Translate this page into:
Clinical and dermoscopic patterns of idiopathic guttate hypomelanosis

*Corresponding author: Devinder Mohan Thappa, Department of Dermatology and STD, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. dmthappa@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Murugan K, Thappa DM. Clinical and dermoscopic patterns of idiopathic guttate hypomelanosis. CosmoDerma. 2024;4:113. doi: 10.25259/CSDM_138_2024
Abstract
Depigmented skin lesions are of great concern in society, especially in the Indian subcontinent. These comprise many infective and inflammatory conditions that cause apprehension and anxiety among patients due to the social stigma attached to these conditions. Idiopathic guttate hypomelanosis (IGH) appears similar to many depigmented lesions and differentiation of IGH from these conditions is difficult clinically as well as histopathologically. IGH is one of the common causes of acquired leukoderma. It is also called disseminated lenticular leukoderma. The etiology is yet not clearly delineated, probably multifactorial. Various etiological factors have been proposed including ultraviolet (UV) exposure, post-phototherapy (psoralen and UVA monotherapy, narrowband-UVB), aging, genetic factors, trauma, and autoimmunity. Clinically, it is characterized by multiple, discrete porcelain-white round to oval macules of 2–5 mm average size. It most commonly occurs in the elderly. The most common sites observed are chronically sun-exposed areas such as the arm, pretibial regions, and forearm extensors. It is not easy to differentiate IGH from other hypo and depigmented conditions such as vitiligo, pityriasis versicolor, extragenital lichen sclerosus et atrophicus, guttate morphea, and post-inflammatory hypopigmentation. It poses a diagnostic challenge to dermatologists. One has to differentiate depigmented lesions from vitiligo as it carries tremendous social implications as social stigma, especially in India. Research on dermoscopic evaluation is uncommon in the literature, despite the abundance of clinic-epidemiological and histological studies of IGH. Four dermoscopic patterns, namely, petaloid, amoeboid, feathery, and nebuloid have been described. These patterns are specific to IGH and help clinicians to differentiate many depigmented skin lesions from IGH in clinical practice. Patients often seek cosmetic treatment. There has been no standard therapy for this condition. Newer treatment modalities range from topical agents to procedure-based therapies and have enhanced the therapeutic armamentarium.
Keywords
Idiopathic guttate hypomelanosis
Clinical patterns
Dermoscopy
INTRODUCTION
Idiopathic guttate hypomelanosis (IGH) is one of the common causes of acquired leukoderma.[1] It is also called disseminated lenticular leukoderma.[1] This entity was first described as symmetric progressive leukopathy of extremities by Costa in 1951.[2] In India, the prevalence of IGH ranges from 20% in those below 30 years to 80% in those above 70 years. It has also been reported as early as three years.[3]
ETIOLOGY
The etiology needs to be clarified. However, various etiological factors have been proposed including ultraviolet (UV) exposure, post-phototherapy (psoralen and UVA monotherapy, narrowband-UVB), aging, genetic factors, trauma, and autoimmunity.[1,4] Human leukocyte antigen-DQ3 was observed to correlate with IGH positively. The prevalence increases with aging due to a progressive decrease in the number of melanocytes (about 10–20% every 10 years). Others observed malfunction in the autophagy of keratinocytes over decreased melanocyte density or morphology.[3] However, further studies are warranted. Repeated microtrauma surmounted by decreased subcutaneous tissue also predisposes to IGH, explaining the pretibial and lower central back being one of the most common sites.[3]
CLINICAL PROFILE
It is characterized by multiple, discrete porcelain-white round to oval macules of average size of 2–5 mm [Figure 1]. It most commonly occurs in the elderly. The most common sites observed are chronically sun-exposed areas such as the arm, pretibial regions, and extensors of the forearm. It can occur both on the sun-exposed and sun-covered areas. Marginal area of face involvement has been reported in 6% of patients.[4] Earlier, three variants of IGH were proposed. The first one is single or multiple hypopigmented macules on sun-damaged areas, the second one is solitary ivory white sclerotic stellate well-defined macules seen both on sun-covered and sun-exposed areas, and the third one is multiple small well-defined hypopigmented macules with thick keratotic crust with scalloped border.[3,4] They may increase in number over time, but usually do not increase in size or coalesce. However, there are also case reports with a 16% complaining increase in the size of the lesions. The younger the age group, the lesser the number, and size of the lesions (<5 and usually <2 mm) compared to older age groups (usually >30 or 50, >3–5 mm). Hair within those areas are usually uninvolved.[4] It is difficult to differentiate IGH from other hypo and depigmented conditions such as vitiligo, pityriasis versicolor, extragenital lichen sclerosus et atrophicus, guttate morphea, and post-inflammatory hypopigmentation.[4] It poses a diagnostic challenge to dermatologists.
