Translate this page into:
Clinical presentation, diagnosis, differential diagnosis and management of contact allergy

*Corresponding author: Suzana Ljubojevic Hadzavdic,Department of Dermatology and Venereology, University Hospital Clinic Zagreb, Kispaticeva 12, Zagreb, 10000, Croatia. suzana.ljubojevic@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kuric I, Ljubojevic Hadzavdic S. Clinical presentation, diagnosis, differential diagnosis and management of contact allergy. CosmoDerma 2022;2:26.
Abstract
Contact allergy is an acquired immunological alteration caused by skin, or occasional mucosal or systemic, contact to low molecular weight substances. With skin involvement, this process manifests as contact dermatitis. Contact dermatitis includes both irritant contact dermatitis and allergic contact dermatitis. The approach to patients with contact dermatitis should consist of a detailed (work and leisure) history, skin examination, patch tests with allergens based on history, physical examination, education on materials that contain the allergen and adequate therapy and prevention. A classification based on the types of clinical presentation was therefore suggested by the International Contact Dermatitis Research Group which is presented in this article.
Keywords
Contact allergy
Contact allergic dermatitis
Management
Patch test
Treatment
INTRODUCTION
Contact allergy (CA) is an altered immune status of an individual in which a sensitizing substance causes the proliferation of allergen-specific T cells. Re-exposure activates the specific T cells leading to a clinically visible disease. With skin involvement, this process manifests as contact dermatitis (CD).[1] Contact dermatitis includes both irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD). ICD is the more common variant occurring by cumulative exposure to weak irritants. An inflammatory response is triggered by direct injury to the skin’s epidermis without the involvement of allergen-specific T cells.[2] ACD is a type IV delayed hypersensitivity reaction to a particular allergen to which a patient had developed sensitivity.[1] The golden standard to diagnose CA is patch test.[1]
EPIDEMIOLOGY
The prevalence of contact sensitization in the general population is 20.1%.[3] It is reported significantly higher in women 27.9%, than in men 13.2%.[3] In the pediatric and adolescent population, the prevalence is up to 16.5%.[3] Contact dermatitis is the third most common skin disease in the general European population behind common warts and acne.[4] The self-reported lifetime prevalence of CD is almost twice as high (15.0%) as the prevalence diagnosed by doctors (8.3%) which indicates that almost half of the affected patients don’t visit their physician for this condition.[4]
PATHOGENESIS
The pathogenesis of ACD consists of two phases: a sensitization phase followed by an elicitation phase. In the sensitization phase allergens activate innate immunity by releasing a host of cytokines (IL-1α, IL-1β, TNF-α, GM-CSF, IL-8, and IL-18) from epidermal keratinocytes.[5] These induce vasodilation, cellular recruitment and infiltration. Langerhans cells and dermal dendritic cells then encounter the allergen escorting it to draining lymph nodes and activating hapten-specific T cells, which include Th1, Th2, Th17, and regulatory T (Treg) cells. These T cells enter the circulation and the site of initial exposure enabling the elicitation phase. On re-encountering, the allergen the haptens, along with other inflammatory cells, induce an inflammatory cascade.[5] The elicitation of dermatitis in a sensitized person occurs within one to four days of re-exposure.[6]
CLINICAL PRESENTATION
The traditional presentation of ACD comes in three morphological patterns: (I) The acute phase is characterized by pruritic papules and vesicles on erythematous base accompanied by edema, oozing, crusting or tenderness [Figure 1]. (II) The subacute phase with more prominent crusts, scales and hyperpigmentation [Figure 2], with repeated exposure, eventually leading to (III) the chronic phase with a dry, scaly, thicker skin and lichenification [Figure 3].[7] With a vast majority of the causative agents, CA represents a challenge for practitioners in everyday settings. A classification based on the types of clinical presentation was therefore suggested by the International Contact Dermatitis Research Group which is presented in this paper.[8]
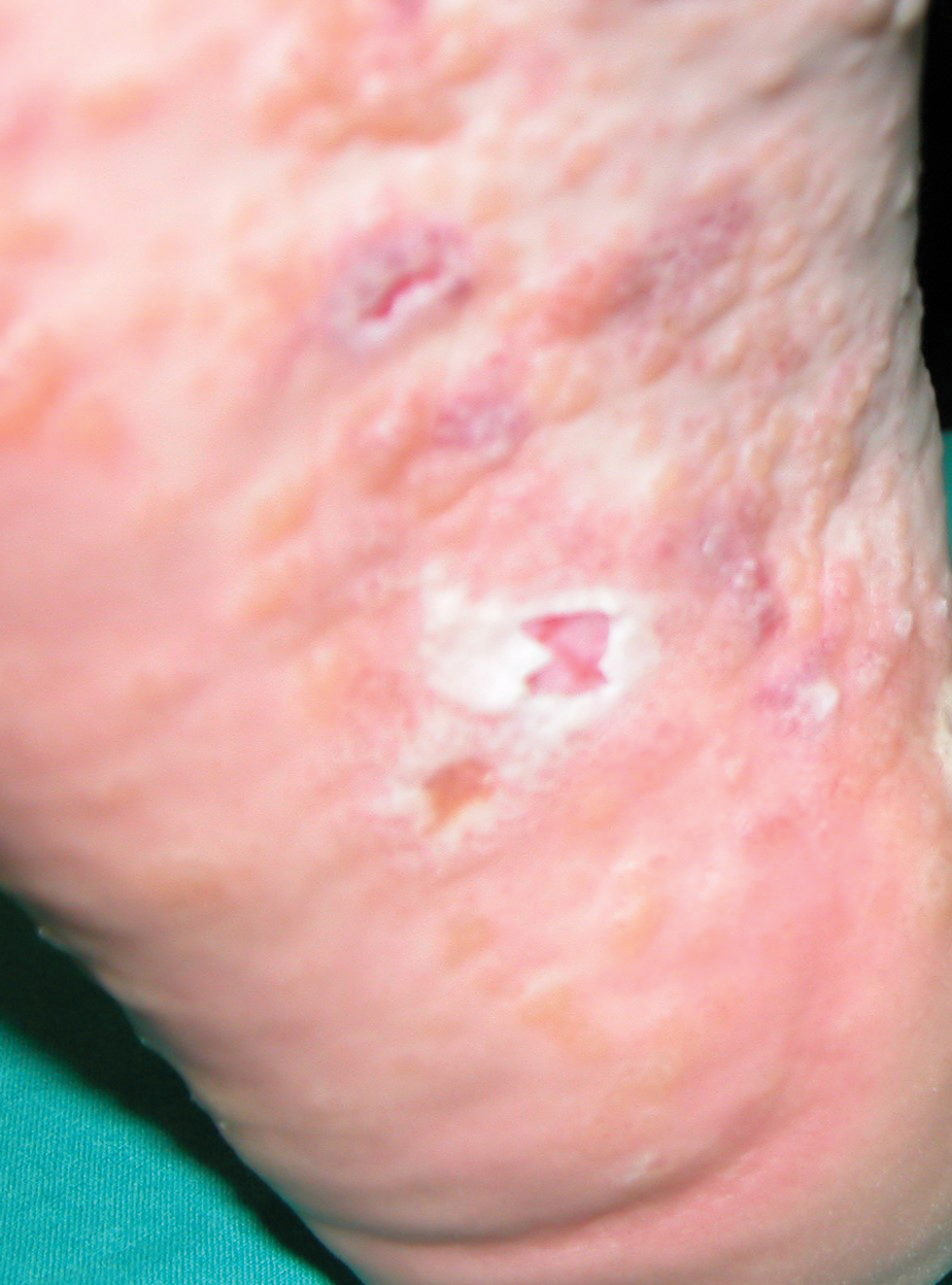
- Acute contact dermatitis.
