Translate this page into:
Combination therapy with oral minoxidil and dutasteride in the treatment of male patterned baldness: A case report

*Corresponding author: Shashank Bansod, Consultant Dermatologist, Nagpur, Maharashtra, India. hitech.skin@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Bansod S. Combination therapy with oral minoxidil and dutasteride in the treatment of male patterned baldness: A case report. CosmoDerma 2022;2:4.
Abstract
Androgenetic alopecia is a medical condition with a deep social and psychological impact on the affected individuals and is characterized by progressive hair thinning, leading to hair loss over the scalp in both males and females. Minoxidil in oral form is primarily an antihypertensive drug, whose mechanism of action is not completely known. Dutasteride is a 5-alpha reductase inhibitor, acting on both alpha-1 and alpha-2 receptors. The author combined these two agents for the treatment of male patterned baldness and found that this combination imparts a visible increase in hair thickness, density, and new hair growth in the patient, within a short period causing minimal side effects.
Keywords
Androgenetic alopecia
Dutasteride
Male patterned baldness
Oral minoxidil
INTRODUCTION
Male patterned baldness is a common type of alopecia where topical minoxidil and finasteride are the first-line therapy and have been approved by the FDA for the same.[1] The author tried minoxidil in the oral formulation and combined it with dutasteride simultaneously as a novel treatment option in a male patient with Grade 5 alopecia.
CASE REPORT
A 28-year-old male presented to our clinic with Grade 5 male patterned baldness, for which he had been using minoxidil 5% solution on and off for the past few years without any satisfactory result. He wanted to get treated with medications only and denied any procedural intervention. After a thorough history and examination, the patient was prescribed tablet minoxidil 2.5 mg once a day and tablet dutasteride 0.5 mg alternate day orally. In addition, he was prescribed ketoconazole shampoo for hair washing twice a week.