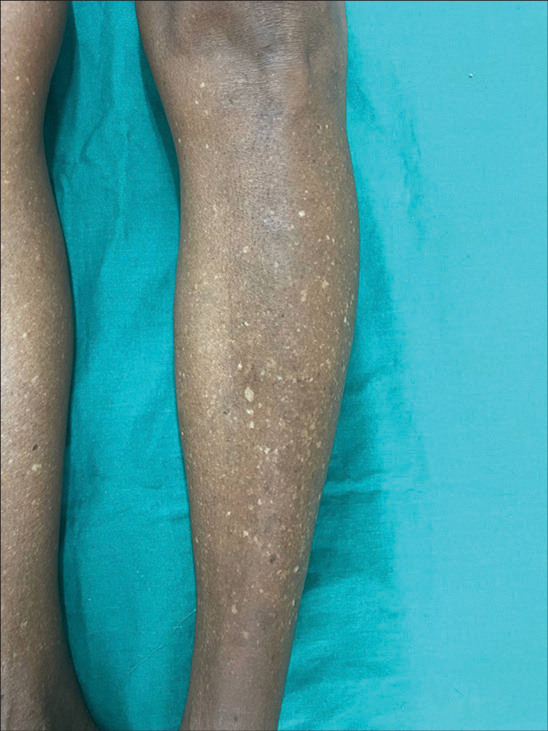
- Clinical picture of idiopathic guttate hypomelanosis.
HISTOPATHOLOGY
Histological findings of IGH include hyperkeratosis, flat rete ridges, atrophic epidermis, reduced number of melanocytes, and reduced melanin content.[1] Less common histological features observed are thicker Grenz zone due to deposition of glycosaminoglycans.[3] Furthermore, Luna stain demonstrated thick, branched, and curled elastic fibers termed “elastosis.”[4] Various studies showed a decrease in the number of dopa-positive melanocytes. Eighty percent of IGH lesions demonstrated small areas of normal melanin alternating with large areas of loss of melanin.[4] Later, Kim et al.[5] found reduced melanin pigment by Fontana-Mason staining. Furthermore, this was added on by weak expression of an immunohistochemical marker, MART-1, and NK1/beteb.[5]
Ultrastructural features noted in IGH are the absence or attenuation of dendrites of melanocytes, endoplasmic reticulum dilation and mitochondrial swelling (together called “senescence of melanocytes”), and a decrease in the number of melanosomes.[3]
DERMOSCOPIC FEATURES
Research on dermoscopic evaluation is uncommon in the literature, despite the abundance of clinic-epidemiological and histological research on IGH. Bambroo et al.[6] recorded four dermoscopic patterns, namely, petaloid, amoeboid, feathery, and nebuloid.[6] A diagrammatic representation of dermoscopic patterns of IGH is given in Figure 2.[7]
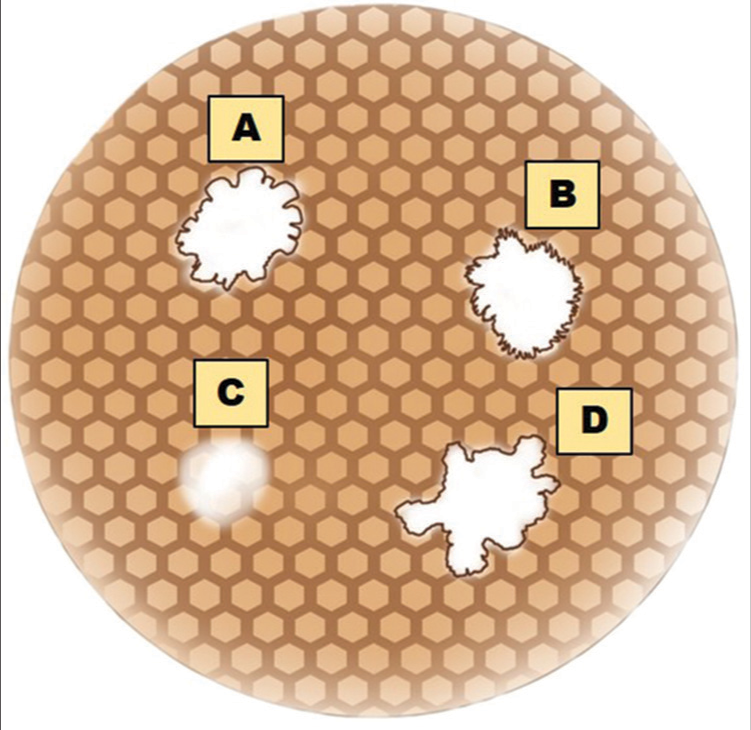
- Dermoscopic patterns of idiopathic guttate hypomelanosis. (A) Petaloid pattern. (B) Feathery pattern. (C) Nebuloid pattern. (D) Amoeboid pattern.
