Translate this page into:
Evaluation of comedogenic potential of a ceramide-based moisturizer (lotion/cream): A prospective, randomized, double-blind, parallel-group comparative study

*Corresponding author: Biswajit Aich, Medical Affairs, Dr. Reddys Laboratories Ltd., Hyderabad, Telangana, India. biswajitaich@drreddys.com
-
Received: ,
Accepted: ,
How to cite this article: Aich B, Dhanaki GD, Sanghavi A, Muchhala S, Katare S, Kotak B. Evaluation of comedogenic potential of a ceramide-based moisturizer (lotion/cream): A prospective, randomized, double-blind, parallel-group comparative study. CosmoDerma. 2025;5:28. doi: 10.25259/CSDM_203_2024
Abstract
Objectives
Comedogenicity is a crucial consideration for cosmetic preparations. While most of the ingredients used in moisturizers may have the ability to cause comedones, the study was done to assess the non-comedogenic potential of test products (Venusia CeraPlus Moisturizers). The primary objective of the study was to evaluate the comedogenic potential of the test products using light microscopy.
Materials and Methods
This was a prospective, randomized, double-blinded, positive- and negative-controlled, two-cohort study to evaluate the comedogenic potential of Venusia CeraPlus moisturizers. Thirty female volunteers with follicular orifices in the upper back region were included in the study after obtaining written informed consent. The four sites were marked for the application of each of the two test products and controls occluded by the patch. A follicular biopsy using the cyanoacrylate method was performed for each site. Product application using a patch was done after 2 h of the biopsy on alternate days. Microcomedones grading was done using rating scale (0 = Non comedogenic, 1 = Small microcomedones, 2 = Moderately sized microcomedones, 3 = Large globoid microcomedones over the entire field).
Results
The mean comedogenic grade for the coconut oil (positive control) and glycerine (negative control) was 1.92 and 0.42, respectively. The significant difference (P = 0.001) between the positive and negative control validated the method. The mean comedone grade in Venusia CeraPlus Cream (P = 0.001) and Venusia CeraPlus Lotion (P = 0.001) was significantly less than that of the positive control. No adverse events were reported during the study.
Conclusion
Based on the results, Venusia CeraPlus moisturizers were non-comedogenic, effective, and well tolerated by participants.
Keywords
Ceramides
Comedogenicity
Comedones
Cosmetics
Moisturizers
INTRODUCTION
The cosmetic market is growing widely with moisturizers being the most used. However, it is important to evaluate the ingredients, safety, and efficacy of each product, as well as the patient’s skin type, when recommending moisturizers.[1] However, the most commonly used oils in moisturizers include olive oil, cocoa butter, and linseed oil, which are comedogenic.[2]
Comedones develop from the obstruction of sebum secretion in a pilosebaceous duct by an excessive proliferation of keratinocytes.[3] Follicle hyperkeratinization, or the creation of microcomedones, is the initial stage in the life cycle of an acne vulgaris lesion, according to the conventional model of acne pathogenesis, which can also be defined as acne lesion development and progression.[4] Although it can appear at any time together with inflammatory papules and pustules, comedonal acne is more common in young teenagers.[5]
Comedogenic chemicals such as lanolin, isopropyl myristate, oleic acid, and stearyl alcohol are present in some cosmetics and can exacerbate or cause acne.[6] When developing cosmetics, topical drugs, and skin care items, comedogenicity has been a crucial factor to take into account.[7] Skin irritations can happen, and using dermo-cosmetics is advised to avoid retinoid side effects and adhere to therapy.[8] Owing to their comedolytic and anti-inflammatory properties, topical retinoids and antibiotics are a mainstay of acne treatment.[9] Today, many dermatologists confront significant issues due to the increasing prevalence of antibiotic resistance and the likelihood of local delayed hypersensitivity reactions.[10] As an adjuvant therapy, noncomedogenic moisturizers are often administered to reduce cutaneous irritations caused by acne drugs.[11]
Ceramides play an important role in stratum corneum functioning as they are the main lipid component of the intercellular gaps of the stratum corneum, which comprise the epidermal permeability barrier, along with cholesterol, free fatty acid, and other minor components.[12,13] It has been claimed that acne patients have a deficiency in total ceramides and free sphingosine.[14] Thus, ceramide-containing moisturizers (CCMs) have the potential to improve adherence and enhance current acne treatments.[15] The ingredients derived from Avena sativa are used as abrasives, antioxidants, skin conditioning agents, absorbents, and bulking agents in cosmetics.[16] Products containing A. sativa are used as cutaneous moisturizers in dermatology.[17] According to certain research, moisturizers can help alleviate acne’s symptoms on their own.[18]
The study assessed the comedogenic potential of Venusia moisturizers (lotion and cream) with topical application under occluded skin patches in healthy adults.