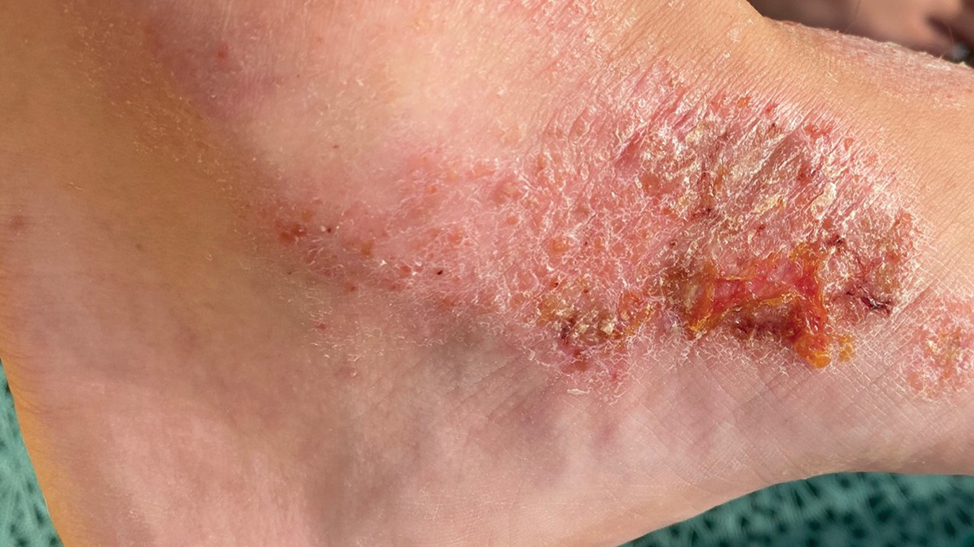
- Subacute contact dermatitis.
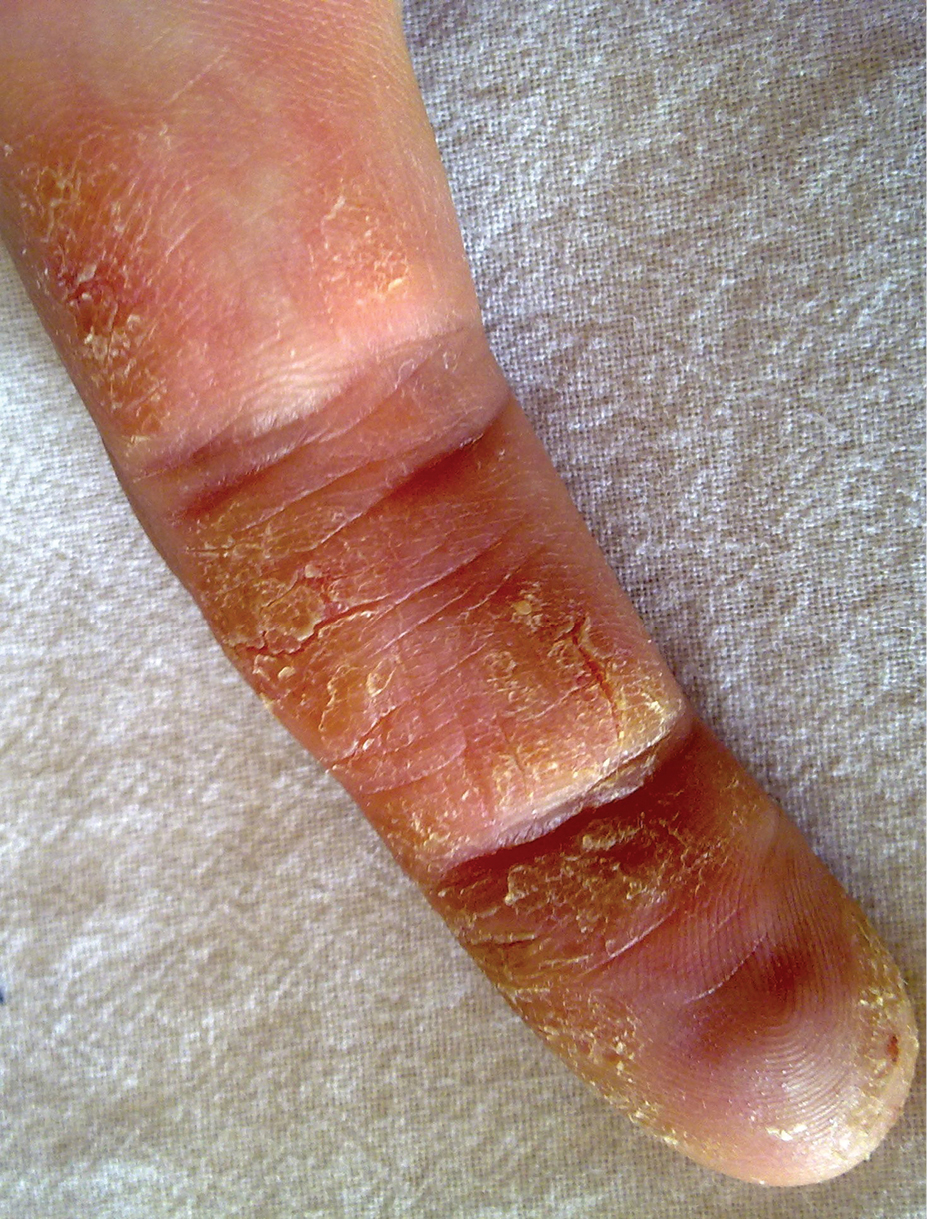
- Chronic contact dermatitis.