Ankad and Beergouder[1] also demonstrated four dermoscopic patterns. The sample size recruited was 30, with 16 females and 14 males. The mean age of the study was 54.5 years ranging from 24 to 85 years. They observed amoeboid, feathery, petaloid, and nebuloid patterns in 46.6%. 40.0%, 23.3%, and 3.3% of patients, respectively. A combination of these patterns was observed in 13.3% of patients. The summary of their study findings is shown in Table 1.
They described
Feathery pattern – feather-like extensions into the surrounding skin seen in younger age groups, and longer duration of skin lesions
Amoeboid pattern characterized by pseudopods with extension at the periphery
A petaloid pattern having well-defined petal-like borders
Nebuloid having ill-defined borders.
The latter three patterns had older age preponderance. A nebuloid pattern was observed in skin lesions of shorter duration and a feathery pattern in skin lesions of longer duration. This is in contrast with the study done by Harish et al. which showed nebuloid pattern in a longer duration of IGH and petaloid pattern in a shorter duration.[8] Dermoscopic patterns of IGH are depicted in Figures 3-6. They performed a histopathological examination, which correlated with dermoscopy findings. Whitish areas in center of the feathery pattern corresponded to basket-weave hyperkeratosis, and depigmentation corresponded to the loss of melanin globules.[1]

- Dermoscopy (×10) showing amoeboid pattern.

- Dermoscopy (×10) showing petaloid pattern (note the borders).

- Dermoscopy (×10) showing feathery pattern.
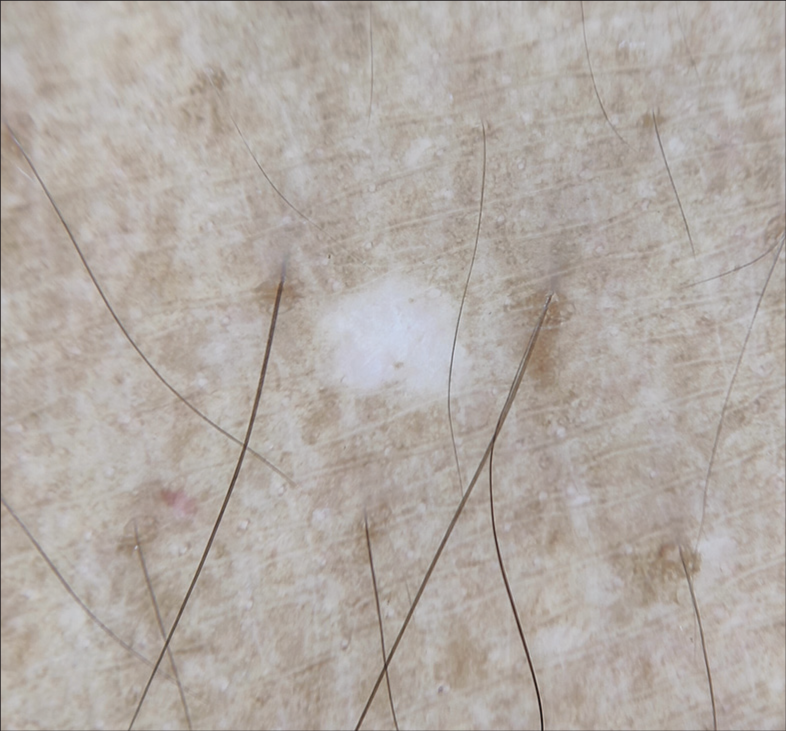
- Dermoscopy (×10) showing nebuloid pattern (note the ill-defined borders).