MATERIALS AND METHODS
Study design and setting
It was a prospective, randomized, double-blinded, positive- and negative-controlled, two-cohort study to evaluate the comedogenic potential of test products (cream and lotion). The clinical study protocol and related documents were reviewed and approved by the Independent Ethical Committee of C.L.A.I.M.S. (Clinical and Aesthetic Investigative Management Services) (Approval Form No.: CL/033/0524/STU; Date of approval: July 15, 2024). The study was conducted in compliance with the good clinical practice guidelines international council for harmonization (ICH), the Indian Council of Medical Research, and the Declaration of Helsinki (Taipei 2016). Approval was obtained from the institutional ethics committee before study initiation and written informed consent was obtained from all the participants before any study-related procedures. The study was registered at the clinical trials registry of India (CTRI/2024/07/071597; dt. July 30, 2024).
Study procedure
The study duration was approximately 1 month, with 24 visits. Once the participants signed the informed consent form, they were screened for eligibility in the study. Participants fulfilling the eligibility were enrolled in the study. The four sites (3 × 3 cm2 each) on the upper back were marked using a template for the application of two test products (lotion and cream) and two controls (positive- coconut oil and negative- glycerine). The positive control was coconut oil due to its comedo-causing potential, while the negative control was glycerine, as it is noncomedogenic.[6] Initially, follicular biopsy of all four sites was performed using 1–2 drops of cyanoacrylate liquid adhesive application on the glass microscope slide. The slide was then applied gently and carefully on the marked zones and the cyanoacrylate was allowed to set for 30–60 s. After that, the slide was gently removed to yield a follicular biopsy specimen. Product application was then carried out as per randomization on 3 × 3 cm2 marked zones, approximately 2 h after the follicular biopsy test. These were occluded using patch chambers (2 × 2 cm2 patches). Each subject had a patch placed on their upper back, covering the corresponding sites with 0.025 g or mL of the test and control materials, applied by clinical research associates (CRAs). Figure 1 shows the diagrammatic representation of the procedure for the study.
