Translate this page into:
Clinical evaluation of a topical ceramide lotion on skin hydration and skin barrier in healthy volunteers with dry skin

*Corresponding author: Dr. Biswajit Aich, Department of Medical Affairs, Dr Reddy’s Laboratories Ltd., Hyderabad, Telangana, India. biswajitaich@drreddys.com
-
Received: ,
Accepted: ,
How to cite this article: Aich B, Kumbhar P, Muchhala S, Sanghavi A, Katare S, Kotak B. Clinical evaluation of a topical ceramide lotion on skin hydration and skin barrier in healthy volunteers with dry skin. CosmoDerma. 2024;4(148). doi: 10.25259/CSDM_175_2024
Abstract
Objectives:
Ceramides are epidermal lipids important for normal skin barrier function. Reduced ceramide content is associated with dry skin and atopic dermatitis (AD) due to increased transepidermal water loss (TEWL) and worsening symptoms. Topical ceramide application restores barrier function and improves hydration by reducing TEWL. The objective of the present study was to evaluate the effect of a test product on skin hydration and TEWL on the volar forearms of adults, by comparing measurements at 12 and 24 h post-application.
Materials and Methods:
This single-center and non-randomized study evaluated the effect of Venusia CeraPlus lotion on volar forearms in volunteers with dry skin. Primary outcomes included MoistureMeterSC reading for hydration and VapoMeter reading for TEWL at 12- and 24-h post-application, comparing occluded and unoccluded sites.
Results:
The study enrolled 32 subjects with a mean age of 34.03 ± 9.41 years, out of which 87.5% were female. Application of Venusia CeraPlus lotion enhanced skin hydration compared to the control under both occluded and unoccluded conditions. Hydration increased from 13.57 ± 2.67 at baseline to 31.61 ± 11.34 at 12 h and 36.36 ± 10.77 at 24 h under occlusion (P < 0.001). TEWL was lower at the test site, with VapoMeter readings of 3.08 ± 2.32 at 24 h compared to 4.54 ± 2.76 at the control site, suggesting that the test product effectively preserved skin hydration and reduced TEWL compared to the control. No adverse reactions were reported.
Conclusion:
This study demonstrates that Venusia CeraPlus Lotion effectively maintains optimal skin hydration for up to 24 h and enhances barrier function, making it a promising option for treating dry skin and improving overall skin health in adults.
Keywords
Ceramide
Dry skin
Hydration
Skin barrier
INTRODUCTION
Dry skin, or xerosis, is a common dermatological condition, impacting a substantial segment of the adult population. Prevalence rates can vary significantly based on environmental and demographic factors, with estimates ranging from 41.2% to 99.1% with a higher incidence observed in older adults.[1] Dry skin arises due to a compromised skin barrier and is marked by symptoms such as scaling, rough texture, and sometimes inflammation.[2,3] This condition is triggered by both external factors (e.g., environmental conditions and lifestyle) as well as endogenous factors (e.g., sebum, sweat, and hormones), age as well as immunologic factors.[4]
The epidermis, as the outermost layer of the skin, is essential in regulating permeability and protecting underlying tissues. A key component of this barrier is the stratum corneum (SC), whose lipids create a hydrophobic layer that works in conjunction with tight junctions and desmosomes beneath it which help to maintain skin hydration by minimizing water loss and protecting against external irritants.[5] The SC is generally composed of 50% ceramides, 25% cholesterol, and 15% fatty acids. As a consequence, the depletion of ceramides in the epidermis leads to significant disruption of the skin’s barrier function, leading to a significant increase in transepidermal water loss (TEWL).[6] Consequently, this results in reduced skin hydration, compromised barrier function, and the manifestation of dry skin.[1,2,6,7]
Topical lipid supplementation, especially with ceramides, is essential for strengthening the skin barrier and maintaining hydration across age groups.[7] When applied topically in sufficient concentrations, ceramides can enhance both the permeability barrier and the skin’s ability to retain moisture. Topical lipids not only create an occlusive layer on the surface of the SC but also contribute to forming lamellar structures in the intercellular spaces.[8] Contrary to popular belief, moisturizers do not add water but prevent evaporation, enhancing hydration by reducing TEWL through occlusion.
