Translate this page into:
Efficacy and safety of Nyumi beauty sleep gummies for the management of sleep deprivation and skin condition: A single-arm clinical study

*Corresponding author: Ananya Agarwal, Department of Research and Development, Ikaria Wellness Private Limited, Mumbai, Maharashtra, India. ananya@nyumi.com
-
Received: ,
Accepted: ,
How to cite this article: Karmase A, Agarwal A, Joshi P, Sethi S, Shrivastava A. Efficacy and safety of Nyumi beauty sleep gummies for the management of sleep deprivation and skin condition: A single-arm clinical study. CosmoDerma. 2025:5:15. doi: 10.25259/CSDM_208_2024
Abstract
Objectives:
Sleep plays a critical role in optimizing physical and mental health. The aim of this clinical study was to evaluate the efficacy and safety of Nyumi beauty sleep gummies for improvement in sleep quality in healthy adult subjects with sleep deprivation.
Materials and Methods:
A single-arm, clinical study was conducted. Sleep quality was assessed by the Sleep Quality Scale (SQS), Insomnia Severity Index, Perceived Stress Scale (PSS), and Daytime fatigue using Fatigue Severity Scale (FSS), along with improvement in sleep and skin quality using a subjective assessment questionnaire. In addition, skin radiance using the Modified Griffiths scale by Dermatologist and Skin Glow by Skin Glossymeter GL 200 instrument was assessed. Changes in serum cortisol levels were also recorded.
Results:
After consuming Nyumi beauty sleep gummies for 15 days and 25 days, a statistically significant reduction was observed in the SQS score by 35.2% and 60.8%, Insomnia Severity Index score by 52.7% and 100.0%, PSS score by 44.6% and 93.4%, and in FSS score by 33.9% and 81.0% (all P < 0.0001), respectively, implying an improvement in sleep quality. Significant improvement by 25.2% on day 15 and 53.7% on Day 25 for dermatological assessment of skin radiance by Modified Griffiths Scale and 1.16-folds on day 15 and 2.11-folds on day 25 (all P < 0.0001) for skin glow by Skin Glossymeter GL200 instrument was also recorded. Improvement in sleep quality and skin glow was recorded by subjective assessment from baseline to the end of the study. Furthermore, no adverse event was recorded during the study conduction.
Conclusion:
Nyumi beauty sleep gummies significantly improved the sleep quality (in terms of total sleep time, sleep latency, sleep efficiency, time to sleep onset, number of awakenings, wake time after sleep onset, and sleep efficiency), daytime mood, ability to function at work, concentration, memory; reduced perceived stress, fatigue severity, and insomnia severity score. It also helped in improving skin glow and skin radiance.
Keywords
Sleep deprivation
Insomnia
Skin glow
Nyumi beauty sleep gummies
Sleep quality
INTRODUCTION
The role of good sleep is vital for the mental and physical well-being of a person. It plays a critical role in optimizing metabolism, appetite regulation, and the functioning of the immune, hormonal, and cardiovascular systems.[1] Different processes occur during sleep, help to promote healthy brain activity, and maintain overall good health. Good sleep helps in consolidating memories that play a significant role in emotional regulation. Sleep is critical for waking perception, i.e., to be able to think clearly, to be attentive and vigilant, and to sustain accurate thoughtfulness.[2]
The term “Sleep deficiency” refers to the inability to get adequate quality sleep. This may happen due to sleep deprivation, not getting adequate sleep or just because of other underlying causes such as a sleep disorder. For proper functioning of the body, at least 6–8 h daily sleep is required. It is estimated that nearly 50% of the population suffers from any type of sleep disorder in their lifespan.[3] Approximately one-fifth of the apparently healthy Indian population in the age group 16–55 years finds trouble in following a consistent sleep-wake schedule.[4] Needless to say, the sleep quality is as essential as the sleep quantity. Sleep deprivation leads to fatigue[5] and stress[6] and increases the risk of numerous ailments. It can adversely affect the immune system, which is primarily responsible for protecting from infections and diseases. Sleep deprivation affects facial expressions and features relating to the eyes, mouth, and skin.[7] Chronic poor sleep quality is also associated with aging signs, reduced skin barrier function, and lower satisfaction with appearance. Good quality sleep on the other hand provides better overall appearance of skin.[8] Various factors ascertain the quality of sleep, ranging from lifestyle and environmental factors to sleep disorders and/or any medical conditions. Sleep disruptions have considerable confrontational short and long-term health concerns.[9] Sleep disruption is of prime concern that causes increased mortality in otherwise healthy men. For those with medical conditions, sleep disruption may adversely affect the health-related quality of life. Recent studies have established the close relationship between scanty sleep and varied disorders such as hypertension,[10] cardiovascular disease,[11] obesity and type-2 diabetes,[12] impaired immune functioning,[13] arrhythmias,[14] mood disorders,[15] neuro-degeneration and dementia,[16,17] and even loneliness.[18]
Sleep disorders comprise a wide range of ailments with significant individual health consequences and high economic costs to society.[19] The medications used for the clinical management of sleep disorders are usually habit-forming and have tendencies of withdrawal symptoms.[20] Alternate therapies such as products formulated with natural and botanical-based ingredients have more acceptance looking to comparatively lesser side effects. Complementary and Alternative Medicine systems can be defined as a system of treatment that is not a conventional therapy but helps in a way to treat various health conditions and ailments. These therapies encompass multiple healing approaches that include but are not limited to Ayurveda, naturopathy, homeopathy, yoga and meditation, herbal medicines, nutritional supplements, etc. The history of these alternative and traditional medicine can be traced back thousands of years. The nutraceutical industry is mostly consumer-driven and has become most popular in the recent past due to enhanced awareness of potential health benefits and to fulfill the need for improved wellness. Evidence-based scientific validation and quality improvement through clinical proof might be some of the key reasons behind the growing acceptance of these alternative medications.
The test product (Nyumi beauty sleep gummies) falls under the nutraceutical category. It has been formulated with multivitamins, nutraceutical, and plant-based ingredients, which have proven scientific background for sleep disorders. Nutraceuticals being natural in nature and quality of being non-toxic, non-habit forming, may serve as a safe alternative, suggested for the prevention and as an adjuvant therapy for sleep deprivation and associated conditions in alternative healing systems. Henceforth, the present study was planned to assess the effect of the combination of these ingredients on healthy adult subjects with sleep deprivation.
MATERIALS AND METHODS
Study design
The present study was a single-arm, clinical study to evaluate the efficacy and safety of Nyumi beauty sleep gummies in healthy adult subjects with sleep deprivation, often characterized by poor quality and quantity of sleep during night, disturbed sleep, daytime sleepiness, etc. Patients diagnosed with clinical insomnia were not in the scope of the present study. The study consisted of 03 visits [Figure 1]. The potential subjects were screened as per the inclusion and exclusion criteria only after obtaining written informed consent from the subjects. All eligible volunteers consumed Nyumi Beauty Sleep Gummies for 25 days. All subjects underwent evaluation by Investigator/Dermatologist, Instrument Evaluation, and Subjective Evaluation. In addition, 2 mL of blood was retrieved from the subjects at visit 01 and visit 03 to determine serum cortisol levels. Safety was assessed throughout the study by monitoring adverse events (AEs), if any.