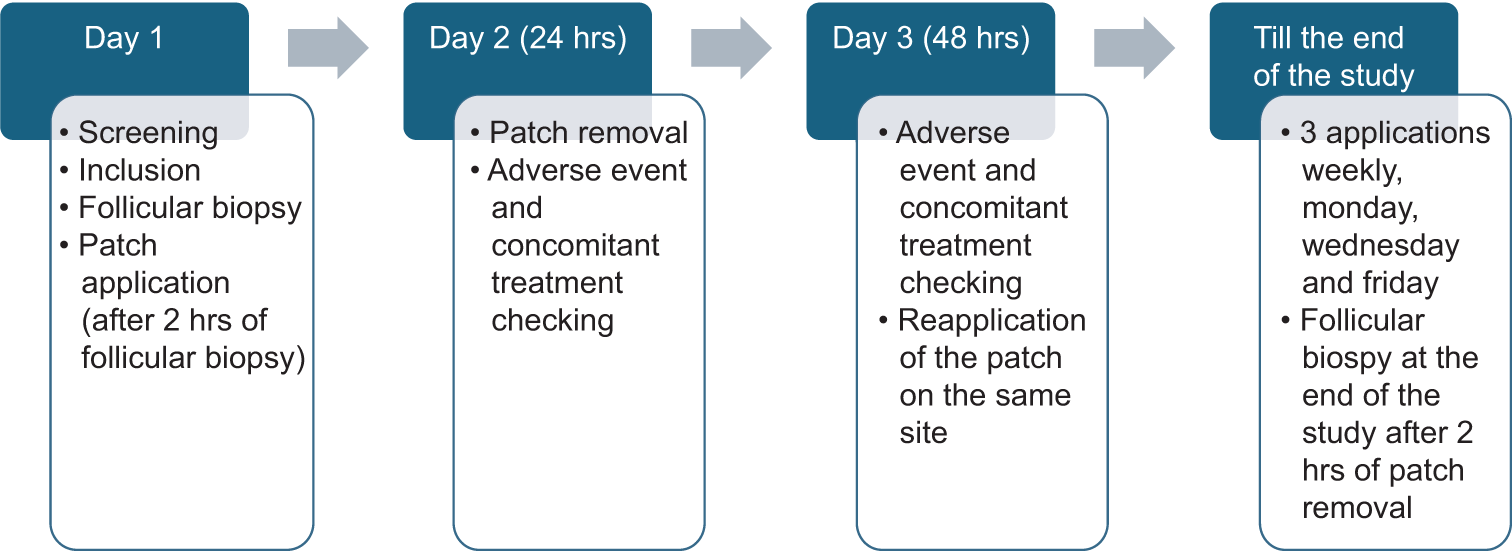
- Diagrammatic representation of study procedure.
Study participants
Healthy women aged between 18 and 55 years with prominent follicular orifices on the upper region of the back were enrolled. Those who were ready to avoid the intense ultraviolet (UV) exposure as well as contact with water were considered for inclusion. Participants having healthy skin test areas and were ready to wear loose cotton clothes were enrolled in the study. Volunteers with regular dermatological infections and scans/tattoos on the area to be studied were excluded. Pregnant or lactating women, prone to hypersensitivity, cutaneous or systemic disease were not included. Those participants who were receiving any medical treatment either systemic/hormonal or topical which might interfere with the performance of the study treatment were an exclusion.
Randomization and blinding
Randomization was done based on the sites on which the test products and controls were applied. Products were coded by C.L.A.I.M.S. Pvt. Ltd. Block randomization was carried out by C.L.A.I.M.S. Pvt. Ltd.’s QA Department. A randomization sheet was generated by the QA Department, which randomly allocated test products and controls to any of the marked test sites. Blinding was ensured with respect to different sites (site 1, site 2, site 3, site 4). Product application was carried out as per randomization by the CRA and evaluation was done by the investigator. It was ensured that the participants and the investigator were blinded to the four treatments.
Test product
Venusia CeraPlus Cream (Ceramide 1,2,3,4,6 II, Butyrospermum Parkii, A. sativa [Oat]. Kernel Oil, A. sativa Kernel Extract, Sodium Hyaluronate, Glycerin, Light Liquid, Paraffin, Dimethicone 200, Cetyl Alcohol, Laminaria digitata Extract) and Venusia CeraPlus lotion (Ceramide 1,2,3,4,6II, B. Parkii, A. sativa [Oat], Kernel Oil, A. sativa Kernel Extract, Sodium Hyaluronate, Glycerin, Light Liquid, Paraffin, Dimethicone 200, Cetyl Alcohol, Laminaria digitata Extract) commercially available as ceramide 1,2,3,4,6II containing moisturizers (manufactured by: Dr. Reddy’s Laboratories, India). The formulation provides deep hydration while repairing the skin barrier, skin’s barrier restoration, and delivers more soothing and skin relief. Coconut oil and glycerine as controls and the test moisturizers were applied on marked zones and occluded under the patch on the upper back. Patch application/removal was done 12 times during the study.
Study outcome
The primary outcome was the grading of micro-comedones using light microscopy by evaluating the biopsy slides.