Contact allergic dermatitis
Contact allergic dermatitis can be established if a patient has a history of direct exposure to a causative allergen, accompanied by a clinically matching dermatitis and a relevant patch test reaction. The most common examples include: a nickel/cobalt ACD found in costume jewelry, (necklaces, bracelets and rings); ACD to fragrances on neck and wrists; glove dermatitis caused by rubber chemicals and chromate in leather; cheilitis induced by ACD to lanolin in lipsticks; ACD caused by various topical agents in cosmetics [Figure 4]; hair dye ACD, etc.[8]
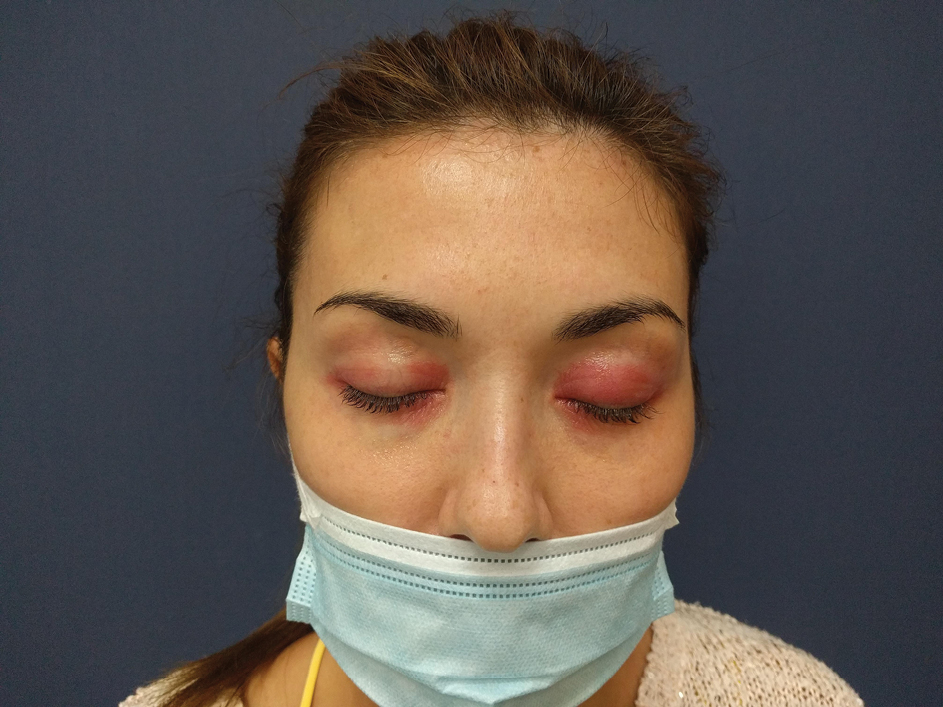
- Eyelid dermatitis caused by personal cosmetics.
ACD can mimic and/or exacerbate preexisting dermatoses like seborrheic dermatitis, atopic dermatitis, nummular dermatitis, etc.
Beside with direct contact, ACD can appear by proxy. Connubial dermatitis (synonyms: by proxy or consort dermatitis) appears in situations where allergen is brought on by another individual, either by direct contact, airborne or with contaminated items.[8] This type of dermatitis often comes in an atypical presentation with bizarre patterns.[9] The causative allergens most commonly include fragrances,[10] preservatives in cosmetics,[11] hair dyes,[12] plant products and in the genital area, due to medicated and spermicidal creams, diaphragm rubber and semen.[8]
Airborne allergic contact dermatitis
Airborne contact dermatitis (ACD) occurs when particles dispersed in the air in the form of dust, pollen, sprays or powders are deposited on the skin. Most often these particles cause an irritative reaction, while airborne allergic reactions are less common. Clinically it involves exposed areas of the skin such as the face, neck, hands and forearms and can resemble photodermatoses.[8] Plant-derived allergens from the Compositae family are the most common cause of airborne ACD due to sensitization to their essential oils (sesquiterpene lactones).[13] Other popular causes include fragrances including balsam of Peru, propolis and colopholny, epoxy resins, acrylates and methacrylates, rubber additives and accelerators, preservatives like methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI), metal dust (exp. nickel, cobalt and gold), and corticosteroids inhalers such as budesonide used in bronchodilators.[14]
Photoinduced contact dermatitis
An exogenous substance may cause photosensitivity by phototoxic or photoallergic mechanisms, or by inducing a dermatosis that is exacerbated by exposure to ultraviolet radiation. Photoinduced contact allergic dermatitis presents with sharply demarcated erythematous lesions sparing light-protected areas such as retroauricular and submandibular areas. The presentation can also be polymorphic and can range from erythema multiforme like lesions, lichenoid, urticarial, hyperpigmented, purpuric etc. Most often reported contact photoallergens include sunscreen components, nonsteroidal anti-inflammatory drugs (NSAID), pesticides, cadmium in tattoos, etc.[15]
Systemic contact dermatitis
Systemic contact dermatitis (SCD) is systemically reactivated allergic contact dermatitis in individuals with cutaneous sensitization to a certain allergen. A susceptible person who is exposed to that substance (allergen) via a systemic route (oral, inhalational, intramuscular, intravenous, etc.) reacts to that same (or a cross-reactive) allergen.[16] It is a type IV hypersensitivity reaction, however, some studies suggest a type III hypersensitivity due to the rapid appearance of cutaneous symptoms.[16] The appearance varies from skin lesions on the previously affected sites, the reappearance of the erythema on the previously positive patch test sites to widespread eczema, vasculitis-like lesions and erythroderma. The nomenclature and classification thus vary with suggested terms like ACD syndrome divided in four clinical stages along with Baboon syndrome or SDRIFE (symmetrical drug-related intertriginous and flexural exanthema).[16] SCD is commonly reported on medication, either from topical absorption (like corticosteroids, ampicillin, NSAIDs, acetylsalicylic acid, anesthetics) or systemic (thylenediamine, neomycin, nystatin, erythromycin, corticosteroids).[16] Food-induced SCD is another common variant due to nickel-rich food products, balsam of Peru, artificial sweetener aspartame, propolis and propylene glycol.[17]