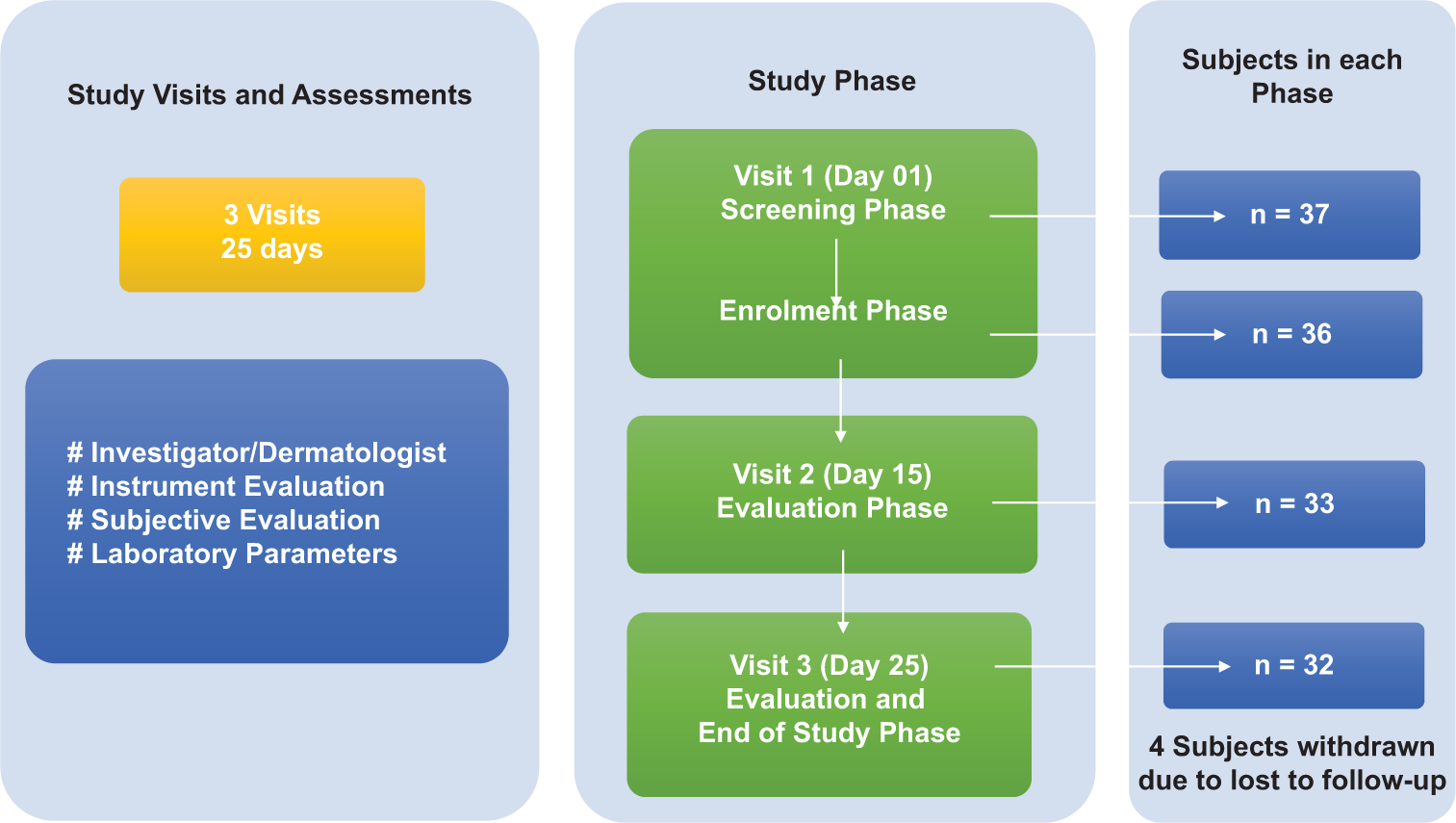
- Study design.
Study participants
Healthy males and non-pregnant, non-lactating females (in ratio of 1:2) between 18 and 60 years (both inclusive at the time of consent) were included in the study. Considering various limitations, sample size calculation was not performed and was based on earlier studies wherein, the investigator chose a feasible sample size rather than picking an arbitrary one. The study design was kept single arm to ensure data availability of adequate subjects for statistical analysis. A total of 36 subjects were enrolled, of which 32 subjects completed all study phases.
Ethics
Protocol and study documents were reviewed and approved by the Institutional Ethics Committee registered with the Central Drugs Standard Control Organization. The trial was registered in the Clinical Trial Registry of India before enrolment, #CTRI/2022/08/044862. The study details including the test product being evaluated, the potential hazards involving allergies, and possible anticipated reactions were explained to all prospective subjects by the investigator or person designated by the investigator before commencement of the study. A signed informed consent form was taken from the volunteers, and a copy of the same was also provided to them before enrolment. The subject’s identity was kept confidential and the data generated in the study was handled as per in-house Standard Operating Procedures and applicable regulations.
This clinical study was carried out in accordance with “The Code of Ethics of the World Medical Association (Declaration of Helsinki), The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH)-GCP (E6 R2), and applicable ethical guidelines for experiments involving humans.
Test product(s)
Nyumi Beauty Sleep Gummies (Ikaria Wellness Pvt. Ltd.) are formulated with a blend of Indian and Western ingredients having botanical and nutraceutical in nature. Active ingredients of the formulation consist of L-theanine, Melatonin, Chamomile Extract (flower), Multivitamin (Vitamin A, C, E, D2, B3, B6), Tagara (Valeriana wallichii) Root Extract, and Pomegranate Extract. Subjects were instructed to simply chew and swallow 2 gummies together, 30–60 min before sleeping. The product was taken once a day for a duration of 25 days.
Inclusion criteria
A healthy representative population of male or non-pregnant/non-lactating females in a ratio of 1:2, aged between 18 and 60 years (both inclusive) at the time of consent were included in the study. Participants with a ≥42 score of the Sleep Quality Scale (SQS) at the screening visit were included. Subjects with at least one sleep symptom (difficulty initiating/maintaining sleep, early morning awakening, non-restorative or non-refreshing sleep or insomnia) and one wake symptom (comprising of sleep-associated daytime impairment, e.g., sleepiness, fatigue, mood disturbances, cognitive difficulties, social impairment or occupational impairment) in past six months were the key inclusion criteria. The other main inclusion criteria were healthy subjects willing to follow the usual wake-sleep schedule during the study period; females of childbearing potential must had a negative urine pregnancy test performed during screening; subjects willing to refrain from any other treatment for the main indications for which the study test products were being given during the course of the study; subjects willing to consume test product throughout the study period as instructed; subjects in good general health as determined by the Investigator based on medical history and vital signs; subjects must be able to understand and provide written informed consent to participate in the study; subjects willing and able to follow the study protocol and study directions, to participate in the study.
