Translate this page into:
Clinicodermoscopic and pathological features of a rare pigmented tumor arising from a scar

*Corresponding author: Rachita S. Dhurat, Department of Dermatology, Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai, Maharashtra, India. rachitadhurat@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Kowe P, Ranka A, Maity A, Gitte DV, Dhurat RS. Clinicodermoscopic and pathological features of a rare pigmented tumor arising from a scar. CosmoDerma. 2024;4:66. doi: 10.25259/CSDM_48_2024
Abstract
A scar due to any underlying pathology is prone to develop various other pathologies over the same site. Among the tumors, squamous cell carcinoma, basal cell carcinoma, and dermatofibrosarcoma protuberans (DFSP) are known to arise from preexisting scar tissue. Being non-invasive and quick, dermoscopy plays an important bedside tool in differentiating various skin tumors from each other till further confirmation by histopathology and immunohistochemistry. We report a rare case of DFSP arising from a scar.
Keywords
Spindle cell
Dermatofibrosarcoma protuberans
Dermoscopy
Storiform pattern
INTRODUCTION
Dermatofibrosarcoma protuberans (DFSP) is a rare mesenchymal soft-tissue neoplasm of lowto-intermediate-grade malignancy. It is an aggressive sarcoma that originates from the dermal fibroblast tissue and may extend deeper structures such as subcutis, fascia, muscle, and bone.[1] Although it displays local aggressiveness, it seldom spreads to vital organs through metastasis. A pigmented multinodular asymptomatic mass may mimic malignant melanoma, basal cell carcinoma (BCC), DFSP, and lupus vulgaris and deep fungal infection among the infective etiologies and can pose a diagnostic dilemma; however, dermoscopy can help narrow down the differentials. We are highlighting clinical, dermoscopic, and pathological features of DFSP arising from a scar in a young patient.
CASE REPORT
A 46-year-old man of skin phototype V presented with an asymptomatic gradually progressive dark-colored mass over the back arising from a scar for the past seven years with a history of ulceration for 15 days. These lesions were present over a preexisting scar that occurred due to a pea-sized lesion (details of which were unknown) approximately 10 years back which on self-manipulation healed with a scar. Cutaneous examination revealed skin-colored to bluish-black, non-tender firm plaque of size 6 × 5 cm associated with overlying multiple nodules, a few areas of ulceration and scarring over the upper back in the right paraspinal region [Figures 1a and b]. The lymph node and systemic examination were unremarkable. Polarized dermoscopy using 3 Gen DermLite DL4 (CA, USA) × 10 magnification showed white homogenous areas, a pigmentary network in the background, linear vessels, and red-brown homogenous areas [Figure 2]. The differential diagnoses of pigmented BCC, melanoma, and DFSP were considered. Contrast-enhanced computed tomography of the neck, thorax, and abdomen did not reveal any metastatic changes. Histopathology from the nodule revealed increased pigmentation of the basal layer and diffuse interlacing cords of spindle cells in the entire dermis extending up to the subcutaneous tissue with few areas showing a characteristic storiform pattern [Figures 3a-c]. Immunohistochemistry (IHC) staining was positive for CD 34 and negative for anti-Human Melanoma Black (HMB)-45 and S 100 stain [Figure 3d]. Thus, correlating clinical findings, dermoscopic examination, skin biopsy, and IHC a final diagnosis of DFSP from a scar was made. After consultation with surgical oncologists, the patient was planned for wide local excision of the tumor with a 3 cm tumor-free margin.

- (a) Skin-colored to bluish-black, non-tender firm plaque of size 6 × 5 cm associated with overlying multiple nodules, areas of ulceration and scarring over the upper back in the right paraspinal region. (b) A closer view of the skin lesion.
