), or activity which may cause sweating during the study period. The study excluded women who were pregnant/lactating, had jobs involving water contact/perspiration, had scars/tattoo on the area to be studied, were diagnosed with any kind of systemic/cutaneous disease, hypersensitivity, allergy antecedent, or undergoing/ underwent (in the past 3 months) any medical therapy either systemic/hormonal or topical that may interfere with the performance of the study treatment.
Study plan and outcome
This study was conducted to evaluate the comedogenic potential of Venusia Max Cream which is a paraben-free intense moisturizing cream enriched with the plant-based butters by Dr. Reddy’s Laboratories Ltd., India, hereafter referred to as the test product. Comedogenic potential of the test product was evaluated in comparison to positive (coconut oil) and negative (glycerin) controls in women. Three test zones of about 3 × 3 cm2 each were marked on the upper back of the participants using a template. Before the treatment, a follicular biopsy of the test zones was performed using cyanoacrylate liquid adhesive Loctite 420 Instant Adhesive (Manufactured by Henkel Adhesives Technologies India Pvt. Ltd.) microscope slide, applied gently on the marked zones, and allowed to set for 30–60 s and then removed obtaining a follicular biopsy specimen [Figure 1]. The study procedure is presented. Weekly cycle (Monday–Friday) and procedure were followed till patches were applied 12 consecutive times on alternate days for each participant. On the past day of the study, 2 h after patch removal, follicular biopsy was taken from the product application zones for the assessment of microcomedones histologically. Microcomedones were graded using light microscopy and were based on scale rating as presented in [Table 1].

- Study procedure.
| Scale of grading | |
|---|---|
| 0 | Non-comedogenic |
| 1 | Small microcomedones |
| 2 | Moderately sized microcomedones over most of the field |
| 3 | Large globoid microcomedones over the entire field |
Blinding
A randomization sheet was generated, which randomly allocated the test products and control to any of the marked test sites.
Statistical methods
A convenient sample of 24 women participants was taken. Statistical analysis was carried out using SPSS version 10.00. Test of significance was determined using Mann–Whitney U-test and P-values were reported based on two-sided significance test and statistical test was interpreted at 5% level of significance.
RESULTS
A total of 24 participants were recruited in the study, out of these, one patient was lost to follow-up and the data of another participant were non-evaluable; thus, data of total 22 (91.67%) participants were analyzed and evaluated. The study population were females in the age range of 19–53 years with a mean age of 35.77 ± 10.86 years.
Mean comedones grades were assessed between positive versus negative control and positive control versus test product. The mean comedones grade was significantly less 0.82 in the negative control (glycerine) as compared to 2.09 in the positive control (P = 0.001). Further, the mean comedones grade was significantly higher 2.09 in the positive control as compared to 0.41 in the test product (P = 0.001). No adverse events were reported during the study period [Figure 2]. Mean comedones grades are presented.
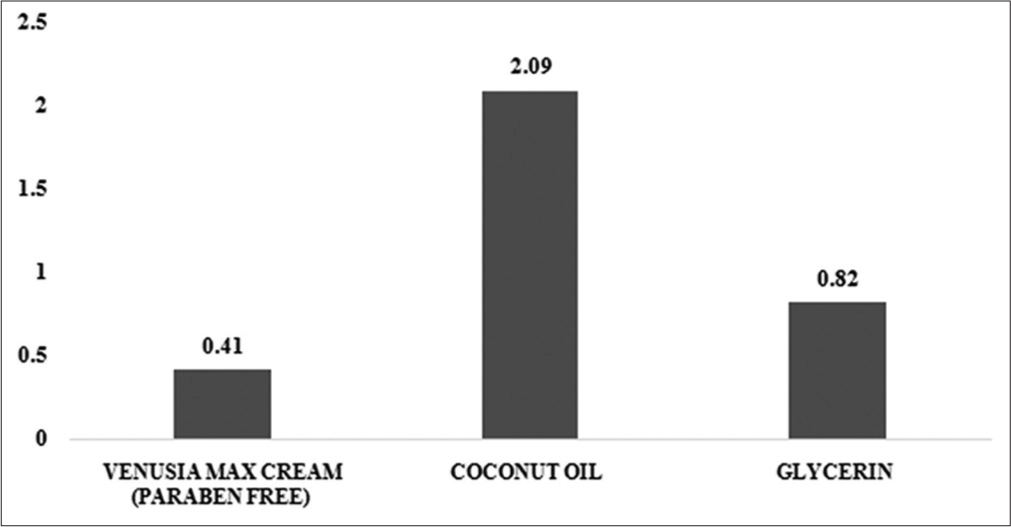
- Mean comedone grades of Venusia Max Cream (paraben free) (test product), coconut oil (positive control), and glycerin (negative control).