To combat dry skin, topical treatments often focus on restoring the skin’s barrier and replenishing moisture. Among the most effective ingredients in combating dry skin are ceramides[9] and hyaluronate.[10] Ceramides, a type of lipid, are naturally found in high concentrations within the cell membranes of the SC and are essential for maintaining the skin’s barrier function and hydration.[9] Hyaluronate, or hyaluronic acid (HA), a key component of the extracellular matrix, plays a crucial role in tissue hydration and water transport due to its high water-binding capacity, holding up to 1000 times its weight in water molecules.[11]
Despite the abundance of moisturizers on the market, there is a pressing need for effective skincare products that provide reliable and sustained hydration, especially for individuals with dry skin. The need for this study arises from the growing consumer demand for scientifically validated products that demonstrate proven efficacy. It aims to address this gap by providing empirical evidence of the effectiveness of Venusia CeraPlus Lotion in enhancing skin hydration and reducing TEWL. By doing so, the study ensures that the product meets the needs and expectations of consumers seeking superior skin hydration.
MATERIALS AND METHODS
Study design
A monocentric, non-randomized, and site-control comparative study was conducted at C.L.A.I.M.S. Pvt. Ltd. in Mumbai, India. The study documents were reviewed and approved by the Independent Ethics Committee of C.L.A.I.M.S. Pvt. Ltd. (Protocol No.: CL/170/0124/STU, February 20, 2024), and the study was overseen by a qualified investigator. The research was conducted in accordance with the Protocol, the Declaration of Helsinki, Good Clinical Practice guidelines, and the Indian Council of Medical Research guidelines for medical research in humans. The study was registered with the Clinical Trials Registry of India (CTRI) on March 26, 2024 (Registration No.: CTRI/2024/03/064776). Before screening, the Principal Investigator or Co-Investigator explained the study, including potential risks and benefits, to the participants. All participant queries were addressed, and informed consent was obtained from those willing to participate.
Participants
The study involved male and female volunteers aged 18–55 with dry skin (MoistureMeter SC Readings <20) and healthy skin at the test sites (free from scars, moles, papules, etc.). Females who were menopausal or pregnant (confirmed by urine pregnancy test), lactating mothers, individuals allergic to any cosmetic product, and participants deemed non-compliant with study requirements by the investigator were not included. In addition, those on any medical treatment, either systemic or topical, that could interfere with the study treatment, as well as those with chronic illnesses affecting skin sensitivity (such as atopic dermatitis [AD], psoriasis, eczema, hypothyroidism, anemia, hormonal problems, or those on anti-cholesterol drugs), were also not part of the study. Subjects in an exclusion period or already participating in another similar cosmetic or therapeutic trial were also excluded from the study.
Test product
The test product used was Venusia CeraPlus lotion (Dr. Reddy’s Laboratories Ltd., Hyderabad, India) a cosmetic product containing essential ceramides, HA, light liquid paraffin, glycerin, Butyrospermum parkii (shea) butter, cetyl alcohol, glyceryl monostearate, cetomacrogol 1000, dimethicone, phenoxyethanol, polyacrylate-13, Avena sativa (oat) kernel oil and bran extract, polyisobutylene, ethylhexylglycerin, disodium ethylenediaminetetraacetic acid (EDTA), polysorbate 20, sorbitan isostearate, sodium hyaluronate, xylitylglucoside, anhydroxylitol, xylitol, ceramide complex, glucose, and purified water.
Application procedure
Approximately 0.03 g of the product was applied to the test sites and massaged into the skin for 30s [Figure 1]. To simulate different real-world scenarios, certain sites were occluded (sites 1, 2, 3, and 4) while others were not (sites 5 and 6). Occlusion enhances moisturizer effectiveness by reducing water loss and creating a more humid environment, which aids in better absorption and retention of hydration. In contrast, unoccluded sites represent typical daily use where the skin is exposed to the environment, testing the moisturizer’s ability to hydrate and protect without additional barriers. This dual approach provides a comprehensive understanding of the moisturizer’s performance in both enhanced and everyday use scenarios, demonstrating its overall efficacy.
- Application procedure of Venusia CeraPlus cream.