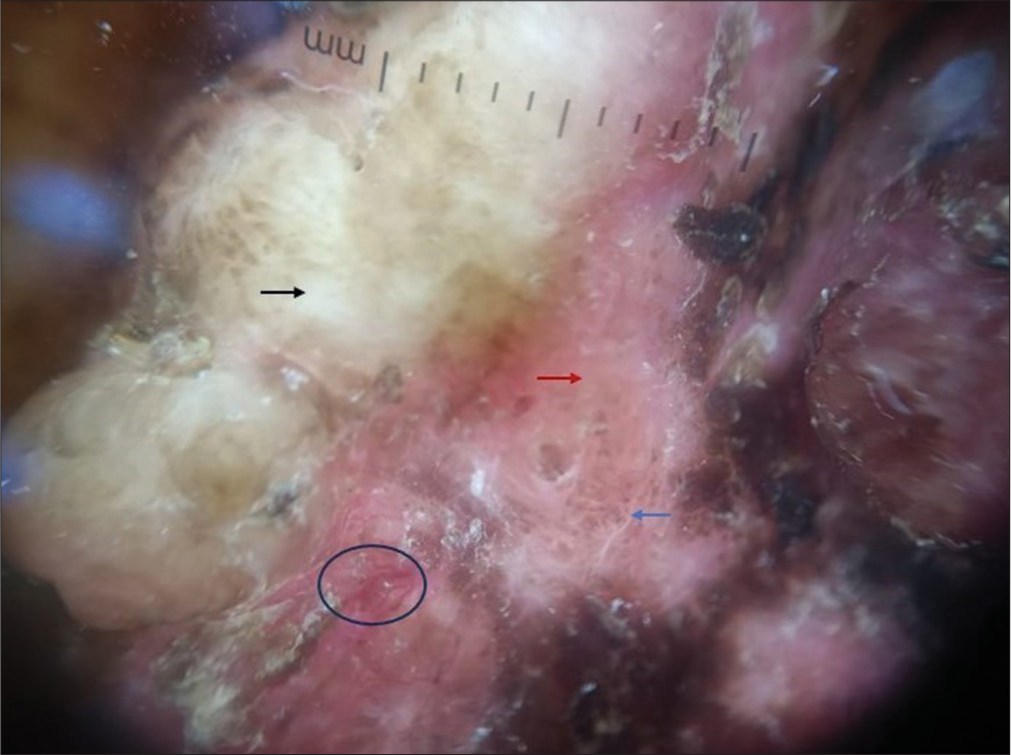
- Polarized dermoscopy showed a white homogenous area (black arrow), brown pigment in the periphery (blue arrow), red-brown homogenous area (red arrow), and linear vessels (black circle).
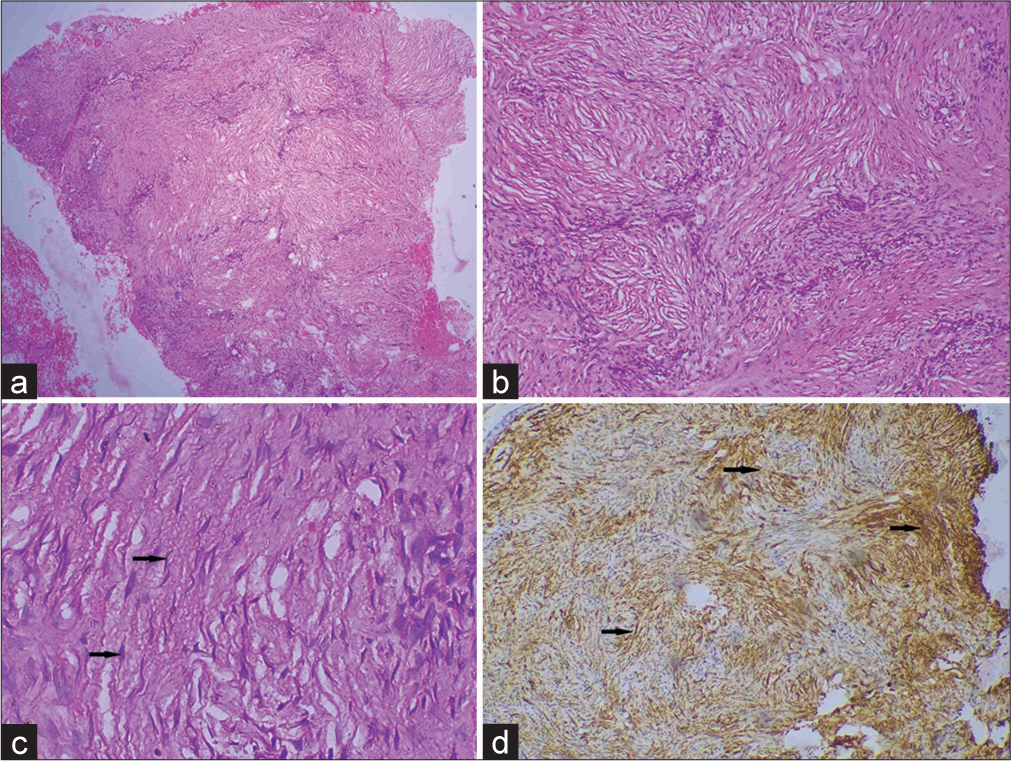
- (a) Scanner view showing interlacing cords of spindle cells involving the entire dermis and subcutis (H&E, × 40), (b) storiform pattern of spindle cells in the deep dermis (H&E, × 100), (c) dermal fibroblast cell (black arrow) (H&E, × 400), and (d) immunohistochemistry (IHC) showing tumor cells densely positive for CD 34 marker (black arrow) (IHC stain × 400).