Contact urticaria
Contact urticaria (CU) is characterized by wheals that occur 10 to 60 minutes within exposure to an external substance. There are two types of CU, immunologic and non-immunologic. Immunologic CU, is a type I hypersensitivity reaction caused either by high molecular weight proteins (like plant or animal-derived proteins), or smaller hapten chemicals. Symptoms vary from localized urticaria, to generalizes with systemic manifestations, gastrointestinal symptoms and anaphylaxis. The more common but less severe variant is a non-immunologic CU where histamine plays no role and there are no specific antigens against the causative agents.[18] It is caused by animals, food, fragrances, medications, metals, plants and preservatives.[19]
Protein contact dermatitis
Protein contact dermatitis (PCU) is an allergic skin reaction induced by proteins of either animal or plant origin. The clinical presentation is that of the CD characterized by eczematous, vesicular, dyshidrotic and urticarial lesions appearing minutes after contact with the causative substances.[20] PCU is often an occupational disease commonly found in food workers.[21,22]
Allergic contact stomatitis
Allergic contact stomatitis (ACS) is a T-cell mediated hypersensitivity reaction to an allergen in contact with the oral mucosa. Possible causative agents include chlorhexidine in mouthwashes, topical anesthetics and steroids, dental implants, metal orthodontic devices, chewing gum etc. It manifests clinically as erythematous plaques, vesiculation, erosions, ulcers or hyperkeratosis accompanied by pain, burning and itchiness.[23] ACS can also present as a lichenoid reaction mimicking oral lichen planus. This variant is usually brought by mucosal contact with amalgam restorations and is located on the posterior buccal mucosa and ventral or lateral edges of the tongue.[24]
DIAGNOSIS
The first step to diagnose ACD is to take good and detailed personal history along with getting occupational and recreational information. Details about patient’s workplace like exposure to potential allergens and irritants, usage of protective equipment as well as free time and leisure activities are mandatory in order to find the potential culprits. All suspected allergens should be patch tested. Patch test is a gold standard for diagnosis of ACD. In case of a positive patch test result and a positive correlation with the patient’s history of dermatitis, the diagnosis of ACD can be made.[25]
Patch test
Patch testing should be considered in individuals with acute recurrent dermatitis, chronic contact dermatitis, chronic dermatitis that isn’t improving with treatment and with eruptions on skin and mucous membranes.[1] There are over 4300 contact allergens known with only a few hundred of commercially available preparations for testing.[26] A positive patch test reaction may only indicate sensitivity, and it has to be correlated with patient history in order to find its relevancy.[27] A baseline series should always be used with additional selected allergens based on the patient’s history of exposure. The preferred patch test site is the upper back due to best reactivity but lateral sides of upper arms and thighs are also acceptable.