Safety/adverse events
No adverse events were reported during the study period.
DISCUSSION
Kligman and Mills introduced the concept of “acne cosmetica” in 1972 and linked the relation of female acne to the use of cosmetic formulations, stating that the ingredients used in the cosmetic formulations have the potential to evoke a comedogenic response or produce comedones.[13] Several studies have examined comedogenicity using diverse materials.[14-16] An epidemiological study evaluating factors associated with acne in the Indian population, stated that 35% of patients reported aggravation of acne after using some form of cosmetics. Therefore, there is a need for an efficacious, non-comedogenic, moisturizing skin care product for the treatment of dry skin conditions. The purpose of this study was to evaluate the comedogenic potential of the test product, a plant-based moisturizer applied topically on the skin under occlusive patch. The evaluation criteria included grading of microcomedones using a light microscope.
The value of comedogenicity assessment based on histologic grading is indicated in lowering the possibility of misinterpretation. The entire follicle is tested histologically, whereas only follicular dilatation is measured visually, which can be misunderstood.[5] In the present study, microcomedones were assessed based on a grading scale of 0–3 along with its size and field area involved [Table 1]. The mean comedones grading was assessed between positive versus negative control, and positive control versus test product. The mean comedone grades were significantly less in test product 0.41 ± 0.50 and negative control 0.82 ± 0.73 in comparison to positive control 2.09 ± 0.68. The test product was least comedogenic in this study.
It has been stated that to assess the severity of acne, one needs to consider its distribution involving the areas such as back, chest, and upper arms.[17] In our study, the upper back region was considered as the test site, where the test and control products along with occlusive patches were applied. The previous studies have also suggested back/trunk for comedogenicity assessment.[5,9] Patch testing of the test product is done to make a diagnosis and predict the potential for primary skin irritation or allergy;[18] thereby, determining the safety of the product. In our study, occlusive patches were applied to the patient’s upper back and no adverse events were reported during examination throughout the study.
The procedure of pre- and post-patching follicular biopsy and alternate day patch application for 12 consecutive times, carried out in our study is in accordance with the study conducted by Draelos and DiNardo, in which they assessed the comedogenic potential of cosmetic products in six individuals, where they applied patches on the upper back, saturated with 0.2–0.5 ml of finished cosmetic products, 3 times weekly for 4 weeks along with pre- and post-patching follicular biopsy specimens.[5]
Millis and Kligman used the cyanoacrylate adhesive method for obtaining follicular biopsy and emphasized that this method is useful in acne areas of face and trunk where sebaceous follicles are abundant, and the contents are removed in a non-invasive way with follicular material obtained well below the surface.[9] Similarly, pre- and post-treatment follicular biopsy using cyanoacrylate adhesive was done to assess and compare the microcomedones formation based on a grading scale.
Coconut oil has been used extensively for centuries in tropical regions.[19] Virgin coconut oil is recommended as a suitable emollient and treatment option for acne. However, its comedogenicity in literature is not well established. Francis and Shojan assessed the comedogenic potential and antimicrobial properties of commonly used oils and concluded that application of coconut oil, mustard oil, and liquid paraffin is not advisable in acne prone skin condition, as they were found to be equally comedogenic with no antibacterial properties.[8] In our study, coconut oil was used as a positive control and the mean comedone grade was found to be higher in comparison to the test product and negative control.