The patient denied having any comorbidity including hypertension, liver, and kidney disorders. He was a non-smoker but an occasional drinker.
Before starting the combination therapy of oral minoxidil and dutasteride, the patient was counseled thoroughly regarding probable adverse effects and use of drugs as an off-label indication, to which he agreed on after which written consent was taken and treatment started.
A baseline ECG, serum electrolytes, and liver and kidney function tests were ordered and were found to be within normal limits. Baseline blood pressure was checked using digital apparatus in a supine position, which was also found to be within normal limits. The patient was strictly advised a restricted salt intake of 2 g/day.
Follow-up visits were planned every 4 weeks and blood pressure was recorded, any history of increased heartbeat, foot swelling, and excess hair growth over the face was elicited during each visit.
After 1 month, the patient experienced a slight increase in hair density over the vertex area. Subsequently, there was a visible increase in hair density overall, with new hair growth seen over the vertex and frontal area [Figure 1].
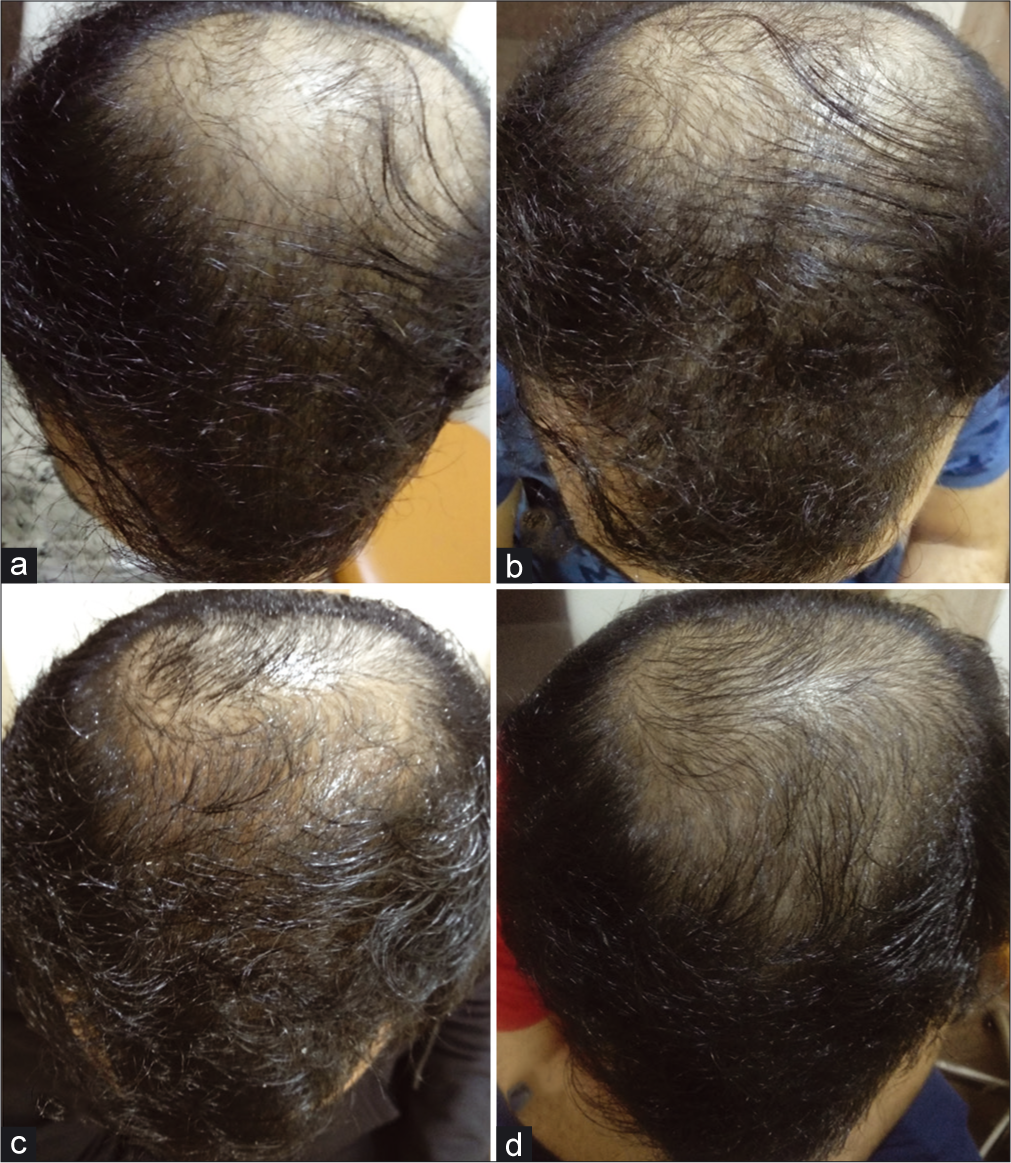
- Progression of hair growth after treatment with 2.5 mg/day oral minoxidil (a). Baseline before starting the treatment (b). One month after starting the treatment, slight increase in hair density seen (c). After 2 months, appreciable increase in hair density (d). After 3 months, new hair growth visible with progressive increase in hair density.
At the end of the 4th month, increased hair volume and new hair growth were seen in all the areas of the scalp which was evident on comparative digital global photography [Figure 2].
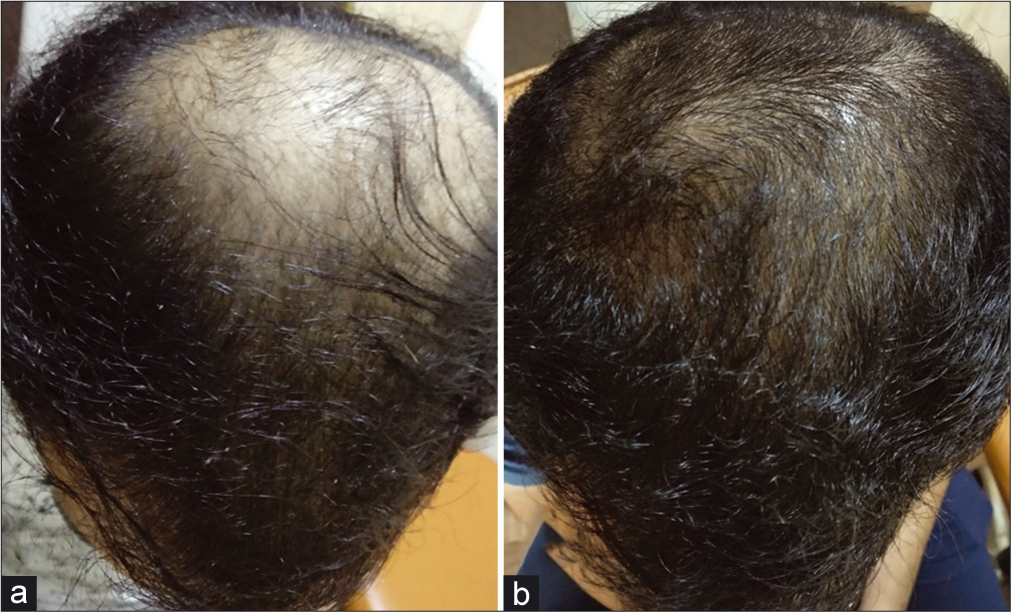
- (a and b) Appreciable increase in hair density, with new hair growth over whole scalp at the end of 4 months of treatment as compared to baseline.