[28] The skin on the tested site needs to be clear of any dermatitis and patients should be instructed to avoid excessive exercise and exposure to water during the test. Natural sun or other UV light exposure should be avoided several weeks before testing. Topical corticosteroids and calcineurin inhibitors should be avoided on the test site for at least 1 week before testing.[29] Immunosuppressants can suppress or minimize hapten response and they might show weaker test reactions.[30] Oral steroids should be discontinued if daily doses exceed 20 mg of prednisolone equivalent, while testing at doses 20 mg and lower can be considered while keeping in mind the possibility of false-negative reactions.[29] Azathioprine does not interfere with patch testing.[30] Cyclosporine may inhibit weaker reactions.[29] Tumor necrosis factor inhibitors, anti-IL17/22 and dupilumab should be tested right before the next dose.[31] Testing with mycophenolate mofetil can be attempted using the lowest possible dose.[31] Patch testing can be done with methotrexate though it is suggested to consider skipping one weekly dose.[31,32] Oral antihistamines do not affect patch test results and can be continued during the test.[33]
The test area is cleaned with ethanol or water and a standardized amount of allergen is applied to each test chamber and fixed with adhesive tape. The position of chambers are marked with a highlighter to ensure accurate reading. The test chambers need to be removed after 48h and the reactions are evaluated on day 2, day 3 or 4 and finally on day 7 (if possible).[27] The most positive reactions in the patch test are found on day 4.[34] Up to 13.5% of positive reactions would be missed if late readings are skipped.[35] The late reading is particularly recommended when testing corticosteroids, antibiotics (neomycin), formaldehydes, p-phenylenediamine and metals.[27]
The results are recorded as: (-) no reaction, (IR) irritant reaction consisting of varied morphology that’s well defined with sharp margins and no induration, (-/+) a doubtful reaction of minimal erythema, (+) a weak positive reaction with erythema, low induration and some papules, (++) a strong positive reaction with well-defined erythema, infiltration, papules and vesicles, (+++) extreme positive reaction with intense erythema, infiltration and coalescing vesicles or ulceration [Figure 5].[27] Once the patch test is completed the relevance of positive allergens should be established. Along with patch test results, patients should receive written handouts with explanations of where each particular allergen can be found.[36]
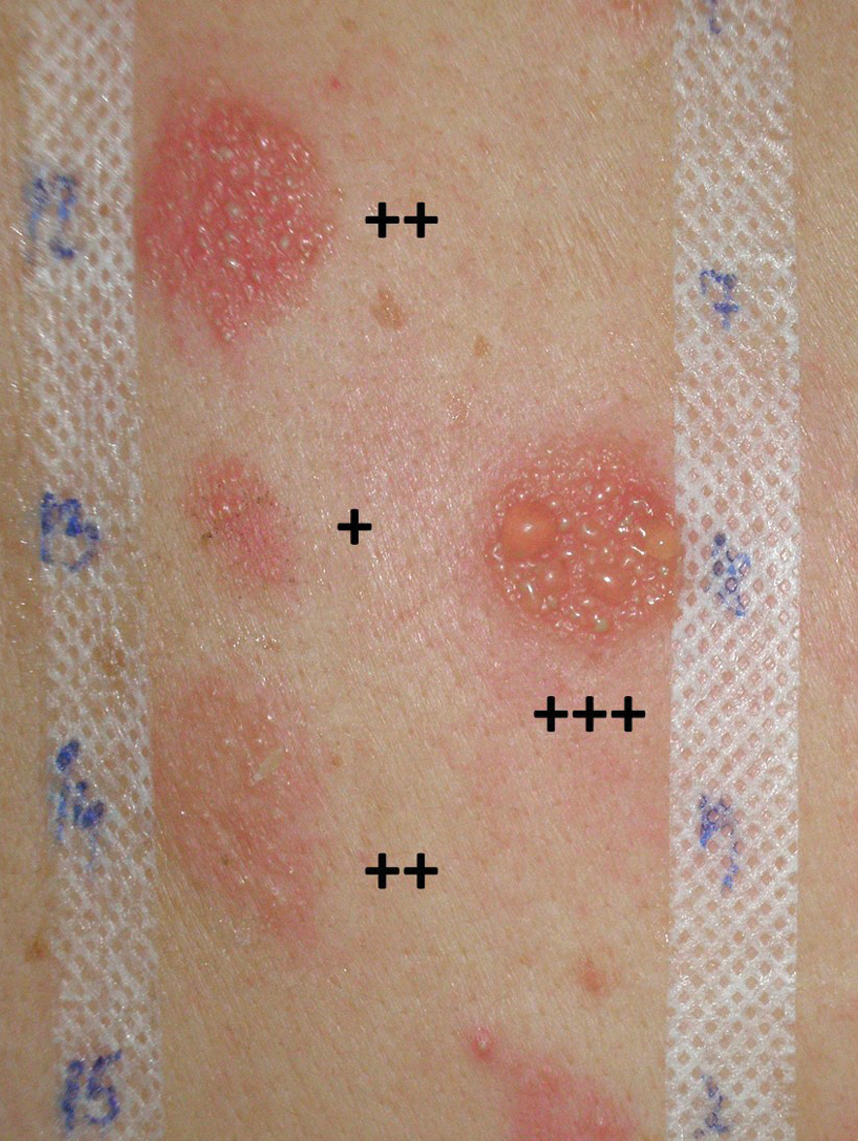
- Positive reactions (+; ++; +++) in patch testing.
Repeated open application test
Repeated open application test (ROAT) is a skin test used to confirm or rule out the presence of ACD. It is useful to identify a clinically relevant allergen.[37] It can be used to test a clinical relevance of an allergen identified as a positive in a patch test or to test a compound not available in commercially available patch tests.[38,39] The test evaluates one substance at a time and can be applied to both leave-on and rinse-off products. No occlusion is used in order to minimize irritation and false-positive findings. The products are usually applied twice a day on the antecubital fossa over 1 to 2 week period.[37]
Semi-open test
The semi-open test is helpful when testing a product with possible irritant properties (like shampoos, cosmetics, liquid soaps, nail varnish, glues, paints, inks etc.). A small amount of the material is applied with a cotton swab to a small area of the skin (1-2 cm2), left to dry and then covered with adhesive tape. The site is evaluated after 48 to 96 hours.[40,41]
Photopatch testing