Measurement of skin hydration
Skin hydration of the SC was measured using the MoistureMeterSC (MMSC), which is a portable instrument used to detect moisture content in skin. A higher MMSC reading indicates a greater moisture content [Table 1]. TEWL was measured using the VapoMeter, which contains sensors for relative humidity and temperature. Measurements were taken at 3 time points, 0 h or baseline (before product application), 12 h, and 24 h after product application.
| Skin type | MMSC Range |
|---|---|
| Dry skin | <20 |
| Normal skin | 20–40 |
| Well hydrated skin | >40 |
MMSC: MoistureMeterSC
Baseline MMSC and VapoMeter measurements were obtained from all six test sites before product application. At 12 h, the occlusion was removed from one test site and one control site (Sites 1 and 2), and measurements were taken for these sites as well as for the sites that remained unoccluded (Sites 5 and 6). Similarly, at 24 h, the occlusion was removed from another test site and another control site (Sites 3 and 4), and measurements were again taken for these sites along with the unoccluded sites (Sites 5 and 6).
Statistical analysis
Statistical analysis was conducted using the Statistical Package for the Social Sciences version 10.0. Continuous variables were summarized by treatment groups using descriptive statistics, including the number of observations, mean, standard deviation, or median with range (minimum and maximum). To assess the significance of the test product results compared to the control site, the mean differences of continuous variables, such as MoistureMeter SC and VapoMeter readings, were evaluated using Student’s t-test and repeated measures analysis of variance, with post hoc analysis performed using the Bonferroni method. A significant difference between test and control sites at specific time points indicates that the test product was effective in significantly hydrating the skin at those particular time points.
RESULTS
Baseline characteristics
The study enrolled 32 subjects with a mean age of 34.03 ± 9.41 years, out of which 87.5% were female. The baseline characteristics, including age, gender, and clinical parameters, were compared across the study groups, as summarized in Table 2.
| Parameters | Test | Control | P-value |
|---|---|---|---|
| Age–(mean±SD) | 34.03±09.41 | ||
| Gender–n(%) | |||
| Male | 04 (12.5) | ||
| Female | 28 (87.5) | ||
| Baseline MMSC at occluded site (mean±SD) | |||
| Site 1,2 | 13.57±02.67 | 13.30±02.35 | P=0.669 |
| Site 3,4 | 13.85±02.81 | 13.67±02.55 | P=0.789 |
| Baseline MMSC at unoccluded site (mean±SD) | 13.22±2.85 | 12.9±2.64 | 0.642 |
MMSC: MoistureMeterSC, SD: Standard deviation
Changes in skin hydration
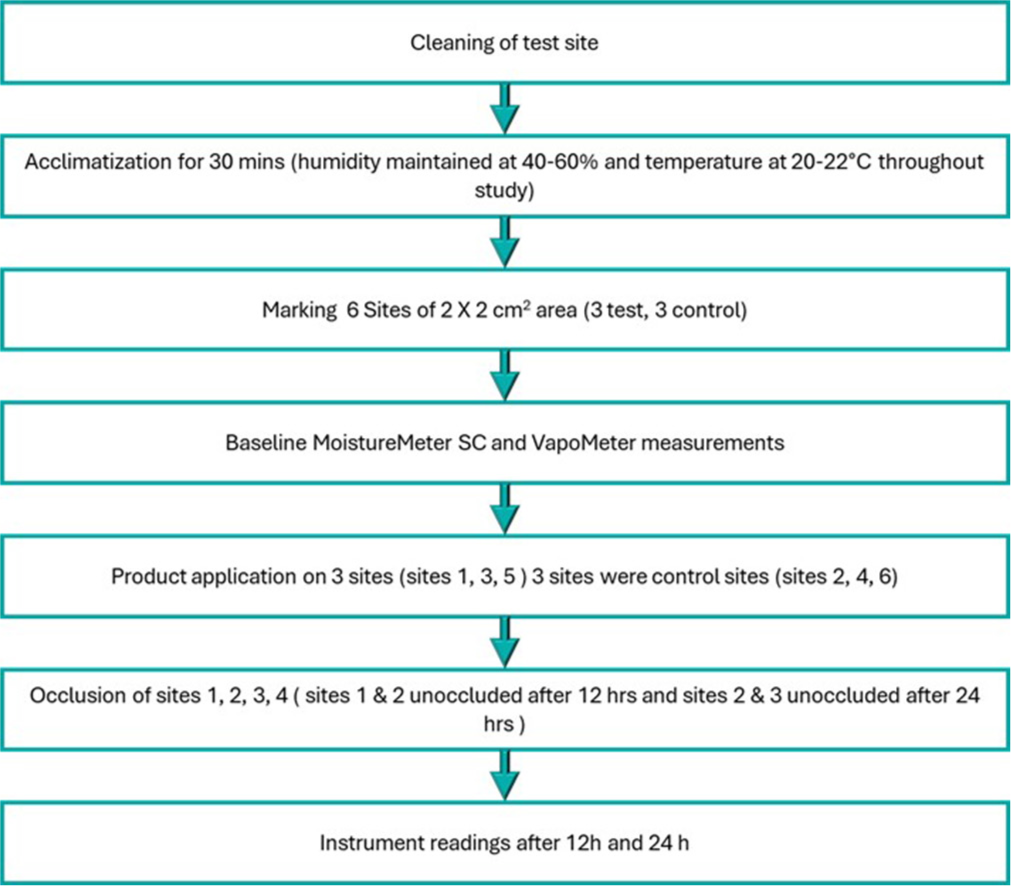