Study assessment
Study parameters
Grading of micro-comedones using light microscopy.
Methods and timing for assessing study parameters
The Principal Investigator (Dermatologist) evaluated the biopsy slides and graded the comedones using the below-mentioned scale.
Scale for grading
| 0 | No comedones |
| 1 | Small microcomedones |
| 2 | Moderately sized microcomedones over most of the field |
| 3 | Large globoid microcomedones over the entire field |
Sample size
No formal sample size calculation was done. However, 30 participants were enrolled in the study, out of which 26 were analyzed (total drop-out of 4 patients).
Statistical methods and data analysis
All scores were averaged and recorded. Statistical analysis was carried out using the Statistical Package for the Social Sciences version 10.00. All the P-values were reported based on a two-sided significance test and all the statistical test was interpreted at 95% level of significance. Mann–Whitney U-test was used for tests of significance. Statistical comparisons included mean comedone grades of positive control versus negative control for method validation and mean comedone grades between test product and positive control.
RESULTS
Demographic and other baseline characteristics
A total of 30 healthy female volunteers were enrolled in the study. Out of which, 4 participants were drop-outs, hence the data analysis was done for 26 participants. All the participants were of an average age of 38.27 years, with 22.00 years being the minimum and 55.00 years being the maximum.
Individual participant comedone grading data
Table 1 shows the participants’ comedone grading data. The large globoid microcomedones were maximum at the site with positive control. A total of 8 (30.8%) and 11 (42.3%) participants were non-comedogenic for Venusia CeraPlus lotion and cream, respectively. However, there was only one (3.8%) large microcomedone for each of the Venusia CeraPlus lotion and cream.
| Venusia-Plus Cream (n=26) | Venusia-Plus Lotion (n=26) | Coconut oil (Positive) (n=26) | Glycerine (Negative) (n=26) | |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Non-comedogenic | 11 (42.3) | 8 (30.8) | 0 (0.0) | 18 (69.2) |
| Small microcomedones | 10 (38.5) | 11 (42.3) | 9 (34.6) | 6 (23.1) |
| Moderately sized microcomedones over most of the field | 4 (15.4) | 6 (23.1) | 10 (38.5) | 1 (3.8) |
| Large globoid microcomedones over the entire field | 1 (3.8) | 1 (3.8) | 7 (26.9) | 1 (3.8) |
| “P”* | 0.001 | 0.001 |
*P-value: Mann–Whitney U-test versus positive control (Coconut oil), No.: Number of females
Statistical comparison of mean comedone grades-positive control (coconut oil). Versus negative control (glycerine) (for method validation)
The positive and negative control method was validated by evaluating the comedogenic potential for both test controls. The mean (Standard deviation [SD]) values were 1.92 (0.80) and 0.42 (0.76) for coconut oil (positive control) and glycerine (negative control), respectively.
Statistical comparison of mean comedone grades between test products - Venusia CeraPlus cream and Venusia CeraPlus lotion versus positive (coconut oil) and negative control (glycerine)
Test products were compared with positive control to conclude on comedogenic potential of the test products. Figure 2 illustrates the average mean (SD) comedone grading value for Venusia CeraPlus cream and lotion being 0.81 (0.85) and 1.00 (0.85), respectively. The average mean (SD) of comedones causing the potential for 26 participants in the coconut oil (positive control) site was 1.92 (0.80) and for the glycerine (negative control) site was 0.42 (0.76).