The photo patch test is used to diagnose an allergen that requires UV exposure in order to induce a hypersensitivity reaction causing a photo contact allergic disease. The test is applied as a duplicate set on the patient’s back, and it is occluded for 2 days after which one set is exposed to 5 J/cm2 of UVA while the second set is completely covered from light.[1] Readings are performed before exposure, immediately after and a minimum of 2 days afterward [Figure 6]. A positive test on the exposed site with a negative on the covered site confirms the diagnosis of photo contact allergy.[1]
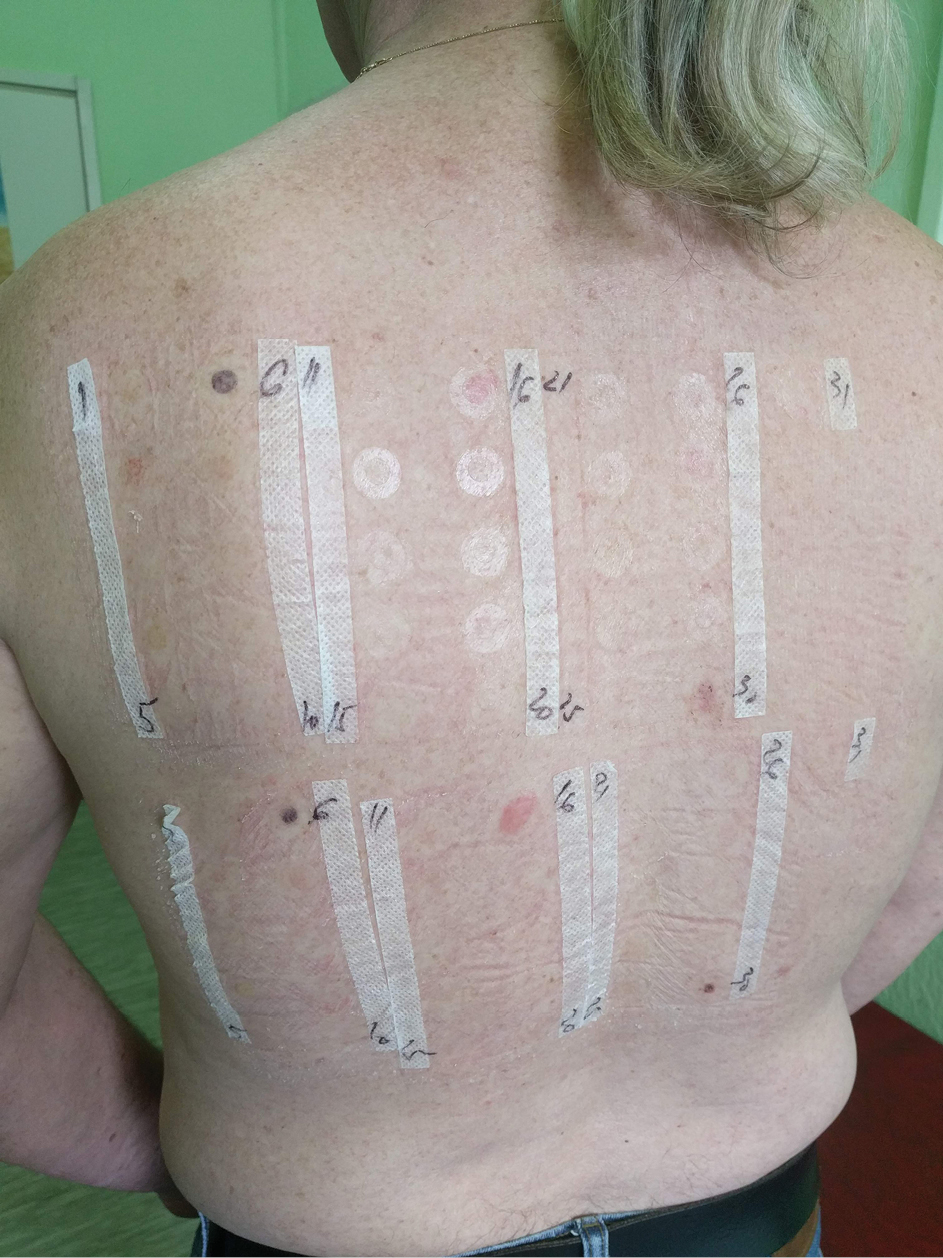
- Photopatch testing. The proximal set was exposed to UVA after 48 h while the distal set was covered with photoprotective material. Both sets showed a positive reaction (++) to thimerosal 0.1% at day 4 of testing (2 days after UVA exposition) suggesting only contact sensitivity.
Dimethylgloxime test
The Dimethylgloxime (DMG) test is a fast and simple solution in identifying nickel or cobalt release in metallic objects. A positive test indicates the presence of nickel or cobalt is concentration that are sufficient to provoke CD. The test shows modest sensitivity of around 60% and can be used for screening purposes.[42]
Skin biopsy
Diagnosis of ACD is suspected from patients’ history and clinical signs and it is confirmed by patch testing. In the early stages of ACD the lesional skin shows spongiosis in the lower epidermis.[43] At later stage shows spongiotic vesicles in various levels of the epidermis, frequently with eosinophilic exocytosis and infiltration of lymphocytes, Langerhans cells and macrophages around superficial vessels of the upper dermis.[43] A chronic skin sample shows less spongiosis and vesiculation, with more prominent epidermal hyperplasia, scale crust, papillary dermal fibrosis and hypergranulosis.[44] This histology of ACD is similar to other common inflammatory diseases like atopic dermatitis, nummular dermatitis, ICD, dyshidrotic dermatitis etc., that are presented with spongiosis.[45]
Potassium hydroxide preparation
Potassium hydroxide preparation and fungal culture is used to exclude tinea, especially when hands and feet are involved.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of an ACD includes a wide range of dermatoses. The ICD is in form of chronic dermatitis that shares a similar clinical presentation with ACD but can be differentiated by patch test.[46] ACD of the face can mimic periorificial dermatitis, rosacea or seborrheic dermatitis. Groin ACD can resemble inverse psoriasis or inverse lichen planus, while intertriginous area involvement can be confused with erythrasma, candida infection or the Hailey-Hailey disease. In patients with prominent photo distribution cutaneous lupus erythematosus, dermatomyositis or polymorphous light eruption should be excluded. Papular forms of ACD can resemble folliculitis or Grover’s disease. Other common skin infections like scabies or fungal infections should also be considered.[43]
MANAGEMENT
Education
The management of ACD includes patient education about causes of contact allergy, avoidance of potential triggers, prevention and adequate treatment according to the clinical picture. To prevent a recurrence, it is necessary for the patient to avoid further contact with the allergen. The treatment of ACD starts with the avoidance of offending allergen. Avoidance requires educating the patient on the nature of contact allergens and the presence of causative agents in everyday products. This information should be explained to the patient but also, they should be given written handouts with information on allergen contact.[47] There are online databases available which are cataloging products with known allergens that allow patients to access a list of allergens on a product by scanning its barcode, such as the Contact Allergen Management Program by the American Contact Dermatitis Society[48] or the SkinSafe app.[49] If the exposure cannot be avoided the patient needs to be instructed in the use of protective equipment like barrier creams, gloves or clothing.