The patient did not experience a reduction in libido, tachycardia, and pedal edema. Only slight hypertrichosis was noticed over the face, which was acceptable to the patient.
The patient is still under treatment with the same regimen on the day of article submission.
DISCUSSION
Oral minoxidil is primarily an antihypertensive drug. Its mechanism of action in hair growth is not completely known, but it is said to increase hair diameter, prolong the anagen phase, and simultaneously shorten the telogen phase.[2]
A major advantage of oral minoxidil over topical form is that it bypasses the percutaneous barrier and thereby increases drug absorption significantly.[3]
Hypertrichosis, pedal edema, and postural hypotension are the common adverse effects encountered.[4] It may also cause water and salt retention; hence, a salt restriction of 2 g/day is advised in all the patients, which if not followed strictly, may cause headache, palpitation, and lethargy.[5] Oral minoxidil should be given with caution in hypertensive and cardiac patients as it is known to cause ECG changes too. Minoxidil in oral form is metabolized by the liver and excreted by kidneys.[6]
The author recommends that baseline investigations of serum electrolytes, liver function, kidney function, and ECG should be done for the same reason and should be repeated every 6 months. Blood pressure recording in supine position should be done at baseline and should be repeated every 4 weeks. Along with this, the patient should be asked and examined for the said side effects too. Best candidates are normotensive individuals with no comorbidity. The exact duration for which this drug can safely be given is not yet known due to a paucity of the literature, but the author has used it for 8–10 months continuously without any significant adverse effects.
Dutasteride is a potent, specific, and competitive inhibitor of both type 1 and type 2, 5-alpha reductase[7] and has been shown to have better efficacy than finasteride with quicker hair growth and side effect profile similar to finasteride. Common side effects include altered libido, erectile dysfunction, and ejaculation disorder, which are dose and duration-dependent and reduce gradually after drug withdrawal.[8]
The author prescribed oral minoxidil in the dose of 2.5 mg/day, as quite a few studies reported this to be the optimum dose, in case of a lesser than expected response, the dose can be increased to 5 mg/day as per some studies.[9] Dutasteride was prescribed as a 0.5 mg dose alternate day due to its long half- life, hence, better compliance with the patients.[10]
CONCLUSION
A combination therapy with oral minoxidil, 2.5 mg/day, and dutasteride, 0.5 mg alternate day was found to produce quick results in male patterned baldness of advanced grades with minimal adverse effects. To confirm the findings of the author, large-scale multicentric studies are required.
Declaration of patient consent
Patient consent is not required as the patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Androgenetic alopecia: New approved and unapproved treatments. Dermatol Clin. 2000;18:47-61.
- [CrossRef] [Google Scholar]
- Mechanism of action of minoxidil in the treatment of androgenetic alopecia is likely mediated by mitochondrial adenosine triphosphate synthase-induced stem cell differentiation. J Biol Regul Homeost Agents. 2017;31:1049-53.
- [CrossRef] [PubMed] [Google Scholar]
- Oral minoxidil treatment for hair loss: A review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-46.
- [CrossRef] [PubMed] [Google Scholar]
- Case series of oral minoxidil for androgenetic and traction alopecia: Tolerability and the five C's of oral therapy. Dermatol Ther. 2018;31:e12707.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of oral minoxidil in the treatment of alopecia areata. Arch Dermatol. 1987;123:1488-90.
- [CrossRef] [PubMed] [Google Scholar]
- Oral minoxidil: A possible new therapy for androgenetic alopecia. J Cutan Med Surg. 2020;24:88-9.
- [CrossRef] [PubMed] [Google Scholar]
- Dutasteride: A review of its use in the management of prostate disorders. Clin Med Insights. 2011;3:CMT-S1956.
- [CrossRef] [Google Scholar]
- Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): A systematic review. J Clin Aesthet Dermatol. 2016;9:56-62.
- [Google Scholar]
- Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: An open-label and global photographic assessment. Dermatol Ther. 2020;10:1345-57.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6(Suppl 9):S31.
- [Google Scholar]