Exclusion criteria
The key exclusion criteria of study were as follows: Subjects having sleep schedule changes associated with shift work; subjects on any medications or supplements known to affect sleep-wake function must not had been taken within two weeks before enrollment; subjects with a history of sleep apnea, chronic obstructive pulmonary disease, seizures, anxiety, depression, schizophrenia, bipolar disorder, mental retardation, a cognitive disorder, disturbance in sleep due to snoring, etc.; subjects with history of drug addiction or abuse within 12 months of the study; subjects with a history of any acute illness in the preceding one month which is likely to cause sleep disturbance; subjects with a history of cigarette smoking or medications; subject with history of allergies to oral care/personal care consumer products or their ingredients; subjects on any prescription medicines that might interfere with the study outcome; pregnant or breastfeeding or planning to become pregnant during the study period; and subjects participating in other similar study within the past four weeks. Any other condition that could warrant exclusion from the study, as per the Dermatologist’s/investigator’s discretion was also excluded.
Efficacy endpoint(s)
Primary endpoint(s)
The primary endpoint of the present study was to assess the effect of the test product on changes in SQS from baseline to each assessment visit.
Secondary endpoint(s)
The secondary endpoints of the study were to assess the effect of the test product on changes in the Perceived Stress Scale (PSS) Questionnaire, Fatigue Severity Scale (FSS), Insomnia Severity Index, the subject-reported total sleep time, sleep latency, sleep efficiency (total sleep time/time in bed × 100), the subject reported time to sleep onset, the subject reported number of awakenings, the subject reported wake time after sleep onset, daytime mood, ability to function at work, concentration and memory, skin glow by subjective assessment questionnaire and reduction in Serum Cortisol levels, Determination of skin radiance using Modified Griffiths scale by Dermatologist and skin glow by Skin Glossymeter GL 200 instrument were the other endpoints.
Safety endpoint(s)
The incidence of undesirable/AE or serious AE (SAE) was assessed at the time of each study visit. Subjects were also instructed to contact the Investigator for any expected and unusual AE/SAE at any timepoint throughout the study conduction.
Study measures/evaluation
SQS
SQS consists of 28 items and evaluates six domains of sleep quality, i.e., daytime symptoms, restoration after sleep, problems initiating and maintaining sleep, difficulty waking, and sleep satisfaction.[21] It is an exhaustive tool for the evaluation of sleep quality in a variety of research populations and can range from 0 to 84 scores. A higher score denotes poor quality of sleep, while a lower score on the scale shows good sleep. Assessment of SQS was done at baseline on Day 01, Day 15 (±2 Days), and Day 25 (±2 Days) after consumption of the test product.
PSS
PSS is widely used to assess stress levels.[22] It is a 10-item questionnaire and the score can range from 0 to 40. A score ranging from 0 to 13 = Low Stress, 14 to 26 = Moderate Stress, and 27 to 40 = High perceived stress was considered for interpretation. The assessment was done at baseline on Day 01, Day 15 (±2 Days), and Day 25 (±2 Days) after consumption of the test product.
FSS
FSS is the most commonly used fatigue-specific questionnaire.[23] It is a 9-item self-report questionnaire scale. FSS was used to determine daytime fatigue (score <36 = You may not be suffering from fatigue and ≥36 = You may need further evaluation by a physician). Assessment of FSS was done at baseline on Day 01, Day 15 (±2 Days), and Day 25 (±2 Days) after consumption of the test product.
Insomnia Severity Index
The Insomnia Severity Index focuses on assessing the subject’s perception and quantifying subjective dimensions of insomnia (Bastien et al. 2001).[24] The score ranges from 0 to 28. A score of 0–7 = No clinically significant insomnia, 8–14 = Subthreshold insomnia, 15–21 = Clinical insomnia (moderate severity), and 22–28 = Clinical insomnia (severe) was taken into account for the determination of Insomnia severity. The assessment was done at baseline on Day 01, Day 15 (±2 Days), and Day 25 (±2 Days) after consumption of the test product.
Assessment of skin radiance using modified Griffiths scale
Assessment of Skin radiance using the Modified Griffiths Scale[25] was done by scale being: 0 (None) = Extremely Bright, clear, radiant skin, 1–3 (Mild) = “Very” to “mildly” to “mildly to moderately” bright, clear, radiant skin, 4–6 (Moderate) = Moderately bright, clear, radiant skin, but with some matte appearance to mildly dull/matte appearance to moderately dull/matte appearance, and 7–9 (Severe) = “Moderately to pronounced” to “pronounced” skin. The assessment was done by the Dermatologist at baseline on Day 01, Day 15 (±2 Days), and Day 25 (±2 Days).
Instrumental assessment by skin Glossymeter GL 200
Skin Glossymeter GL 200 (Courage-Khazaka Electronic, Köln, Germany) is an instrument that measures skin gloss[26] based on the principle of reflection. Instrumental assessment by Skin Glossymeter GL 200 was performed at baseline on day 01, day 15 (±2 Days), and after treatment on day 25 (±2 Days).
Subject assessment questionnaire
The subjective assessment questionnaire[27] was based on subject-reported total sleep time, sleep latency, sleep efficiency (total sleep time/time in bed × 100), subject-reported time to sleep onset, subject-reported number of awakenings, subject-reported wake time after sleep onset, daytime mood, ability to function at work, concentration and memory, and skin glow. The subjective Assessment Questionnaire of the product was taken into account at baseline, i.e., day 01 and after treatment on day 15 (±2 days) and day 25 (±2 Days) after consumption of the test product.
Serum cortisol level
Cortisol is a steroid hormone often known as “Stress hormone.” Stress and sleep quality are closely associated. A serum cortisol test measures the level of cortisol in blood that is used to help diagnose medical conditions of stress. Sleep fragmentation increases cortisol levels.[28] Reducing cortisol levels might be a very effective approach to addressing sleep disturbances.[29] 02 mL blood was withdrawn as per the laboratory manual and determination of cortisol level was done on visit 01 (day 01) at baseline and post-product consumption on visit 03 (day 25 (±2 days) at the end of the study.
Statistical analysis
The statistical analysis was done using Statistical Analysis System (SAS)® statistical software (Version: 9.4 or higher; SAS Institute Inc., USA). Categorical variables were summarized using counts and percentages. Continuous variables were described by descriptive statistics (i.e., Mean, Median, Min, Max). For continuous variables, the within-treatment analyses were conducted to compare baseline to post-treatment data using paired t-test. For categorical variables, the within-treatment analysis was conducted to compare baseline to post-treatment analysis using the Wilcoxon signed-rank test. All statistical tests of the hypothesis were employed at a level of significance of 0.05. If the P-value was observed ≤0.05, the test was considered statistically significant otherwise non-significant.