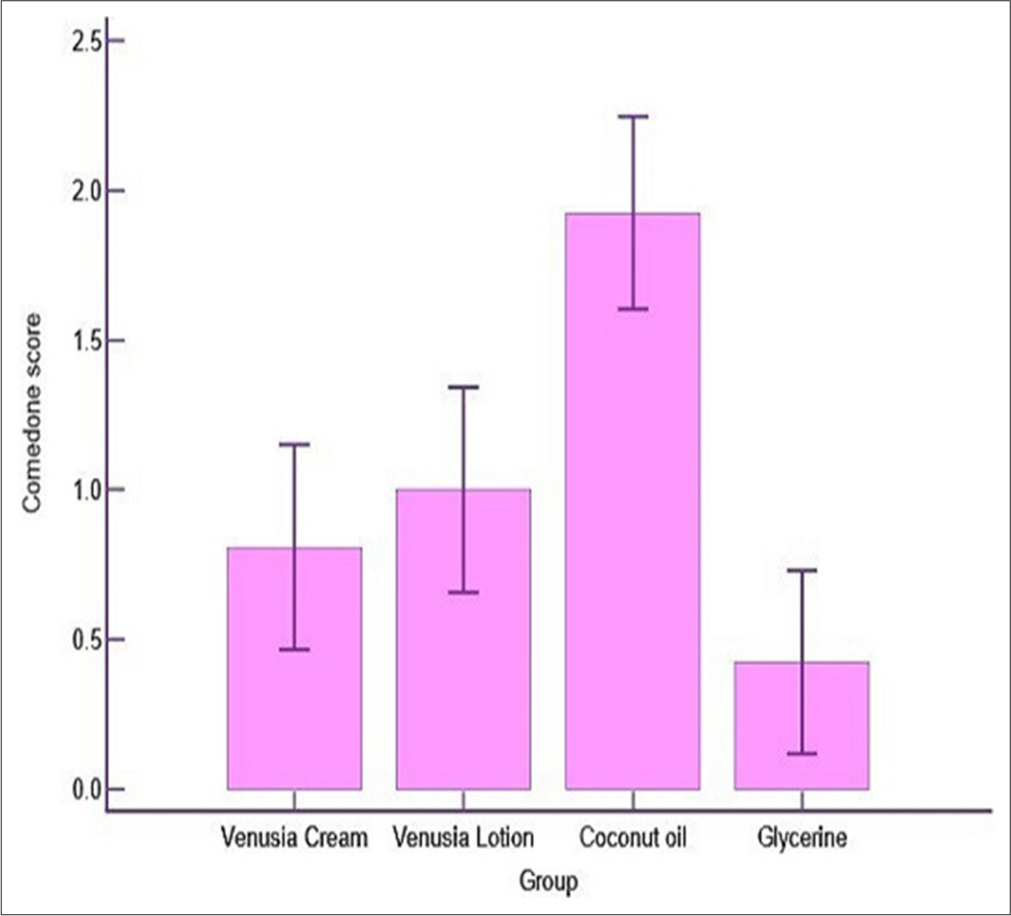
- Statistical Comparison of Mean Comedone grades between Test Products- Venusia CeraPlus Cream and Venusia CeraPlus Lotion versus Positive (Coconut Oil) and negative control (Glycerine).
DISCUSSION
A moisturizer is a topical solution made up of ingredients designed to replenish the skin’s natural protective layer and make it more hydrated.[19] Many moisturizers claim to be effective in treating acne.[18] The application of cosmetics, such as moisturizers, may result in delayed horny cell desquamation or comedone hyperkeratinization of the follicular epithelium.[7]
Due to extreme sensitivity, it has been reported that the substances that are weakly comedogenic in the rabbit are probably safe for human use.[20] The most common ingredients that are used in cosmetics as vehicles that are proven to be comedogenic in a rabbit study conducted by Nguyen et al., 2007 included isopropyl palmitate, isopropyl myristate, butyl stearate, isopropyl isostearate, decyl oleate, isostearyl neopentanoate, isocetyl stearate, myristle myristate, cocoa butter, while the products that do not cause comedogenicity in rabbit ear model included cocoa butter, cetyl alcohol, paraffin, stearyl alcohol sodium lauryl Sulfate, and petrolatum.[21]
The aim of comedogenic testing on human models is to measure the amount of microcomedones that form on the backs of the participant. Acne cosmetica prevalence is rising along with the cosmeceutical industry’s expansion. As a result, comedogenicity is a crucial factor for consumers to decide. Comedones of acne cosmetica are smaller, closed, and exhibit less involution of the sebaceous gland.[22]
The cornerstone of skin disease treatment now includes moisturizers that are high in ceramides. Dermatologists frequently advise applying such as CCM, to mimic skin moisturization for a variety of skin issues brought on by acne treatment.[11] Our formulations mainly consisted of ceramide 1,2,3,4,6II; in the previous study, ceramide-based ointment has proven to be effective in lack of comedogenicity. According to the results of a previous study, the mean value of ceramide-based ointment was 0.12, while for positive and negative control, it was 0.59 and 0.18, respectively.[23] With its occlusive and emollient properties, dimethicone reduces transepidermal water loss (TEWL) without leaving a greasy aftereffect. It is suitable for those with sensitive skin types and acne because it is non-comedogenic and hypoallergenic. Glycerine and B. parkii play an important role in hydrating the stratum corneum.[18,24] To improve the skin’s ability to retain water, simple moisturizers blend occlusives with humectants.[25] Therefore, the ingredients in the Venusia CeraPlus moisturizers provide the benefits of noncomedogenicity and hydrating.