Our test product is composed of glycerin, dimethicone, shea butter, aloe butter, cocoa, and mango butter as their active ingredients. Glycerin and dimethicone both cover the three main properties of a moisturizer: Humectant, occlusive, and emollient. Dimethicone is an ingredient with emollients and occlusive properties, whereas glycerin is a humectant.[3] Humectant is a hygroscopic compound that attracts water from the dermis into the epidermis and occlusive ingredients block the transepidermal water loss in the stratum corneum creating a hydrophobic barrier over the skin.[20] A real-world clinical study with the constituents of our formulation concluded that plant-based intense moisturizing cream can be considered as an adjuvant for dry skin in the management of psoriasis in India.[2]
The observations obtained from available statistical data indicate that our test product is a non-comedogenic product as compared to the positive control – coconut oil. In addition, there were no adverse events reported throughout the study period.
CONCLUSION
Histological grading evidence suggests that Venusia Max Cream (paraben free) is a non-comedogenic, plant-based intense moisturizing cream. A generous application of Venusia Max Cream can be a valuable component of daily skin care routine, especially for acne prone and dry skin as it improves the skin hydration levels.
Acknowledgment
The author acknowledges Knowledge Isotopes Pvt. Ltd. (http://www.knowledgeisotopes.com) for the medical writing assistance.
ETHICAL APPROVAL
Study approval statement
The study was approved by the Institutional Ethics Committee and conducted in compliance with the protocol and principles of the Declaration of Helsinki, Indian regulatory guidelines (ICMR and Indian GCP guidelines), and Schedule Y guidelines.
Authors’ Contributions
All the authors contributed to study design, critical review, and data interpretation.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Dr. Reddy’s Laboratory Ltd., Hyderabad, India.
Conflicts of interest
All the contributing authors are employees of Dr. Reddy’s Laboratories Ltd.
References
- The role of moisturizers in addressing various kinds of dermatitis: A review. Clin Med Res. 2017;15:75-87.
- [CrossRef] [PubMed] [Google Scholar]
- A real-world, non-interventional Indian study evaluating intensive plant-based butter moisturizing cream in psoriasis. Dermatol Ther. 2019;9:537-46.
- [CrossRef] [PubMed] [Google Scholar]
- Moisturizers for acne: What are their constituents? J Clin Aesthet Dermatol. 2014;7:36-44.
- [Google Scholar]
- The clinical benefit of moisturizers. J Eur Acad Dermatol Venereol. 2005;19:672-88.
- [CrossRef] [PubMed] [Google Scholar]
- A re-evaluation of the comedogenicity concept. J Am Acad Dermatol. 2006;54:507-12.
- [CrossRef] [PubMed] [Google Scholar]
- American academy of dermatology invitational symposium on comedogenicity. J Am Acad Dermatol. 1989;20:272-7.
- [CrossRef] [Google Scholar]
- Factors aggravating or precipitating acne in Indian adults: A hospital-based study of 110 cases. Indian J Dermatol. 2018;63:328-31.
- [CrossRef] [PubMed] [Google Scholar]
- World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81:14-8.
- [Google Scholar]
- National ethical guidelines for biomedical and health research involving human participants, 2017: A commentary. Indian J Med Res. 2018;148:279-83.
- [CrossRef] [PubMed] [Google Scholar]
- Indian GCP by Central Drugs Standard Control Organization. 2013. :1-108. Available from: http://www.cdsco.nic.in/html/gcp1.html [Last accessed on 2021 Aug 31]
- [Google Scholar]
- An improved rabbit ear model for assessing comedogenic substances. Br J Dermatol. 1979;100:699-702.
- [CrossRef] [PubMed] [Google Scholar]
- A human model for assessing comedogenic substances. Arch Dermatol. 1982;118:903-5.
- [CrossRef] [Google Scholar]
- Comedogenicity of squalene monohydroperoxide in the skin after topical application. J Toxicol Sci. 2000;25:77-83.
- [CrossRef] [PubMed] [Google Scholar]
- A new occlusive patch test system comparable to IQ and Finn chambers. Indian J Dermatol Venereol Leprol. 2014;80:291-5.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized double-blind controlled trial comparing extra virgin coconut oil with mineral oil as a moisturizer for mild to moderate xerosis. Dermatitis. 2004;15:109-16.
- [CrossRef] [PubMed] [Google Scholar]
- Moisturizers: The slippery road. Indian J Dermatol. 2016;61:279-87.
- [CrossRef] [PubMed] [Google Scholar]