This study evaluated the mean skin hydration levels for both test and control groups at baseline, 12 h, and 24 h under occluded and unoccluded conditions. In the occluded condition, baseline hydration levels were similar for the test (13.57 ± 2.67) and control groups (13.30 ± 2.35). However, at 12 h, the test group showed a marked increase in hydration (31.61 ± 11.34) compared to the control group (16.06 ± 4.36), with a change from baseline of 18.04 ± 10.28 (P = 0.001) in the test group versus 2.76 ± 4.13 (P = 0.001) in the control group (P = 0.001 between groups). At 24 h, the test group continued to exhibit superior hydration (36.36 ± 10.77) compared to the control (17.67 ± 5.66), with changes from baseline of 22.51 ± 10.38 (P =0.001) and 4.00 ± 5.34 (P = 0.001), respectively (P = 0.001 between groups) [Figure 2].
- Changes in Mean MoistureMeterSC (MMSC) Readings (Skin Hydration) from baseline at 12 h and 24 h for test and control groups. (a) Change in the mean MMSC reading at occluded sites. (b) Change in the mean MMSC reading at unoccluded sites. The MMSC readings at each time point were significantly higher in the test group compared to the control (P < 0.001), indicating a marked improvement in skin hydration with the test product.
In the unoccluded condition, baseline hydration levels were also comparable between the test and control groups. At 12 h, the test group experienced a modest but significant increase in hydration (16.04 ± 3.97) compared to the control (13.81 ± 2.62), with a change from baseline of 2.82 ± 2.69 (P = 0.001) versus 0.91 ± 2.02 (P = 0.015) (P = 0.0023 between groups). At 24 h, the test group showed a slight increase in hydration (15.8 ± 2.84) compared to the control (14.39 ± 2.59), with changes from baseline of 2.58 ± 2.78 (P = 0.001) and 1.49 ± 1.92 (P = 0.001), respectively (P = 0.075 between groups) [Figure 2]. These results are summarized in Table 3.
| Skin site | Duration (Hours) | Mean skin hydration (mean±SD) | P-value (test vs. control) | |
|---|---|---|---|---|
| Test (n=32) | Control (n=32) | |||
| Occluded | Baseline | 13.57±02.67 | 13.30±02.35 | |
| At 12 h | 31.61±11.34 | 16.06±04.36 | ||
| Change from baseline to 12 h | 18.04±10.28 (P<0.001) | 2.76±4.13 (P<0.001) | P<0.001* | |
| Baseline | 13.85±02.81 | 13.67±02.55 | ||
| At 24 h | 36.36±10.77 | 17.67±05.66 | ||
| Change from baseline to 24 h | 22.51±10.38 (P<0.001) | 04.00±05.34 (P<0.001) | P<0.001* | |
| Unoccluded | Baseline | 13.22±2.85 | 12.9±2.64 | |
| At 12 h | 16.04±3.97 | 13.81±2.62 | ||
| Change from baseline to 12 h | 2.82±2.69 (P<0.001) | 0.91±2.02 (P<0.015) | P=0.0023* | |
| At 24 h | 15.8±2.84 | 14.39±2.59 | ||
| Change from baseline to 24 h | 2.58±2.78 (P<0.001) | 1.49±1.92 (P<0.001) | P=0.075 | |
Measurement of TEWL
For the occluded sites, VapoMeter readings showed a significant increase from baseline at both the test and control sites. At 12 h, there was no significant difference in VapoMeter readings between the test (2.70 ± 1.97) and control sites (2.94 ± 2.01) (P = 0.631). However, at 24 h, the control site exhibited a significantly higher increase in VapoMeter readings (4.54 ± 2.76) compared to the test site (3.08 ± 2.32), indicating greater TEWL at the control site [Figure 3]. This suggests that the test product effectively preserved skin hydration and reduced TEWL compared to the control. The detailed changes in VapoMeter readings are summarized in Table 4.