RESULTS
Participant characteristics/subject demography
In this study, among 36 enrolled subjects, there were 24 females and 12 males. Age of the subjects ranged between 19 and 59 years with an average being 38.3 years. At the end of the study, 4 subjects were lost to follow-up and were treated as dropped out of the study [Table 1].
| Gender, n (%) | Female | ([0-9])4 (66.67) |
| Male | ([0-9])2 (33.33) | |
| Race, n (%) | Asian | ([0-9])6 (100) |
| Age (Years) | N | ([0-9])6 |
| Mean (SD) | ([0-9])8.3 (9.67) | |
| Median | ([0-9])9.5 | |
| Min, Max | ([0-9])9, 59 |
SD: Standard deviation
Efficacy assessments
SQS
On consuming the test product for 25 days, the assessment of SQS score showed a significant reduction by 35.2% on day 15 (P < 0.0001) and 60.8% on day 25 (P < 0.0001), as compared to baseline. Thus, Nyumi beauty sleep gummies were found to be effective in improving sleep quality [Table 2 and Figure 2a].
| Sleep parameters | Visit 01 | Visit 02 | Visit 03 | ||||
|---|---|---|---|---|---|---|---|
| Baseline (SD) | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | |
| Mean value | |||||||
| Sleep quality scale | ([0-9])2.4* (±3.88) | ([0-9])3.8* (±2.14) | −18.6 | −35.2 | ([0-9])0.4* (±1.16) | −32.0 | −60.8 |
| Insomnia severity index | ([0-9])3.2* (±0.77) | ([0-9]).2* (±0.75) | −6.9 | −52.7 | ([0-9]).0* (±0.00) | −13.2 | −100.0 |
| Perceived stress scale | ([0-9])4.8* (±4.00) | ([0-9])3.7* (±2.22) | −11.1 | −44.6 | ([0-9]).5* (±1.29) | −23.3 | −93.4 |
| Fatigue severity scale | ([0-9])0.8* (±1.65) | ([0-9])3.5* (±2.37) | −17.3 | −33.9 | ([0-9]).7* (±1.00) | −41.1 | −81.0 |
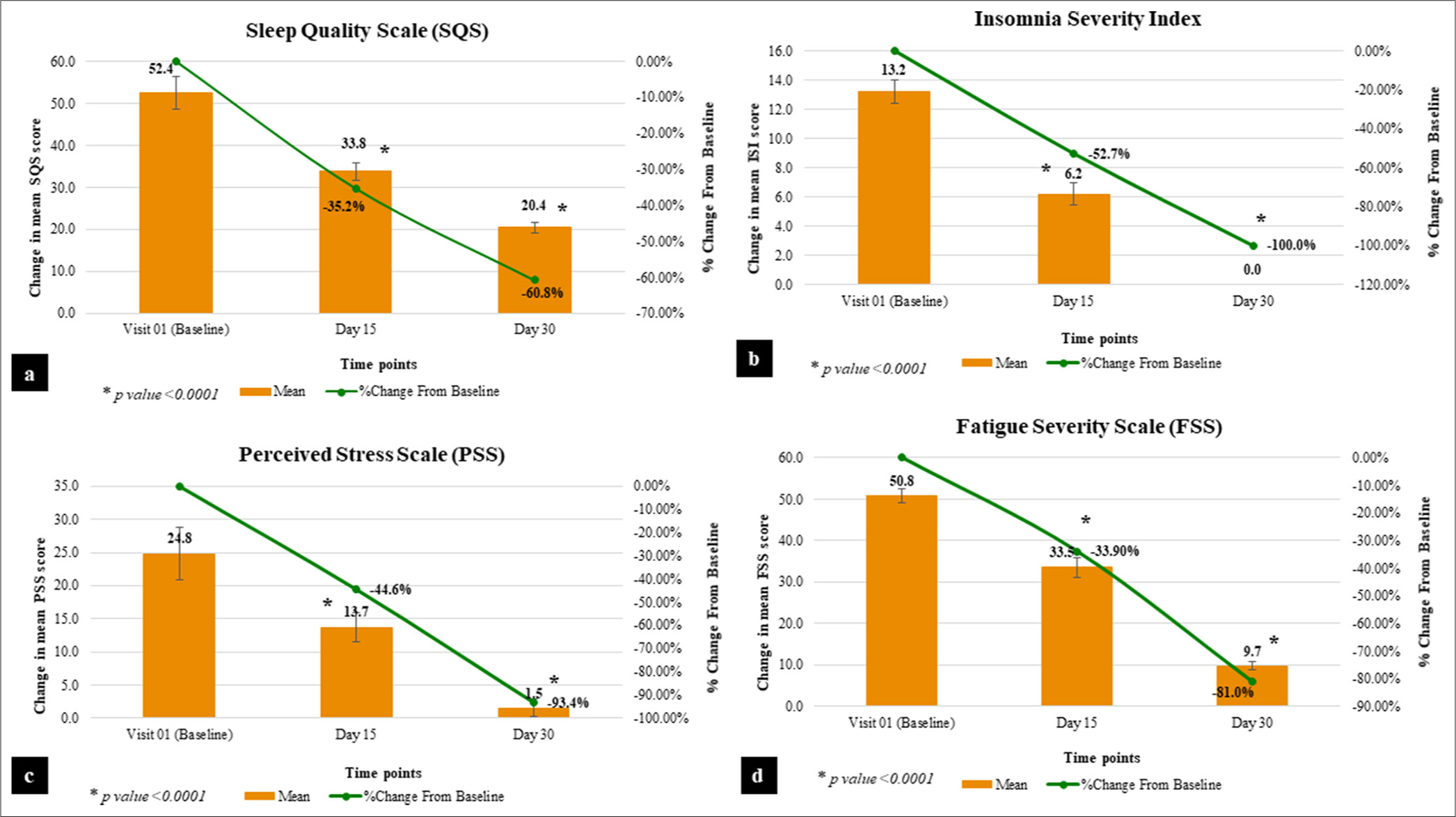
- Graphical representation of sleep quality parameters. (a) Sleep quality scale. (b) Insomnia severity index. (c) Perceived stress scale. (d) Fatigue severity scale.
Insomnia severity index
At baseline, the mean score of severity of insomnia was 13.2. After consuming the test product for 15 days and 25 days, the mean score of severity of insomnia was 6.2 and 0.0, respectively. On consuming the test product for 25 days, assessment of insomnia severity exhibited a significant reduction by 52.7% on day 15 (P < 0.0001) and 100.0% on day 25 (P < 0.0001), as compared to the baseline proving the effectiveness of the test product on the severity of non-clinical insomnia symptoms [Table 2 and Figure 2b]. It is evident from the results that 100% of participants showed a complete absence of insomnia severity symptoms post-25 days of consumption of Nyumi beauty sleep gummies.