The study conducted by Letawe et al., 1998 for topically applied linoleic acid on acne microcomedones showed about a 25% reduction in their overall size being achieved over a 1-month treatment period.[26] However, the comedogenicity of Venusia CeraPlus cream and Venusia CeraPlus Lotion was quite low as compared to the positive control (coconut oil), indicating the test products to be non-comedogenic (P = 0.001). The previous study results state that coconut oil is a highly comedogenic substance, thus it was used as a positive control.[27] As glycerin is a humectant and non-occlusive agent, it does not cause comedones and was used as a negative control in the study.[18] The method validation was done by comparing the mean comedone grades for negative control (glycerine) and positive control (coconut oil). The mean comedone grading for glycerine was significantly less (P = 0.001) as compared to coconut oil. Even when applied to vast parts of the body over extended periods of time, moisturizers are rarely linked to health risks. Minor issues like urticaria, cosmetic acne, dermatitis, and skin irritation may occur with moisturizers.[28] The product has proven to be non-comedogenic, and no skin tolerances or adverse events were reported during the study.
Strengths and future prospects
First, a prospective, randomized, double-blinded study design with both positive and negative controls is the biggest strength of this study. Second, the study being conducted at a single center provides homogeneity to the study participants. Third, all test and control treatment effects were assessed on the same participant making the study design robust. A larger study with multiple centers across India and in diverse study population could be further planned to improve the external validity of the study.
Limitations
A small sample size and short study duration are a possible limitation of this study.
CONCLUSION
Venusia CeraPlus moisturizers (cream and lotion) occluded patch application decreased the number of microcomedones as assessed by light microscopy. The comedogenicity of the positive control (coconut oil) was significantly higher than the test products. The products are proven to be safe for intended use as no adverse events were reported.
Acknowledgments
The authors want to thank Dr. Jayshree Langade for manuscript writing and publication services.
Authors’ contributions
BA, GDD, AS, SM, SK, BK: Concept, design, clinical studies, manuscript preparation, manuscript editing, and review. BA, GDD, AS, SM, SK: Statistical analysis.
Ethical approval
The clinical study protocol and related documents were reviewed and approved by the Independent Ethical Committee of C.L.A.I.M.S. (Approval Form No.: CL/033/0524/STU; Date of approval: July 15, 2024).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
All the authors are the employees of Dr. Reddy’s Laboratories Ltd., Hyderabad, Telangana, India.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: The study was funded by Dr. Reddy’s Laboratories., Hyderabad, Telangana, India.
References
- Moisturizer in patients with inflammatory skin diseases. Medicina (Kaunas). 2022;58:888.
- [CrossRef] [PubMed] [Google Scholar]
- What is comedogenicity, and what ingredients are comedogenic? The full story. Available from: https://www.acne.org/what-is-comedogenicity-and-what-ingredients-arecomedogenic-the-full-story [Last accessed on 2024 Nov 28]
- [Google Scholar]
- Perianal comedones: A Rare Incidental Finding. Case Rep Dermatol Med. 2017;2017:9019682.