Errichetti and Stinco[9] compared the dermoscopic patterns of both chronically sun-exposed and sun-protected areas. The enrolled cases were 21, with 11 females, and 10 males. They recruited biopsy-proven cases with an average disease duration of eight years. They described two different patterns: “Cloudy sky-like pattern” – multiple small macules coalescing to form polycyclic macules with both ill- and well-defined edges, and varying shades of white, surrounded by patchy areas of the hyperpigmented network which was observed more in chronically sun-exposed areas; the other one was “cloudy pattern” – ill- or well-defined homogenous white round areas surrounded by patchy areas of the hyperpigmented network, which was more in sun-protected areas. Other patterns observed include sparse blurry vessels (dotted/branching) (six in sun-exposed, two in sun-protected), scaling (seven in sun-exposed, six in sun-protected), and patchy hyperpigmented network (five in sun-exposed, seven in sun-protected areas, respectively), which were statistically insignificant.[9]
Singhal et al.[10] in their clinicodermoscopic study of IGH from Madhya Pradesh, among the 180 patients, the most affected age group was 51–60 years. A total of 108 (60%) were females and 72 (40%) were males. The most common site of involvement was the distal part of the lower extremity in 152 (84.4%) cases followed by the distal part of the upper extremity in 115 (63.8%) cases. A total of 46 (25.5%) patients had a history of excessive sun exposure. In addition, 21 (11.6%) patients had other associated features of photoaging, such as xerosis, solar lentigo, seborrhoeic keratosis, freckles, and actinic keratosis. The most common dermoscopic pattern observed was amoeboid in 103 (57.2%) patients, followed by feathery in 41 (22.7%), petaloid in 23 (12.7%), nebuloid in 13 (7.2%), and a combination of patterns in 6% of patients.
In a study conducted at our institute from July 2022 to December 2023 on adult cases (>18 years old) with lower leg pigmentation, IGH was observed in 62 patients. They all presented with a porcelain-white colored round to oval macules in guttate distribution, average age of 56.2 years ranging from 32 to 97 years. This observation was almost similar to that of Ankad and Beergouder[1] (54.5 years) and Singhal et al.[10] (57.82 years). Both studies were conducted in India, whereas Errichetti and Stinco[9] (67 years) recorded at a higher age group. This is explained by the fact that the study by Errichetti and Stinco[9] was conducted in a different demographic setting in Korea, where the complexion is lighter and has less contrast, leading to delayed diagnosis. The most common age group affected was 51–60 years (24, 38.7%), followed by 41–50 years (16, 25.8%) in our study. Females (48, 77.4%) outnumbered males (14, 22.5%) with a female: male ratio of 3.4:1 as noted by Singhal et al.[10] (3:2), whereas studies of Ankad and Beergouder[1] (1.14:1) and Errichetti and Stinco[9] (1.1:1) found no gender preponderance. IGH was found more often in females than in males, most likely as a result of their greater concern for appearance which necessitated dermatological consultation.[9]
| Study | Mean age (years) | Findings | |||
|---|---|---|---|---|---|
| Amoeboid | Feathery | Petaloid | Nebuloid | ||
| Ankad and Beergouder[1] (n=30) | 54.5 (24–85) | 14 (46.6%) | 12 (40.0%) | 7 (23.3%) | 1 (03.3%) |
Amongst 62 cases of IGH in our study, the frequently observed dermoscopic pattern was amoeboid (21, 33.87% cases), followed by feathery (19, 30.64% cases), petaloid (13, 20.96% cases), and nebuloid (9, 14.51% cases). This was similar to studies by Ankad and Beergouder[1] and Singhal et al.[10] Ankad and Beergouder[1] observed individuals with skin lesions of longer duration showed a feathery pattern, while individuals with shorter duration had a nebuloid pattern.[1] Singhal et al.[10] also observed perifollicular pigmentation (32, 17.7%), perilesional pigmentation (47, 26.1%), and linear vessels (14, 7.7%) which were not observed in our study participants. Younger people exhibited the feathery pattern more frequently, and elderly people displayed the nebuloid pattern more frequently. Comparison of dermoscopic patterns of IGH between Ankad and Beergouder,[1] Singhal et al.[10] and our study is given in Table 2.