PSS
At baseline, the mean perceived stress score was 24.8. After consuming the test product for 15 days and 25 days, the mean perceived stress score was 13.7 and 1.5, respectively. The results prove that on consuming the test product for 25 days, there is a significant reduction in the score of PSS by 44.6% on day 15 (P < 0.0001) and 93.4% on day 25 (P < 0.0001), as compared to baseline [Table 2 and Figure 2c] indicating significant reduction in stress level.
FSS
At baseline, the mean score was 50.8. After consuming the test product for 15 days and 25 days, the mean score was 33.5 and 9.7, respectively. On consuming Nyumi beauty sleep gummies for 25 days, there is a significant reduction by 33.9% on day 15 (P < 0.0001) and 81.0% on day 25 (P < 0.0001) in the score, as compared to baseline [Table 2 and Figure 2d]. The results imply that there was a significant reduction in daytime fatigue among the participants.
Skin radiance using modified Griffiths scale by dermatologist
At baseline, the mean score of dermatological assessment of skin radiance by Modified Griffiths Scale was 4.7, which was reduced to 3.5 and 2.2 after consuming the test product for 15 days and 25 days, respectively. The results showed that on consuming the test product, there is a significant improvement by 25.2% on day 15 (P < 0.0001) and 53.7% on day 25 (P < 0.0001), as compared to baseline, proving its effectiveness in improving skin radiance [Table 3 and Figure 3a].
| Skin parameters | Visit 01 | Visit 02 | Visit 03 | ||||
|---|---|---|---|---|---|---|---|
| Baseline (SD) | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | |
| Mean Value | |||||||
| Skin radiance using modified Griffiths scale | ([0-9]).7* (±0.77) | ([0-9]).5* (±0.62) | −1.2 | −25.2 | ([0-9]).2* (±0.57) | −2.6 | −53.7 |
| Instrumental assessment by skin Glossymeter GL 200 | ([0-9]).954** (±1.087) | ([0-9]).014** (±1.347) | ([0-9]).060 | ([0-9])16.507 | ([0-9]).423** (±1.302) | ([0-9]).468 | ([0-9])11.685 |
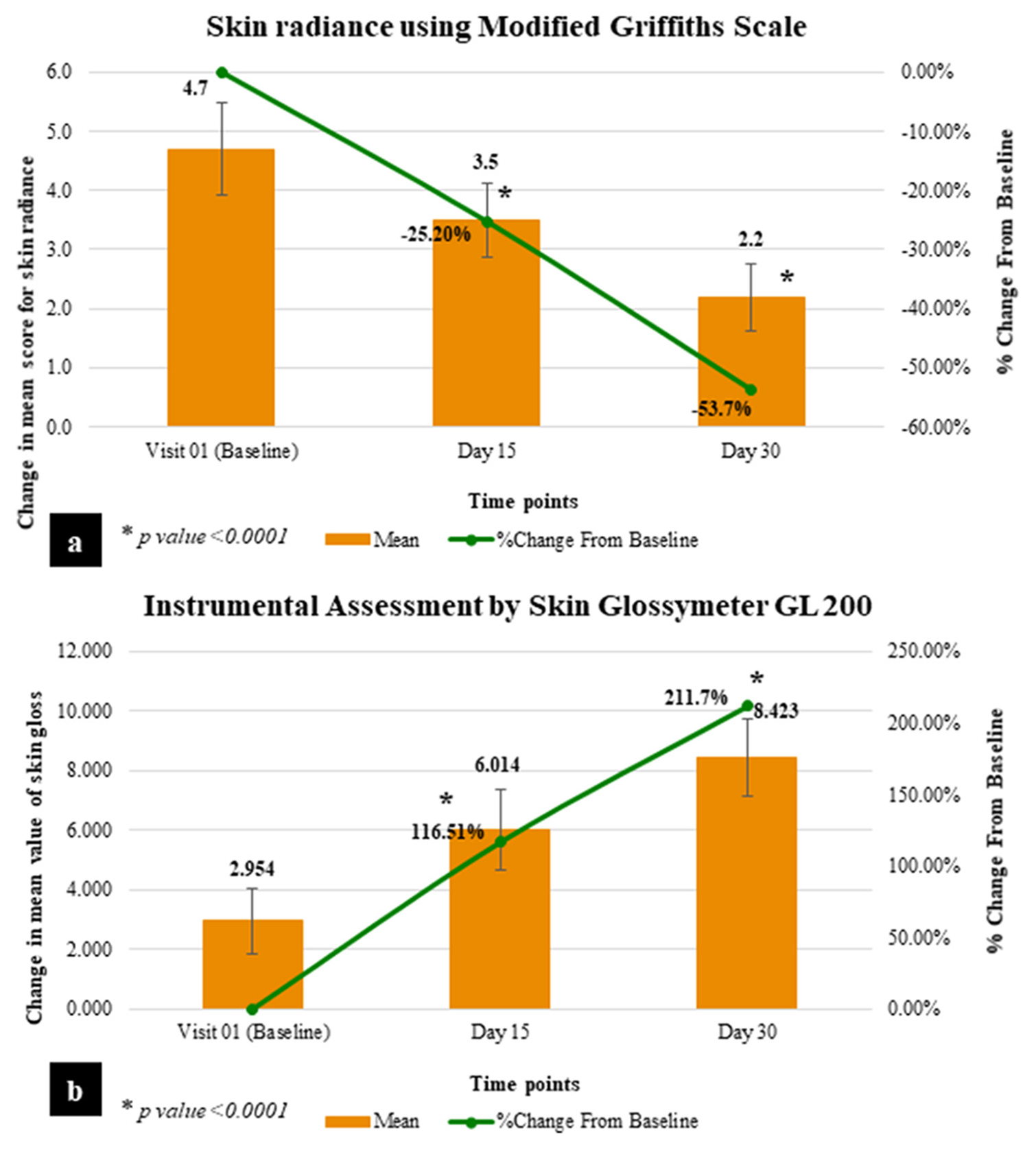
- Graphical representation of skin improvement parameters. (a) Skin radiance using modified Griffiths scale. (b) Instrumental assessment by skin Glossymeter GL 200.
Skin glow by skin Glossymeter GL 200
At baseline, the mean Glossymeter reading was 2.954, which was increased to 6.014 and 8.423 after consuming the test product for 15 days and 25 days, respectively. The instrumental assessment of skin gloss/glow by Skin Glossymeter GL 200 showed significant improvement by 1.16-folds on day 15 (P < 0.0001) and 2.11-folds on day 25 (P < 0.0001), as compared to baseline. This clinically indicates that Nyumi beauty sleep gummies are effective in improving skin gloss/glow [Table 3 and Figure 3b].