Topical treatment
Along with avoidance, most patients will require some form of topical therapy in order to repair the damaged skin barrier. The first line of treatment are barrier creams and moisturizing creams, followed by local anti-inflammatory therapy.[50] Moisturizers use humectants (urea, sorbitol, glycerin etc.,) to bind water molecules hydrating the stratum corneum. Barrier creams form a protective layer on the skin preventing the penetration of harmful substances and the evaporation of water from the skin increasing its hydration. The use of barrier creams is often enough to treat milder cases of contact dermatitis.[50] The maintenance treatment are topical corticosteroids. In acute conditions medium or high potency corticosteroids can be used.[51] Use of moisturizers and skin barrier repair creams along with topical corticosteroids can significantly increase the disease-free time interval.[52] Topical immunomodulators are approved for ACD but can be considered an appropriate corticosteroid substitute on the skin sites such as face, genital or intertriginous regions.[53,54]
Phototherapy
Phototherapy can be of use in chronic hand eczema treatment with psoralen plus ultraviolet-A (PUVA). Phototherapy has limited use, because there is a need for frequent visits (for PUVA therapy 3 times per week, maximum of 3 months and for narrow band ultraviolet-B (nbUVB) 4-5 times per week up to 10 weeks).[55]
Systemic treatment
In widespread contact dermatitis, severe acute and severe acutisation of chronic CD short course of systemic corticosteroids (oral prednisone starting dose of 0.5-1 mg/kg per day tapered over a 2 week period) can provide faster regression.[56,57] Alitretinoin is a vitamin A derivative with affinity for retinoic acid and retinoid X receptors. It is the first EU approved systemic treatment for chronic hand eczema.[58] Prescribed once daily 10-30 mg for up to 24 weeks it showed complete or close to complete clearance of symptoms in 57% of patients that were previously unresponsive to topical steroids.[58] It is generally well tolerated with headaches and dyslipidemia as the most commonly reported side effects.[58] Acitretin is oral retinoid that can be off-label used in hyperkeratotic hand dermatitis.[59] Azathioprine is a purine analog that inhibits the proliferation of rapidly dividing cells as well as B and T cells. Its use in ACD treatment has been modestly documented with refractory chronic hand eczema and widespread recalcitrant dermatitis as preferable therapy candidates.[60,61] A delayed onset of response at 8 to 12 weeks is expected with a relatively favorable safety profile.[57] Cyclosporine is a calcineurin inhibitor that inhibits CD8+ activity. Off-label use for severe ACD cases has been reported with mixed results that included a lack of efficacy and exacerbations during the treatment and successful treatment in atopic dermatitis patients with hand eczema.[57] Methotrexate is a folate acid antagonist with anti-proliferating, immunosuppressive and anti-inflammatory effects that haven’t seen much use in the treatment of CD.[57] One study reported a partial or complete response on 78% of treated patients, with 23% achieving complete clearance.[62] This is comparable to other systemic drugs like cyclosporine and azathioprine.[62] Apremilast is an oral phosphodiesterase 4 inhibitor with a theoretically favorable anti-inflammatory property with regards to ACD.[57,63] The reports on TNF-α antagonists are mostly limited to case reports. Infliximab showed a good response in a patient with both psoriasis and ACD to multiple allergens.[64] Etanercept showed a modest reduction in the acute phase of CD with no effect during the chronic phase.[57] Omalizumab was used successfully in a patient with protein contact dermatitis to wheat.[65] Secukinumab and ustekinumab showed no improvement of ACD.[65] Dupilumab is an anti-IL-4 receptor drug that decreases serum levels of IL4 and IL13. There’s evidence in favor of its use for ACD with also good results reported for several recalcitrant ACD that previously failed years of other systemic therapies.[66,67] The antigens that provoke a Th2 response such as nickel, balsam of Peru, textile dyes and colophony show a particularly good response to dupilumab.[57,65]
CONCLUSION
Allergic contact dermatitis is a common dermatological disease caused by repeated exposure to an exogenous substance. Numerous variations in clinical presentation, as well as an ongoing number of causative substances, can make diagnosing a challenge. Patch testing remains the gold standard enabling the physician to identify specific allergens and work towards educating the patient in their avoidance. Topical therapy with emollients and corticosteroids remains the core treatment with several systemic drugs available for refractory cases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73:195-221.
- [CrossRef] [PubMed] [Google Scholar]
- Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of contact allergy in the general population: A systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of skin disease in a population-based sample of adults from five European countries. Br J Dermatol. 2018;178:1111-18.
- [CrossRef] [PubMed] [Google Scholar]
- The unique molecular signatures of contact dermatitis and implications for treatment. Clin Rev Allergy Immunol. 2019;56:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of irritant and allergic contact dermatitis. Contact Dermatitis 2010:43-90.
- [CrossRef] [Google Scholar]
- Proposed ICDRG classification of the clinical presentation of contact allergy. Dermatitis. 2016;27:248-58.
- [CrossRef] [PubMed] [Google Scholar]
- Consort contact dermatitis to paraphenylenediamine, with an unusual clinical presentation of tumid plaques. Contact Dermatitis. 2007;56:366-7.
- [CrossRef] [PubMed] [Google Scholar]
- Connubial allergic contact dermatitis caused by fragrance ingredients. Dermatitis. 2012;23:e1-2.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic contact dermatitis from cosmetics applied by the patient’s girlfriend. Contact Dermatitis. 2004;50:252-3.
- [CrossRef] [PubMed] [Google Scholar]
- Contact dermatitis to partner’s hair dye. Clin Exp Dermatol. 1976;1:283-4.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological data on airborne contact dermatitis—results of the IVDK. Contact Dermatitis. 2015;73:239-47.
- [CrossRef] [PubMed] [Google Scholar]
- Airborne allergic contact dermatitis: Management and responsible allergens on the American Contact Dermatitis Society Core Series. Dermatitis. 2019;30:106-15.
- [CrossRef] [PubMed] [Google Scholar]
- Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56-65.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic contact dermatitis. Clin Rev Allergy Immunol. 2019;56:9-18.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic contact dermatitis: A review. Dermatol Clin. 2020;38(3):379-88.
- [CrossRef] [PubMed] [Google Scholar]
- Contact urticaria: Present scenario. Indian J Dermatol. 2009;54:264-68.
- [CrossRef] [PubMed] [Google Scholar]
- Available at: Accessed: January 10th, 2022
- [Google Scholar]
- Protein contact dermatitis: Allergens, pathogenesis, and management. Dermatitis. 2008;19:241-51.
- [PubMed] [Google Scholar]
- Occupational protein contact dermatitis. Eur J Dermatol. 2015;25:527-34.
- [CrossRef] [PubMed] [Google Scholar]
- Protein contact dermatitis—case report. An Bras Dermatol. 2013;88:611-3.
- [CrossRef] [PubMed] [Google Scholar]
- Review: Allergic contact stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:559-65.
- [CrossRef] [PubMed] [Google Scholar]
- Oral lichen planus and oral lichenoid lesions; a critical appraisal with emphasis on the diagnostic aspects. Med Oral Patol Oral Cir Bucal. 2009;14:E310-4.
- [PubMed] [Google Scholar]
- A review of the impact of patch testing on quality of life in allergic contact dermatitis. J Am Acad Dermatol. 2017;76:1000-4.