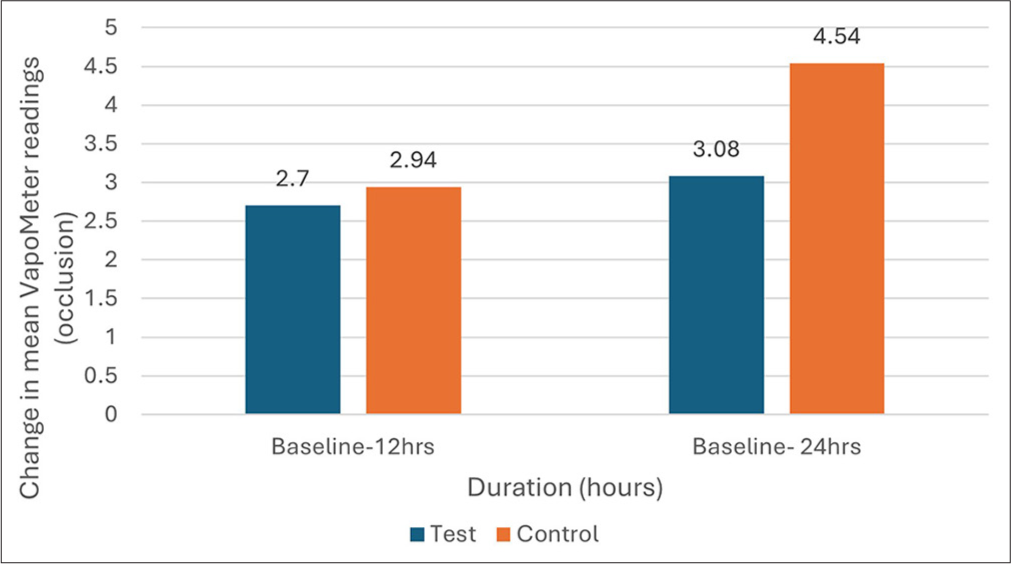
- Changes in mean VapoMeter readings (trans-epidermal water loss) at occluded sites from baseline to 12 h and 24 h, showing a reduction in moisture loss at the test site compared to the control site.
| Skin site | Duration (Hours) | Mean skin hydration (mean±SD) | P-value | |
|---|---|---|---|---|
| Test (n=32) | Control (n=32) | |||
| Site 1 and Site 2 (occluded) | Baseline | 08.03±1.68 | 9.24±2.14 | *0.014 |
| 12 | 10.72±2.17 | 12.17±2.34 | – | |
| Change from Baseline–12 h | 2.70±1.97 (P<0.001)* | 2.94±2.01 (P<0.001)* | ||
| Site 3 and Site 4 (occluded) | Baseline | 08.58±1.71 | 08.88±1.96 | 0.516 |
| 24 | 11.67±2.63 | 13.42±2.69 | – | |
| Change from Baseline–24 h | 3.08±2.32 (P<0.001)* | 4.54±2.76 (P<0.001)* | ||
For the unoccluded sites, VapoMeter readings remained stable and consistent with baseline measurements at both the test and control sites across all time points, as detailed in Table 5.
| Duration (Hours) | Mean TEWL (mean±SD) | P-value | |
|---|---|---|---|
| Test (n=32) | Control (n=32) | ||
| Baseline | 8.52±1.57 | 9.12±1.85 | 0.167 (NS) |
| 12 | 8.48±1.41 | 8.88±1.91 | |
| 24 | 8.96±2.22 | 8.95±1.84 | |
| Mean diff (Baseline–12 h) | −0.04±1.29 | −0.24±1.37 | (NS) |
| Mean diff (Baseline–24 h) | 0.44±1.73 | −0.17±1.63 | (NS) |
NS: Indicates that the results were not significant, TEWL: Trans-epidermal water loss, SD: Standard deviation
Safety evaluation
None of the participants experienced any skin reactions or intolerances following the application of the test product. In addition, no adverse events were reported by any participant throughout the entire duration of the study.