Serum cortisol
On consuming the test product for 25 days, there is no statistically significant change observed in serum cortisol level (P = 0.0967). However, a slight reduction from baseline (−5.28%) was observed at day 25. This indicates that Nyumi beauty sleep gummies slightly reduce serum cortisol levels and maintain them within the normal range [Table 4].
| Statistics | Visit 01 | Visit 03 | ||
|---|---|---|---|---|
| Baseline (SD) | Post baseline (SD) | CFB | %CFB | |
| Mean | ||||
| Serum Cortisol | ([0-9]).961* (±2.576) | ([0-9]).066* (±2.437) | −0.895 | −5.273 |
Subjective assessment questionnaire
Subjective assessment on various sleep and skin glow parameters showed that Nyumi beauty sleep gummies are effective in improving sleep quality (in terms of total sleep time, sleep latency, sleep efficiency, time to sleep onset, number of awakenings, wake time after sleep onset), daytime mood, ability to function at work, concentration, memory, and skin glow. At baseline, the mean sleep efficiency of all subjects was 60.0%. After consuming the test product for 15 days and 25 days, the mean sleep efficiency of all subjects was 74.0% and 89.6%, respectively. Thus, assessment of sleep efficiency showed significant improvement by 24.1% on day 15 (P < 0.0001) and 50.6% on day 25 (P < 0.0001), as compared to baseline [Table 5].
| Statistics | Visit 01 | Visit 02 | Visit 03 | ||||
|---|---|---|---|---|---|---|---|
| Baseline (SD) | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | |
| Mean Value | |||||||
| Sleep Efficiency# | ([0-9])0.0* (±5.06) | ([0-9])4.0* (±3.05) | ([0-9])4.0 | ([0-9])4.1 | ([0-9])9.6* (±3.20) | ([0-9])9.6 | ([0-9])0.6 |
At baseline, 28.13% of subjects reported that they got <5 h of actual sleep at night; whereas 65.63% of subjects reported that they got 5–6 h of actual sleep at night. However, at the end of the study, 59.38% of subjects reported that they got 6–7 h of actual sleep at night; whereas 34.38% of subjects reported that they got more than 7 h of actual sleep at night.
At the end of the study, 93.75% of subjects did not report any waking up time as they did not wake up through the night after sleeping; 100% of subjects reported that it took them 15 min to fall asleep each night; 84.38% subjects reported that they spent more than 7 h in bed; 100% subjects reported that their sleep problem does not affect their memory at all; 96.88% subjects reported that their sleep problem does not affect their concentration at all; 100% subjects reported that their sleep problem does not affect their ability to function at work at all; 100% subjects reported that their sleep problem does not affect their mood at all during the day; and 100% subjects were agreed to strongly agreed that there is an improvement in skin glow on consumption of test product [Figure 4].
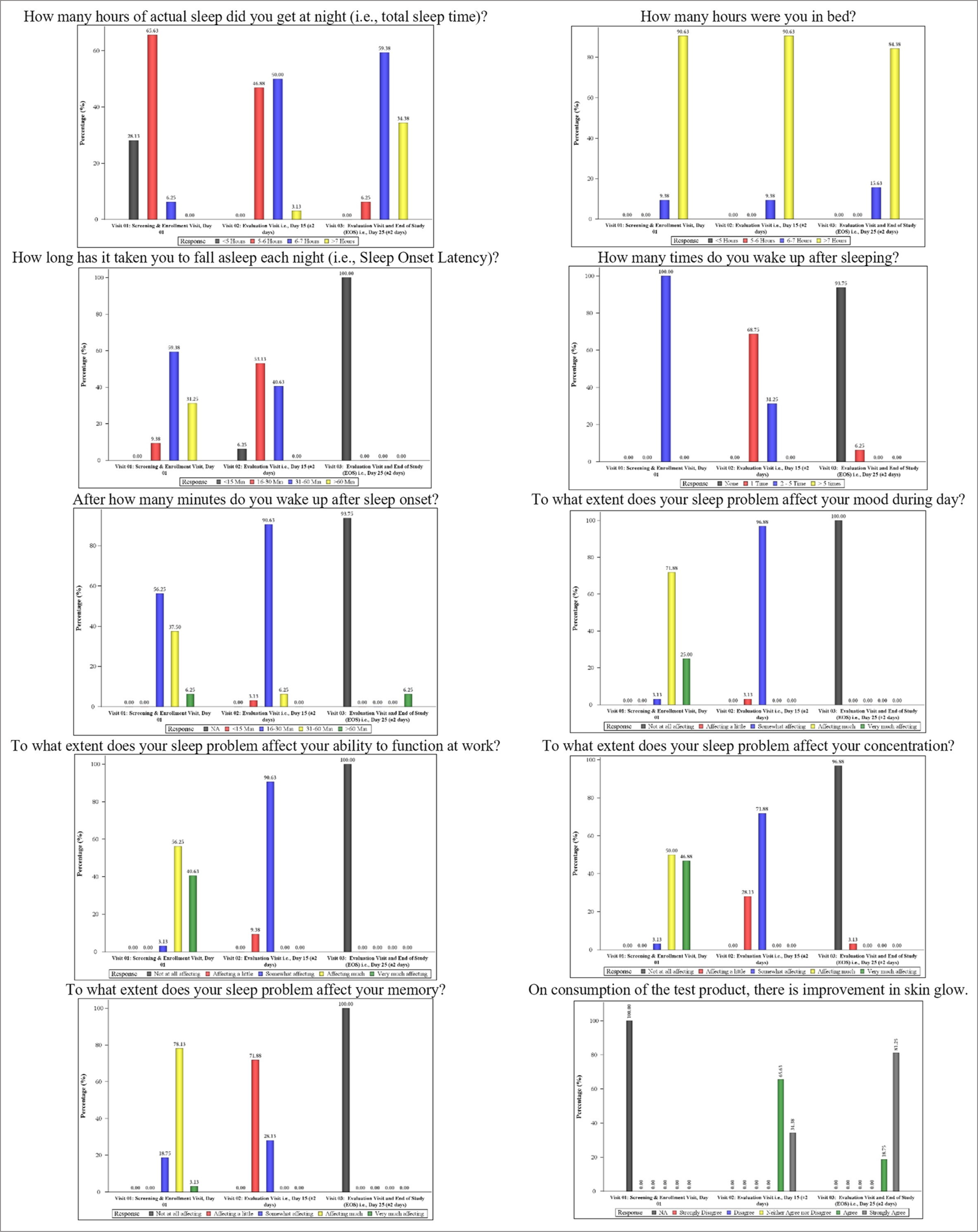
- Graphical representation of subjective questionnaire parameters.
Furthermore, no local intolerances were observed in any of the subjects during the study period confirming the safety of the test product and no AEs were neither reported nor observed during the course of the study.
DISCUSSION
Sleep is the utmost necessity to keep the body healthy and for proper physical and mental functioning as well. A healthy sleep is truly interdisciplinary because it touches every aspect of well-being. The quantity and quality of daily sleep determines the overall health condition of a person. Poor sleep habits may dramatically affect productivity, quality of life, and health of a person. Sleep is certainly a complex physiological state that can be classified into the rapid eye movement (REM) stage - also known as paradoxical sleep, and the non-REM or slow-wave sleep stage. Different regions of the central nervous system, mainly the hypothalamus, in combination with several neurotransmitters, control the sleep-wake cycle.[30]
There are many factors that determine the sleep pattern of a person, including habit, lifestyle, any medical condition, etc. Sleep deprivation has a high prevalence nowadays and is often closely associated with poor physical and psychological activity and primary sleep disorders which are not associated with a medical condition, substance use, or concurrent psychological disorder.[31] The most common indications of sleep deprivation are difficulty initiating or maintaining sleep or early morning awakening with an inability to return to sleep and dissatisfaction with sleep quality or quantity.