- [CrossRef] [PubMed] [Google Scholar]
- Patch Testing: Test Concentrations and Vehicles for 4350 Chemicals Wapserveen, The Netherlands: deGroot; 2008.
- [Google Scholar]
- S3 guidelines: Epicutaneous patch testing with contact allergens and drugs - short version, Part 1. J Dtsch Dermatol Ges. 2019;17:1076-93.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity of various skin sites in the repeated open application test. Dermatitis. 1991;2:102-4.
- [Google Scholar]
- S3 guidelines: Epicutaneous patch testing with contact allergens and drugs - short version, Part 2. J Dtsch Dermatol Ges. 2019;17:1187-207.
- [CrossRef] [PubMed] [Google Scholar]
- Azathioprine does not influence patch test reactivity in parthenium dermatitis. Contact Dermatitis. 2016;74:64-5.
- [CrossRef] [PubMed] [Google Scholar]
- Patch test clinic start-up: From basics to pearls. Dermatitis. 2020;31:287-96.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate does not impede the development of contact allergy. Contact Dermatitis. 2018;78:223-24.
- [CrossRef] [PubMed] [Google Scholar]
- Patch testing 101, part 1: Performing the test. Cutis. 2020;106:165-67.
- [CrossRef] [PubMed] [Google Scholar]
- Day 4 is better than day 3 for a single patch test reading. Contact Dermatitis. 1996;34:402-4.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of a second patch test reading of TRUE tests(R) on D6/7. Contact Dermatitis. 2013;68:94-7.
- [CrossRef] [PubMed] [Google Scholar]
- Patch testing 101, part 2: After the patch test. Cutis. 2020;106:292-96.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical utilization of repeated open application test among American Contact Dermatitis Society members. Dermatitis. 2015;26:224-9.
- [CrossRef] [PubMed] [Google Scholar]
- Repeated open application test with methylisothiazolinone in individuals sensitive to methylchloroisothiazolinone/methylisothiazolinone. Contact Dermatitis. 2014;70:244-6.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic contact dermatitis caused by hydroperoxides of limonene and dose-response relationship—A repeated open application test (ROAT) study. Contact Dermatitis. 2019;80:208-16.
- [CrossRef] [PubMed] [Google Scholar]
- Patch testing without a kit In: In: Guin JD, ed. Practical contact dermatitis. New YorkNY:: McGraw-Hill; 1995. p. :63-74.
- [Google Scholar]
- Alternatives aux patch-tests. Ann Dermatol Venereol. 2009;136:623-5.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity and specificity of the nickel spot (dimethylglyoxime) test. Contact Dermatitis. 2010;62:279-88.
- [CrossRef] [PubMed] [Google Scholar]
- Another great imitator: Allergic contact dermatitis differential diagnosis, clues to diagnosis, histopathology, and treatment. Curr Treat Options Allergy. 2015;2:333-48.
- [CrossRef] [Google Scholar]
- Eosinophilic spongiosis: A clinical, histologic, and immunopathologic study. J Am Acad Dermatol. 1994;30:973-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative histopathology of allergic and irritant patch test reactions in man. Current concepts and new prospects. Arch Belg Dermatol Syphiligr. 1973;28:83-92.
- [PubMed] [Google Scholar]
- Allergic and irritant contact dermatitis. Eur J Dermatol. 2009;19:325-32.
- [CrossRef] [PubMed] [Google Scholar]
- Contact dermatitis, patch testing, and allergen avoidance. Mo Med. 2015;112:296-300.
- [PubMed] [Google Scholar]
- Contact Allergen Management Program. 2022. Available at: contactderm.org/resources/acds-camp—Accessed January 15, 2022.
- SkinSAFE database. 2022. Available at: http://allergyfreeskin.com/faq.htm Accessed; January 15, 2022.
- Best practices, new perspectives and the perfect emollient: Optimizing the management of contact dermatitis. J Dermatolog Treat 2018::241-51.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-55.
- [PubMed] [Google Scholar]
- Treatment with a barrier-strengthening moisturizer prevents relapse of hand-eczema. An open, randomized, prospective, parallel group study. Acta Derm Venereol. 2010;90:602-6.
- [CrossRef] [PubMed] [Google Scholar]
- Contact dermatitis and patch testing for the allergist. Ann Allergy Asthma Immunol. 2018;120:592-98.
- [CrossRef] [PubMed] [Google Scholar]
- Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol. 2009;123:1124-33.
- [CrossRef] [PubMed] [Google Scholar]
- Psoralen plus ultraviolet A (PUVA) soaks and UVB TL01 treatment for chronic hand dermatoses. Dermatol Reports. 2012;4:e3.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic treatments for allergic contact dermatitis. Dermatitis. 2019;30:46-53.
- [CrossRef] [PubMed] [Google Scholar]
- Alitretinoin: A review in severe chronic hand eczema. Drugs. 2016;76:1271-79.
- [CrossRef] [PubMed] [Google Scholar]
- Acitretin as a therapeutic option for chronic hand eczema. Ann Dermatol. 2017;29:385-87.
- [CrossRef] [PubMed] [Google Scholar]
- Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol. 2006;72:24-7.
- [CrossRef] [PubMed] [Google Scholar]
- Azathioprine treatment and drug survival in patients with chronic hand eczema—results from daily practice. Contact Dermatitis. 2017;76:304-7.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate use in allergic contact dermatitis: A retrospective study. Contact Dermatitis. 2018;78:194-8.
- [CrossRef] [PubMed] [Google Scholar]
- A phase 2, open-label, investigator- initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-6.
- [PubMed] [Google Scholar]
- Infliximab in recalcitrant severe atopic eczema associated with contact allergy. Int J Immunopathol Pharmacol. 2006;19:237-40.
- [PubMed] [Google Scholar]
- Review of biologics in allergic contact dermatitis. Contact Dermatitis. 2020;83:179-81.
- [CrossRef] [PubMed] [Google Scholar]
- Dupilumab use in allergic contact dermatitis. J Am Acad Dermatol. 2019;80:280-1.
- [CrossRef] [PubMed] [Google Scholar]
- A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31:e12701.
- [CrossRef] [PubMed] [Google Scholar]