DISCUSSION
The DFSP belongs to the group of fibroblastic/myofibroblastic tumors in the soft-tissue tumor classification of the World Health Organization 2013.[2] The yearly occurrence rate of this tumor ranges from 0.8 to 4.1 cases per million individuals.[3] It is prevalent in the third to fourth decade of life and is rare at extremes of ages.[1] The growth of the tumor is gradual, with a notable inclination for local recurrence (20 – 50%); however, metastasis to the vital organs through the blood or lymph is rare.[3] Its precise cause remains incompletely understood, although studies suggest a link to the chromosomal translocation t(17;22) (q22;q13) involving chromosome17 and chromosome 22, resulting in the fusion of platelet-derived growth factor-beta polypeptide gene and collagen type 1A1 gene. The rearrangement of this gene leads to the upregulation of Platelet-derived growth factor subunit B (PDGFB), causing excessive stimulation of platelet-derived growth factor, continuous autocrine activation of platelet-derived growth factor receptor-beta, cellular proliferation, and tumor formation.[4] It manifests as a gradually enlarging painless indurated plaque followed by multiple reddish-brown nodules that developed subsequently over it and have a predilection for the trunk and extremities.[5] Dermoscopic features of DFSP include a delicate pigment network (most common finding), structureless hypo to depigmented or brown areas, shiny white streaks, a pinkish background, and arborizing and linear vessels.[6] Kowe et al. also reported a structureless blue-colored area that corresponded to engorged blood vessels filled with deoxygenated blood.[7] Histopathology of DFSP includes tightly packed monomorphous plump spindle cells (dermal fibroblast) in a typical storiform or cartwheel-like pattern in the center of the tumor whereas the periphery of the tumor shows diffuse infiltration of the dermal stroma extending up to the subcutaneous tissue in a honeycomb-like pattern with a minimal atypia or mitotic activity.[8] Immunohistochemical study shows strong positivity for CD34 whereas S100, melan-A, factor XIIIa, desmin, α-smooth muscle actin, and cytokeratin are negative.[9] Primary melanoma shows positivity for multiple biomarkers such as anti-HMB 45, melan-A, and S100 protein family [Table 1].[10] Wide local excision with a clear margin of 2 – 4 cm is considered the standard treatment for the resectable tumor. However, Mohs micrographic surgery is a better therapeutic choice as it has a low recurrence rate and is considered to be the gold standard modality wherever facilities are available.[1] Among the non-surgical options, imatinib mesylate is an alternative and radiotherapy is an adjuvant treatment option as DFSP is a radiosensitive tumor.[1] It has a good prognosis if negative margins are achieved after tumor resection. The 5 – 10-year recurrence-free survival rates are 86 – 76%, respectively, however, it has a high chance of local recurrence (20 – 50%) especially if positive margins are left and time from excision to local recurrence was reported to be 32 – 38 months.[9] Hence, long-term follow-up at an interval of 6 – 12 months is considered mandatory for the initial five years and then yearly.[7,9]
| Biomarker | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Anti HMB-45 | 72–100 | 91–100 |
| Melan-A | 83–100 | 81–98 |
| Tyrosinase | 90–100 | 97–100 |
| MITF | 100 | 87–100 |
| S 100 protein | 89–100 | 70–79 |
HMB: Human melanoma black, MITF: Melanocyte inducing transcription factor
CONCLUSION
DFSP can present as a pigmented tumor in a darker skin type which can be a diagnostic challenge. Hence, a detailed workup with biopsy and IHC study is a must to confirm this locally recurrent tumor. Dermoscopy serves as a valuable bedside tool for narrowing down the potential differentials.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Dermatofibrosarcoma protuberans: Case series in a tropical setting and review of literature. Rare Tumors. 2024;16:20363613241234243.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of tumors of soft tissue and bone (medicine) (5th ed). Geneva: World Health Organization; 2019. p. :254-62.
- [Google Scholar]
- Prevalence of dermatofibrosarcoma protuberans in Saudi Arabia over 24 years: A retrospective single-institution study. Saudi Med J. 2021;42:1362-5.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatofibrosarcoma protuberans In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024.
- [Google Scholar]
- Dermoscopy of dermatofibrosarcoma protuberans in skin of colour: A study of four cases. Indian J Dermatol Venereol Leprol 2023:1-3.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy of dermatofibrosarcoma protuberans: What do we know? Dermatol Pract Concept. 2019;9:139-45.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous nodules arising in a surgical scar: Role of dermoscopic examination in a patient with skin of color. Indian Dermatol Online J. 2022;13:789-91.
- [CrossRef] [PubMed] [Google Scholar]
- Tumors of fibrous tissue involving the skin In: Elder DE, ed. Lever's histopathology of the skin (11th ed). Netherlands: Wolter Kluwer; 2015. p. :2326.
- [CrossRef] [Google Scholar]
- Dermatofibrosarcoma protuberans: Update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic and prognostic biomarkers in melanoma. J Clin Aesthet Dermatol. 2014;7:13-24.
- [Google Scholar]