The first-line treatment preferences for sleep deprivation and primary sleep disorders often include psychological or behavioral therapies.[32] Apart from these, various nonpharmacological therapies are also in practice for sleep management. The latter includes sleep hygiene, sleep restriction, stimulus control, aromatherapy, relaxation training, and cognitive therapy which have shown some evidence of efficacy. For more severe conditions, it is clinically managed by pharmacological treatments, namely benzodiazepine receptor agonists, benzodiazepines, sedating antidepressants, and other drugs with sedating features (anxiolytics, antipsychotics, and antihistamines).[31] Although these medications have proven efficacy, they have a range of side effects and often they are dependent forming. The common side effects of pharmacological interventions include daytime drowsiness, depression, hypnotic withdrawal insomnia, and even excess mortality.[33] Moreover, significant barriers exist in the pharmacological treatments that include limited treatment access due to high cost, long duration of the treatment, lack of trained clinicians, lack of understanding of non-pharmacotherapy treatment options, and tendency of self-management.[34]
The use of nutraceutical products for sleep disorders and associated conditions is of prime interest nowadays. Plant-based and nutraceutical ingredients for sleep therapeutics include chamomile, cherries, kava kava, L-tryptophan, and valerian.[3] The test product (Nyumi beauty sleep gummies) is formulated with L-theanine, Melatonin, Chamomile extract (flower), Multivitamin (Vitamin A, C, E, D2, B3, B6), Tagara (V. wallichii) root extract, and Pomegranate Extract. These multivitamins, nutraceutical, and plant-based natural ingredients have proven anti-stress, anti-anxiety, and relaxation benefits on mild symptoms of non-clinical insomnia, thus improving sleep quality. Some of the ingredients have proven efficacy on various skin conditions, including pigmentation, skin glow, and skin radiance. Moreover, there is also a direct association between sleep quality and the expression of skin glow and skin texture, which is an ancillary advantage of good sleep.
Results of various previous research indicate that L-theanine, a non-protein amino acid derived from botanical sources, produces a soothing and calm effect through inhibitory neurotransmitters, thus emitting positive effects on stress and poor sleep management. In the human model, oral dosage of L-theanine effectively improved sleep quality by reducing anxiety and relaxation. The results ascertain a reduction of intermittent awakening and also the recovery from exhaustion and refreshed awakening. It simulates increased parasympathetic nerve system responses and decreases sympathetic nerve system responses, thus supplementation of L-theanine before going to bed may improve sleep quality through anxiolysis but not by sedation.[35] It increases alpha brain wave frequency without producing drowsiness, which is a possible mode of action in improving sleep quality. In another finding, oral administration for four weeks was also found effective in reducing Pittsburgh Sleep Quality Index subscale scores for sleep latency, sleep disturbance, and use of sleep medication, compared to the placebo supplement (P < 0.05).[36] Melatonin is a dietary supplement and might be of interest to medical science in the management of sleep deprivation. It may be one natural solution for poor sleep.[37] Results of the earlier research conducted suggest that it resets the body’s sleep-wake cycle and increases total sleep time when supplemented by the person suffering from sleep restriction or altered sleep schedule. It is also found beneficial in relieving daytime fatigue associated with jet lag. In individuals with delayed sleep phase syndrome, melatonin supplementation reduces the time of falling asleep.[38]
One of the active ingredients of the test product is Chamomile Extract (flower), which has both sedative and anti-anxiety effects and is widely regarded as a mild tranquilizer and sleep-inducer. It may provide modest and mixed clinical benefits to patients with chronic primary insomnia.[39] It is evident from a study that apigenin, a component of chamomile, binds at the same receptor sites in the brain as benzodiazepines like Valium.[40] Tagara (V. wallichii) roots have shown significant improvement in initiation and duration of sleep and help in disturbed sleep in a comparative clinical study.[41] Valepotriates found in the plant are considered primarily responsible for improving sleep quality.[42]
Sleep deprivation is an important condition related to hyperalgesia. The former has been associated with Vitamin D in recent studies.[30] Vitamin D deficiency has both a direct and an indirect role in the regulation of sleep.[43] Several mechanisms have been suggested to describe Vitamin D deficiency and sleep disorders relationship. Vitamin D deficiency regulates the development of symptoms of wakefulness that are commonly related to sleep disorders.[44] Similarly, a deficiency of Vitamin B6 (pyridoxine) has been associated with the symptoms of insomnia and depression. Vitamin B6 acts as a coenzyme in the biosynthesis of melatonin. The latter plays a vital role in regulating the circadian rhythm and sleep-wake cycle, and its deficit results in sleep disturbances.[45] Vitamin B3, often referred to as Niacinamide or Nicotinamide, is known to promote sleep[46] and has been shown to inhibit melanosome transfer and improve facial pigmentation. It can also be utilized as a complementary agent for skin conditions.[47]
It is, therefore, evident from the above discussion that all the ingredients have proven efficacy on the indications associated with sleep disturbance and skin expression. The synergistic effect of active ingredients shows that Nyumi beauty sleep gummies effectively improve the sleep quality in healthy adult volunteers after consuming the recommended dosage, i.e., 2 gummies together once a day for 25 days, as evaluated by SQS and Insomnia Severity Index. It also reduced the score of PSS and Fatigue Severity Index significantly. Furthermore, after consuming the product for 25 days, there was a reduction of 5.28% in serum cortisol level that can be correlated with reduced stress level and good sleep quality of the volunteers, the reduction, however, was not significant. Dermatological assessment of skin radiance by Modified Griffiths Scale and Glossymeter GL 200 instrument also proved that there is a significant improvement in skin radiance and skin gloss. Results of subjective assessment parameters were also in parity of the above results where the test product was found effective on various sleep and skin glow parameters, namely total sleep time, sleep latency, sleep efficiency, time to sleep onset, number of awakenings, wake time after sleep onset, daytime mood, ability to function at work, concentration, memory, along with the skin glow.
There are a few limitations of the present clinical study including short study duration, low sample size, and non-comparative study design.
CONCLUSION
Based on the overall clinical assessments, instrumental evaluation, and subjective analysis, it can be concluded that Nyumi beauty sleep gummies significantly improve sleep quality and reduce perceived stress, fatigue severity, and insomnia severity scores. The test product also helped in improving skin glow and skin radiance at the end of the study as assessed by the Glossymeter GL 200 instrument and the Dermatological assessment by the Modified Griffiths Scale. Furthermore, all subjects showed improvement in sleep quality (in terms of total sleep time, sleep latency, sleep efficiency, time to sleep onset, number of awakenings, wake time after sleep onset, sleep efficiency), daytime mood, ability to function at work, concentration, memory; also, improvement in skin glow was observed as assessed by subjective assessment questionnaire. Moreover, there was no local intolerance and no AEs neither reported nor observed after the use of the product throughout the study. Thus, the Nyumi beauty sleep gummies can be consumed safely to improve sleep and skin quality.