DISCUSSION
This study evaluated skin hydration on the volar forearms of participants after the application of the test product, assessing changes at 12 and 24 h compared to baseline and a control site. At baseline, both sites exhibited dry skin (MMSC <20). However, after 12 h of product application and occlusion, the test site showed a significant increase in mean MMSC values, improving to the normal skin category (MMSC: 20–40), while the control site did not exhibit similar improvement. This enhanced hydration was sustained at 24 h. Similar improvements were noted at unoccluded sites.
TEWL, assessed using the VapoMeter to measure skin hydration, showed a significant decrease in readings, indicating reduced moisture loss from the skin. At 12 h, there was no significant difference in TEWL between the test and control sites. However, at 24 h, the control site showed significantly higher TEWL than the test site when occluded. For unoccluded sites, there were no significant changes or differences in VapoMeter readings at any time point compared to the baseline.
Moisturizers fall into several categories such as occlusives, humectants, and emollients, each serving to prevent water loss, increase skin hydration, and reduce flakiness. Occlusive moisturizers form a barrier that minimizes TEWL, allowing moisture from deeper skin layers to replenish the SC.[12] The Venusia CeraPlus Lotion is distinguished by its incorporation of essential ceramides, which are critical for the structural and functional integrity of the skin’s permeability barrier. As powerful moisturizers, they maintain skin hydration by preventing water loss and protecting against external irritants, making them essential for skin barrier health.[5,13] The findings of our study are consistent with those of a previous study by Spada et al., which demonstrated that a single topical application of a ceramide cream significantly increased skin hydration over time (P < 0.001). At 24 h post-application, the skin hydration levels achieved with the ceramide cream were significantly higher (P < 0.05) than those of three other reference moisturizers. In addition, the ceramide cream significantly reduced TEWL (P < 0.001) over the same period and was found to be non-sensitizing to the skin.[9] Similar results were observed in the double-blind Restore Phase 1 study, where a ceramide-containing cream and lotion in a multi-vesicular emulsion sustained significant skin moisturization for 24 h, potentially alleviating the burden of managing dry skin conditions like AD.[7]
Along with ceramides, the test product also contains a dual oat complex known to enhance skin hydration and elasticity. This is supported by a study conducted by Sacchidanand et al., which demonstrated that an oat extract-based moisturizer significantly improved skin hydration and reduced dryness, with notable improvements (P < 0.0001) lasting up to 24 h post-application.[14] In addition to these ingredients, the study lotion contains potent humectants such as glycerin and HA. Glycerin acts as a natural moisturizer, preserving the SC barrier and improving skin texture by softening the SC and smoothing superficial corneocytes through cell shrinkage.[15] HA, which can absorb up to 6 L of water/g, is multifunctional, modulating cellular immunity, regulating epidermal cell interactions, and integrating into the extracellular matrix.[16] Milani and Sparavigna, in their randomized, assessor-blinded trial, found that a single application of glycerin and HA product significantly improved skin hydration and barrier function for up to 24 h, with lower TEWL at the test site and reduced post-stripping TEWL at 1, 8, and 24 h.[17] These findings are consistent with our present study results. Humectants increase water absorption into the epidermis, leading to potential water loss, so they are often combined with occlusives to improve barrier function and hydration. Emollients further support this by softening and smoothing the skin.[18] By combining occlusives and humectants with specific emollients, the test product not only enhances skin hydration but also improves the esthetic properties and stability of its active ingredients, making it an effective solution for dry skin and suitable for daily use.
However, the present study has a few limitations. Including a comparative analysis of other products could provide additional context for the lotion’s performance. The assessment techniques used were specific and may not have captured all aspects of the lotion’s effectiveness, suggesting that more advanced methods could improve the evaluation. In addition, the 24-h duration may not fully reflect the product’s long-term efficacy and safety.
CONCLUSION
Venusia CeraPlus Lotion exhibited substantial effectiveness in enhancing skin hydration under both occluded and unoccluded conditions, with the most pronounced effects observed within the 1st 12 h of unoccluded use. The lotion successfully maintained skin hydration in the normal range for up to 24 h under occluded conditions. Although the lotion could not entirely prevent the skin from reverting to a drier state under unoccluded conditions, it still demonstrated superior hydration compared to the control. The product’s ceramide content is pivotal in improving skin barrier function, as reflected by its lower increase in TEWL compared to the control site. The test product’s blend of ceramides, dual oat complex, glycerin, and HA enhances both hydration and barrier protection, making it an excellent choice for patients needing sustained moisture retention. Future research could further investigate its long-term effects to provide a more comprehensive assessment of its full range of advantages.