- [CrossRef] [PubMed] [Google Scholar]
- The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: What does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 Suppl):S109-15.
- [Google Scholar]
- Management of comedonal acne vulgaris with fixed-combination topical therapy. J Cosmet Dermatol. 2018;17:227-31.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of comedogenic potential of a paraben-free plant-based butter moisturizing cream: A double-blind, comparative study. CSDM. 2021;1:52.
- [CrossRef] [Google Scholar]
- A re-evaluation of the comedogenicity concept. J Am Acad Dermatol. 2006;54:507-12.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of ceramides and niacinamide-containing moisturizer versus hydrophilic cream in combination with topical anti-acne treatment in mild to moderate acne vulgaris: A split face, double-blinded, randomized controlled trial. J Cosmet Dermatol. 2024;23:1758-65.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring acne treatments: From pathophysiological mechanisms to emerging therapies. Int J Mol Sci. 2024;25:5302.
- [CrossRef] [PubMed] [Google Scholar]
- Topical antibiotics in the dermatological clinical practice: Indications, efficacy, and adverse effects. Dermatol Ther. 2020;33:e13824.
- [CrossRef] [Google Scholar]
- Clinical efficacy of 0.5% topical mangosteen extract in nanoparticle loaded gel in treatment of mild-to-moderate acne vulgaris: A 12-week, split-face, double-blinded, randomized, controlled trial. J Cosmet Dermatol. 2019;18:1395-403.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of topical application of a skin moisturizer containing pseudo-ceramide and a eucalyptus leaf extract on atopic dermatitis: A review. J Clin Med. 2024;13:1749.
- [CrossRef] [PubMed] [Google Scholar]
- Ceramides in skin health and disease: An update. Am J Clin Dermatol. 2021;22:853-66.
- [CrossRef] [PubMed] [Google Scholar]
- New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821-32.
- [CrossRef] [PubMed] [Google Scholar]
- Moisturizers and Ceramide-containing Moisturizers May Offer Concomitant Therapy with Benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- [Google Scholar]
- Web-based international cosmetic ingredient dictionary and handbook United States: Personal Care Products Council; 2023.
- [Google Scholar]
- Moisturizers for acne: What are their constituents? J Clin Aesthet Dermatol. 2014;7:36-44.
- [Google Scholar]
- Moisturizers In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. Available From: http://www.ncbi.nlm.nih.gov/books/NBK545171 [Last accessed on 2024 Oct 21]
- [Google Scholar]
- A human model for assessing comedogenic substances. Arch Dermatol. 1982;118:903-5.
- [CrossRef] [Google Scholar]
- Comedogenicity in rabbit: Some cosmetic ingredients/vehicles. Cutan Ocul Toxicol. 2007;26:287-92.
- [CrossRef] [PubMed] [Google Scholar]
- Myths, truths, and clinical relevance of comedogenicity product labeling. JAMA Dermatol. 2018;154:1131-2.
- [CrossRef] [PubMed] [Google Scholar]
- Multivesicular emulsion ceramide-containing moisturizers: An evaluation of their role in the management of common skin disorders. J Clin Aesthet Dermatol. 2016;9:26-32.
- [Google Scholar]
- Vegetable butters and oils as therapeutically and cosmetically active ingredients for dermal use: A review of clinical studies. Front Pharmacol. 2022;13:868461.
- [CrossRef] [PubMed] [Google Scholar]
- Digital image analysis of the effect of topically applied linoleic acid on acne microcomedones. Clin Exp Dermatol. 1998;23:56-8.
- [CrossRef] [PubMed] [Google Scholar]
- The role of moisturizers in addressing various kinds of dermatitis: A review. Clin Med Res. 2017;15:75-87.
- [CrossRef] [PubMed] [Google Scholar]