Ethical approval
The research/study was approved by the Institutional Review Board at OM Institutional Ethics Committee, number C3B02406, dated August 13, 2022.
Declaration of participant consent
The authors certify that they have obtained all appropriate participant consent.
Conflicts of interest
There is no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
This study was financially supported by Ikaria Wellness Pvt. Ltd., India.
References
- Transcriptional signatures of sleep duration discordance in monozygotic twins. Sleep. 2017;40:zsw019.
- [CrossRef] [Google Scholar]
- The extraordinary importance of sleep: The detrimental effects of inadequate sleep on health and public safety drive an explosion of sleep research. P T. 2018;43:758-63.
- [Google Scholar]
- Updates on nutraceutical sleep therapeutics and investigational research. Evid Based Complement Alternat Med. 2015;2015:105256.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep quality among medical students in Moradabad, Uttar Pradesh, India. Int J Community Med Public Health. 2020;7:274-8.
- [CrossRef] [Google Scholar]
- Do sleep, stress, and illness explain daily variations in fatigue? A prospective study. J Psychosom Res. 2014;76:280-5.
- [CrossRef] [PubMed] [Google Scholar]
- Day-to-day relations between stress and sleep and the mediating role of perseverative cognition. Sleep Med. 2016;24:71-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cues of fatigue: Effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-60.
- [CrossRef] [PubMed] [Google Scholar]
- Does poor sleep quality affect skin ageing? Clin Exp Dermatol 2014. Clin Exp Dermatol. 2015;40:17-22.
- [CrossRef] [PubMed] [Google Scholar]
- Short-and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151-61.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep duration and hypertension: Analysis of > 700,000 adults by age and sex. J Clin Sleep Med. 2018;14:1031-9.
- [CrossRef] [PubMed] [Google Scholar]
- Restless legs syndrome and cardiovascular disease: A research roadmap. Sleep Med. 2017;31:10-7.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metabolism. 2018;84:56-86.
- [CrossRef] [PubMed] [Google Scholar]
- Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: Methodology and discussion. Sleep. 2015;38:1161-83.
- [CrossRef] [PubMed] [Google Scholar]
- OSA and cardiac arrhythmogenesis: Mechanistic insights. Chest. 2017;151:225-41.
- [CrossRef] [PubMed] [Google Scholar]
- Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: A cross-sectional study of 91 105 participants from the UK Biobank. Lancet Psychiatry. 2018;5:507-14.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep and neurodegeneration: A critical appraisal. Chest. 2017;151:1375-86.
- [CrossRef] [PubMed] [Google Scholar]
- Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology. 2017;89:445-53.
- [CrossRef] [PubMed] [Google Scholar]
- Sleep loss causes social withdrawal and loneliness. Nat Commun. 2018;9:3146.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of sleep disorders: A brief review for clinicians. Dialogues Clin Neurosci. 2003;5:371-88.
- [CrossRef] [PubMed] [Google Scholar]
- Nutraceuticals for sleep disorders. Comb Chem High Throughput Screen. 2021;24:1583-92.
- [CrossRef] [PubMed] [Google Scholar]
- Development of the sleep quality scale. J Sleep Res. 2006;15:309-16.
- [CrossRef] [PubMed] [Google Scholar]
- A global measure of perceived stress. J Health Soc Behav. 1983;24:385-96.
- [CrossRef] [PubMed] [Google Scholar]
- The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121-3.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
- [CrossRef] [PubMed] [Google Scholar]
- A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol. 1992;128:347-51.
- [CrossRef] [PubMed] [Google Scholar]
- Taxifolin as a promising ingredient of cosmetics for adult skin. Antioxidants (Basel). 2021;10:1625.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment methodologies in sleep medicine clinical trials. Clin Invest. 2013;3:791-800.
- [CrossRef] [Google Scholar]
- Interactions between sleep, stress, and metabolism: From physiological to pathological conditions. Sleep Sci. 2015;8:143-52.
- [CrossRef] [PubMed] [Google Scholar]
- The role of cortisol in sleep. 2014. Nat Med J. Available from: https://www.naturalmedicinejournal.com/journal/role-cortisol-sleep
- [Google Scholar]
- The interfaces between vitamin D, sleep and pain. J Endocrinol. 2017;234:R23-36.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS One. 2013;8:e63773.
- [CrossRef] [PubMed] [Google Scholar]
- Insomnia: Evidence-based approaches to assessment and management. Clin Med. 2011;11:278-81.
- [CrossRef] [PubMed] [Google Scholar]
- Hypnotic drug risks of mortality, infection, depression, and cancer: But lack of benefit. F1000Research. 2016;5:918.
- [CrossRef] [PubMed] [Google Scholar]
- Medicinal seeds Ziziphus spinosa for insomnia: A randomized, placebo-controlled, cross-over, feasibility clinical trial. Complement Ther Med. 2021;57:102657.
- [CrossRef] [PubMed] [Google Scholar]
- In search of a safe natural sleep aid. J Am Coll Nutr. 2015;34:436-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of L-theanine administration on stress-related symptoms and cognitive functions in healthy adults: A randomized controlled trial. Nutrients. 2019;11:2362.
- [CrossRef] [PubMed] [Google Scholar]
- The effectiveness of melatonin for promoting healthy sleep: A rapid evidence assessment of the literature. Nutr J. 2014;13:106.
- [CrossRef] [PubMed] [Google Scholar]
- Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: A randomized placebo-controlled pilot study. BMC Complement Altern Med. 2011;11:78.
- [CrossRef] [PubMed] [Google Scholar]
- Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995;61:213-6.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative clinical study on the effect of Tagara (Valeriana wallichii DC.) and Jatamansi (Nardostachys jatamansi DC.) in the management of Anidra (primary insomnia) Ayu. 2015;36:46-9.
- [CrossRef] [PubMed] [Google Scholar]
- The scientific basis for the reputed activity of Valerian. J Pharm Pharmacol. 1999;51:505-12.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D and sleep regulation: Is there a role for vitamin D? Curr Pharm Des. 2020;26:2492-6.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012;8:693-7.
- [CrossRef] [PubMed] [Google Scholar]
- A combination of melatonin, vitamin B6 and medicinal plants in the treatment of mild-to-moderate insomnia: A prospective pilot study. Complement Ther Med. 2019;45:104-8.
- [CrossRef] [PubMed] [Google Scholar]
- Nicotinic acid promotes sleep through prostaglandin synthesis in mice. Sci Rep. 2019;9:17084.
- [CrossRef] [PubMed] [Google Scholar]