Authors’ contributions
Dr. Biswajit Aich and Preeti Kumbhar: Contributed to the conception and design of the study. Dr. Snehal Muchhala, Dr. Arti Sanghavi, Dr. Sagar Katare, and Dr. Bhavesh Kotak: Drafted the article and revised it critically for important intellectual content. All authors have read and approved the final manuscript. All authors are accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical approval
The research/study approved by the Institutional Review Board at C.L.A.I.M.S. Pvt. Ltd, number ECR/245/Indt/MH/2015/RR-22), dated February 2024.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr. Biswajit Aich, Preeti Kumbhar, Dr. Snehal Muchhala, Dr. Arti Sanghavi, Dr. Sagar Katare, and Dr. Bhavesh Kotak are employees of Dr Reddy’s Laboratories Ltd.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
This study was funded by Dr. Reddy’s Laboratories Ltd.
References
- The prevalence and severity of dry skin and related skin care in older adult residents in institutional long-term care: A cross-sectional study. Geriatr Nur (Lond). 2023;54:331-40.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and associated factors of dry skin among older inpatients in hospitals and nursing homes: A multicenter cross-sectional study. Int J Nurs Stud. 2022;135:104358.
- [CrossRef] [PubMed] [Google Scholar]
- Epidermal barrier function in dry, flaky and sensitive skin: A narrative review. J Eur Acad Dermatol Venereol. 2024;38:812-20.
- [CrossRef] [PubMed] [Google Scholar]
- Skin barrier function: The interplay of physical, chemical, and immunologic properties. Cells. 2023;12:2745.
- [CrossRef] [PubMed] [Google Scholar]
- Role of omega-hydroxy ceramides in epidermis: Biosynthesis, barrier integrity and analyzing method. Int J Mol Sci. 2023;24:5035.
- [CrossRef] [PubMed] [Google Scholar]
- An investigation of the skin barrier restoring effects of a cream and lotion containing ceramides in a multi-vesicular emulsion in people with dry, eczema-prone, skin: The RESTORE study phase 1. Dermatol Ther. 2020;10:1031-41.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of topical application of a skin moisturizer containing pseudo-ceramide and a eucalyptus leaf extract on atopic dermatitis: A review. J Clin Med. 2024;13:1749.
- [CrossRef] [PubMed] [Google Scholar]
- Skin hydration is significantly increased by a cream formulated to mimic the skin's own natural moisturizing systems. Clin Cosmet Investig Dermatol. 2018;11:491-7.
- [CrossRef] [PubMed] [Google Scholar]
- Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 2018;120:1682-95.
- [CrossRef] [PubMed] [Google Scholar]
- Hyaluronan (hyaluronic acid): A natural moisturizer for skin care Palm Springs, CA: Chemical Publishing Company; 2015. p. :605-22.
- [Google Scholar]
- Moisturizers In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/books/NBK545171 [Last accessed on 2024 Nov 15]
- [Google Scholar]
- The role of ceramides in skin homeostasis and inflammatory skin diseases. J Dermatol Sci. 2020;97:2-8.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, assessorblinded, comparative study to evaluate the efficacy and safety of oat extractbased moisturizer in adult individuals with dry skin. Clin Dermatol Rev. 2018;2:58.
- [CrossRef] [Google Scholar]
- Glycerol as a skin barrier influencing humectant In: Lodén M, Maibach HI, eds. Treatment of dry skin syndrome: The art and science of moisturizers. Berlin, Heidelberg: Springer; 2012. p. :473-80.
- [CrossRef] [Google Scholar]
- Efficacy evaluation of a topical hyaluronic acid serum in facial photoaging. Dermatol Ther. 2021;11:1385-94.
- [CrossRef] [PubMed] [Google Scholar]
- The 24-hour skin hydration and barrier function effects of a hyaluronic 1%, glycerin 5%, and Centella asiatica stem cells extract moisturizing fluid: An intra-subject, randomized, assessor-blinded study. Clin Cosmet Investig Dermatol. 2017;10:311-5.
- [CrossRef] [PubMed] [Google Scholar]
- The role of moisturizers in addressing various kinds of dermatitis: A review. Clin Med Res. 2017;15:75.
- [CrossRef] [PubMed] [Google Scholar]