Errichetti and Stinco[9] described two different patterns: “Cloudy sky-like pattern” – with coalescing polycyclic macules with both ill- and well-defined edges, with varying white shades, surrounded by patchy hyperpigmented network and “cloudy pattern” – ill- or well-defined homogenous white round areas surrounded by patchy areas of the hyperpigmented network. Other patterns they noticed were patchy hyperpigmented networks, scaling, and sparse blurry vessels (dotted/branching), all of which were statistically insignificant. However, this was not observed in our study.
DIAGNOSIS
The IGH is diagnosed based on its clinical features and enhanced depigmentation on Wood’s lamp.[1-3] Investigations such as dermoscopy and histopathology are performed to differentiate IGH from other macular depigmented lesions, especially at atypical sites. However, routine use of such investigations is not necessary.
TREATMENT
The IGH can be a cosmetic concern. Educational intervention should be included in the first visit of a patient to a dermatologist’s office.[3] Sun protection with sunscreens or sunblocks is important to prevent sunburn in depigmented areas. A plethora of treatment modalities including medical and surgical methods have been suggested for IGH with variable success. These include topical calcineurin inhibitors (pimecrolimus 1% and tacrolimus 0.5%), topical retinoids (tretinoin 0.025%), intralesional steroids, topical placental extract with 88% phenol, therapeutic surgical wounding with trichloroacetic acid (TCA), phenol, dermabrasion, liquid nitrogen spray, needling and lasers (ablative or non-ablative), chemical peeling with TCA or phenol, excimer light, tattooing, and finally skin grafting.[3]
Dermatologists usually counsel against treatment of any form as there is a dearth of evidence from well- controlled randomized studies.[3] The primary step is to educate to clear any misconceptions regarding the disease to curb anxiety, advice photoprotection, and avoidance of trauma. If the patient insists, topical calcineurin inhibitors, 50% TCA, and non-ablative fractional 1550-nm Erbium laser as effective and well-tolerated therapeutic options may be offered.[3]
CONCLUSION
This article basically summarizes the clinical, dermoscopic, and histopathological features of IGH and compares these findings with our study. Four dermoscopic patterns, namely, petaloid, amoeboid, feathery, and nebuloid have been described. Their occurrence has been recorded in varied frequencies in various studies. Patients often seek cosmetic treatment. There has been no standard therapy for this condition. Newer treatment modalities range from topical agents to procedure-based therapies and have enhanced the therapeutic armamentarium.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Dermoscopic evaluation of idiopathic guttate hypomelanosis: A preliminary observation. Indian Dermatol Online J. 2015;6:164-7.
- [CrossRef] [PubMed] [Google Scholar]
- Progressive symmetrical leukopathia of the extremities. Ann Dermatol Syphiligr (Paris). 1951;78:452-4.
- [Google Scholar]
- Idiopathic guttate hypomelanosis: Presentation and management. J Cosmet Laser Ther. 2021;23:8-15.
- [CrossRef] [PubMed] [Google Scholar]
- Idiopathic guttate hypomelanosis: A review of its etiology, pathogenesis, findings, and treatments. Am J Clin Dermatol. 2016;17:403-11.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive understanding of idiopathic guttate hypomelanosis: Clinical and histopathological correlation. Int J Dermatol. 2010;49:162-6.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy in the differentiation of idiopathic guttate hypomelanosis (IGH) and Guttate vitiligo In: Khopkar S, ed. Dermoscopy and trichoscopy in diseases of the brown skin. Atlas and short text (1st ed). New Delhi: Jaypee Brothers Ltd; 2012. p. :97-103.
- [CrossRef] [Google Scholar]
- Dermoscopic analysis of idiopathic guttate hypomelanosis:a cross sectional study. Pigment Int. 2021;8:25-9.
- [CrossRef] [Google Scholar]
- Dermoscopy of idiopathic guttate hypomelanosis. J Dermatol. 2015;42:1118-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicodermoscopic study of idiopathic guttate hypomelanosis at a tertiary care hospital in Central India. J Clin Diagn Res. 2023;17:WC06-9.
- [CrossRef] [Google Scholar]






