Translate this page into:
Botulinum toxin beyond aesthetics in dermatology

*Corresponding author: Gulhima Arora, Dermatology, Mehektagul Dermaclinic, Safdarjung Enclave, New Delhi, Delhi, India. gulhima@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Arora G. Botulinum toxin beyond aesthetics in dermatology. CosmoDerma 2022;2:15.
Abstract
Botulinum toxin is a purified protein that was first used in the 1970s for strabismus, a non-cosmetic indication. Increasing knowledge and understanding of the product has led to its use for various indications other than cosmetic ones. It is used in many fields of medicine. This review aims to present the various indications beyond aesthetics in the realm of dermatology with its postulated mechanism of action for which the toxin has been used with success and the technique of administration for a few common dermatological indications.
Keywords
Botulinum toxin
Non-aesthetic indications
Post herpetic neuralgia
Hyperhidrosis
Alopecia
INTRODUCTION
Botulinum toxin (BT) is well-known as the “poison that heals” and not without reason. The beneficial effect of BT for cosmetic indications mostly dynamic wrinkles is well established,[1] backed by both scientific literature and evidence-based medicine.[2] Scientific academia is filled with benefits of this purified protein for non-cosmetic indications as well. In fact, its first use was for strabismus and not for a cosmetic indication.[3] Ever since its approval in 2002 by the US FDA for cosmetic indications, its evidence-based therapeutic use for non-cosmetic indications is well documented and spans the specialties of urology, otolaryngology, gynecology, ophthalmology, plastic surgery, neurology, and dermatology [Table 1]. It has been used for various indications in the head and neck like laryngeal dystonia, vocal tics, stuttering, blepharospasm, hemifacial spasm, rhinitis, facial nerve paresis to name a few.[4] Of special note is its use in pain. It has been used as a successful prophylactic treatment of migraine where it is injected into the muscles innervated by the trigeminal and facial nerves.[5] Masticatory myalgia and trigeminal neuralgia are other painful conditions the toxin has been used for.[4] In surgery, it has been used for skeletal muscle hyperactivity disorders, abdominal wall reconstruction, prosthetic breast reconstruction among other indications.[6]
| Dermatological condition | |
|---|---|
| Disorders of eccrine glands | Hyperhidrosis[7] |
| Eccrine hydrocystomas | |
| Eccrine angiomatous hamartoma | |
| Pitted keratolysis | |
| Chromhidrosis | |
| Hailey-Hailey disease[8] | |
| Dyshidrotic eczema[9] | |
| Disorders of apocrine glands | Hidradenitis suppurativa[10] |
| Bromhidrosis | |
| Disorders of striated muscles | For unopposed muscle action in facial palsy[11] |
| Disorders associated with pain | Post-herpetic neuralgia[12] |
| Leiomyoma associated pain | |
| Notalgia paresthetica | |
| Telalgia | |
| Raynaud’s phenomenon | |
| Disorders associated with Itching | Brachioradial pruritus |
| Vulvodynia | |
| Lichen simplex chronicus | |
| Hair disorders | Androgenetic alopecia[13] |
| Trichodynia[14] | |
| Scars | Traumatic/surgical scar remodeling[15] |
| Hypertrophic scars/keloids | |
| Acne scars (hypertrophic) | |
| Others | Inverse psoriasis |
BT has been used for several indications other than cosmetic ones in dermatology as well.
Mechanism of action
The basic mechanism of action of BT is chemical denervation or preventing the release of acetylcholine into the synaptic cleft. Upon injection into the skin or muscle, the heavy chain binds to the receptors on the cholinergic nerve endings. This is followed by endocytosis of the molecule and attachment of the light chain to the soluble N-ethylmaleimide-sensitive fusion (SNARE complex) protein receptors after getting cleaved from the heavy chain. The SNARE is composed up of three protein receptors namely, synaptobrevin (VAMP), synaptosome associated protein (SNAP-25), and syntaxin. BT type A cleaves SNAP 25 and BT Type B cleaves synaptobrevin. The binding of BT to these receptor proteins prevents the attachment of acetylcholine vesicles to the nerve endings and its further release into the synaptic cleft.[16,17]
The effects of BT on various muscles, glands, and pathways that bring about therapeutic activity in dermatological conditions are outlined below.
Effect of BT on muscles:
The action of BT on both striated and smooth muscles is similar. BT can cause a reduction in muscle hypertrophy and even atrophy of certain muscles with repeated injections. The effects, however, are inconsistent.[18] Dose-duration correlation is present but is not predictable. Lower doses last for shorter durations, but higher doses plateau after 3 months. The correlation between dose and effect is also not predictable. Higher doses do cause greater effect, but it plateaus after some time. Even low doses do produce some effect.[19] This is the rationale behind using it in facial palsy for muscular hypertrophy on the unaffected side.
Effect of BT on eccrine and apocrine glands:
The therapeutic effect of BT for disorders of the exocrine glands is gratifying. The mechanism of action of BT here is not just inhibition of the release of acetylcholine from the cholinergic nerves. This was shown in a study where administration of exogeneous acetylcholine did not induce sweating on the BT treated side but induced it on the control side. This study also showed that the cutaneous vasodilatation was not much affected in response to exogenous acetylcholine, which it should. This showed that BT treatment changed the responsiveness of the exocrine glands to acetylcholine. This study also proposed that inhibition of sweating by BT treatment was not muscarinic-dependent.[20] The postulated mechanism of action is the action on the SNARE complex present in AQP which are the water-channel proteins that inhibit the water flux thus inhibiting sweating.[20-22]
The action of BT on disorders associated with pain:
It has been shown in rabbit muscles that substance P is inhibited along with acetylcholine.[23] BT also inhibits glutamate which is a neurotransmitter involved in nociception.[18] These are the postulated mechanisms of BT being effective for pain-related disorders.
The action of BT on itch-related disorders:
The C-fibers that conduct itch are sensitive to neurotransmitters, substance P, calcitonin gene-related protein (CGRP), histamine, and other inflammatory mediators. BT is known to inhibit the first three mediators mentioned and hence helps with itch-related disorders.[24]
Effect of BT on the central nervous system:
BT which is injected into the muscles has indirect effects on the CNS.[25] A retrograde axonal movement has been proposed as BT does not cross the blood-brain barrier.[18]
Effect of BT in hair disorders:
The proposed mechanism of the action of BT in androgenetic alopecia is that it relaxes the scalp muscle thereby reducing the pressure on the underlying vasculature causing more transcutaneous perfusion of oxygen and washing off the excess dihydrotestosterone that is implicated in the miniaturization of hair follicles.[13] The potential mechanism of action in alopecia areata is the inhibition of neurotransmitters that directly or via neuroimmunologic pathways inhibit the cytokines that are responsible for the mechanism of alopecia areata.[26]
The action of BT on scar tissue:
BT reduces the tensile forces created during wound healing which in turn reduces the inflammatory response during the healing process. It modulates the fibroblast activity by reducing transforming growth factor (TGF), connective tissue growth factor and prevents the differentiation of fibroblasts to myofibroblasts.[27]
Administration techniques of Botulinum toxin for various indications:
Androgenetic alopecia: A dilution of 1ml of saline for 100 U is used. A total dose of 150 U has been used over 30 sites with 5 U injected at each site. The injections are intramuscular in the frontalis, occipital, temporalis, and periauricular muscle sites[13] [Figure 1].
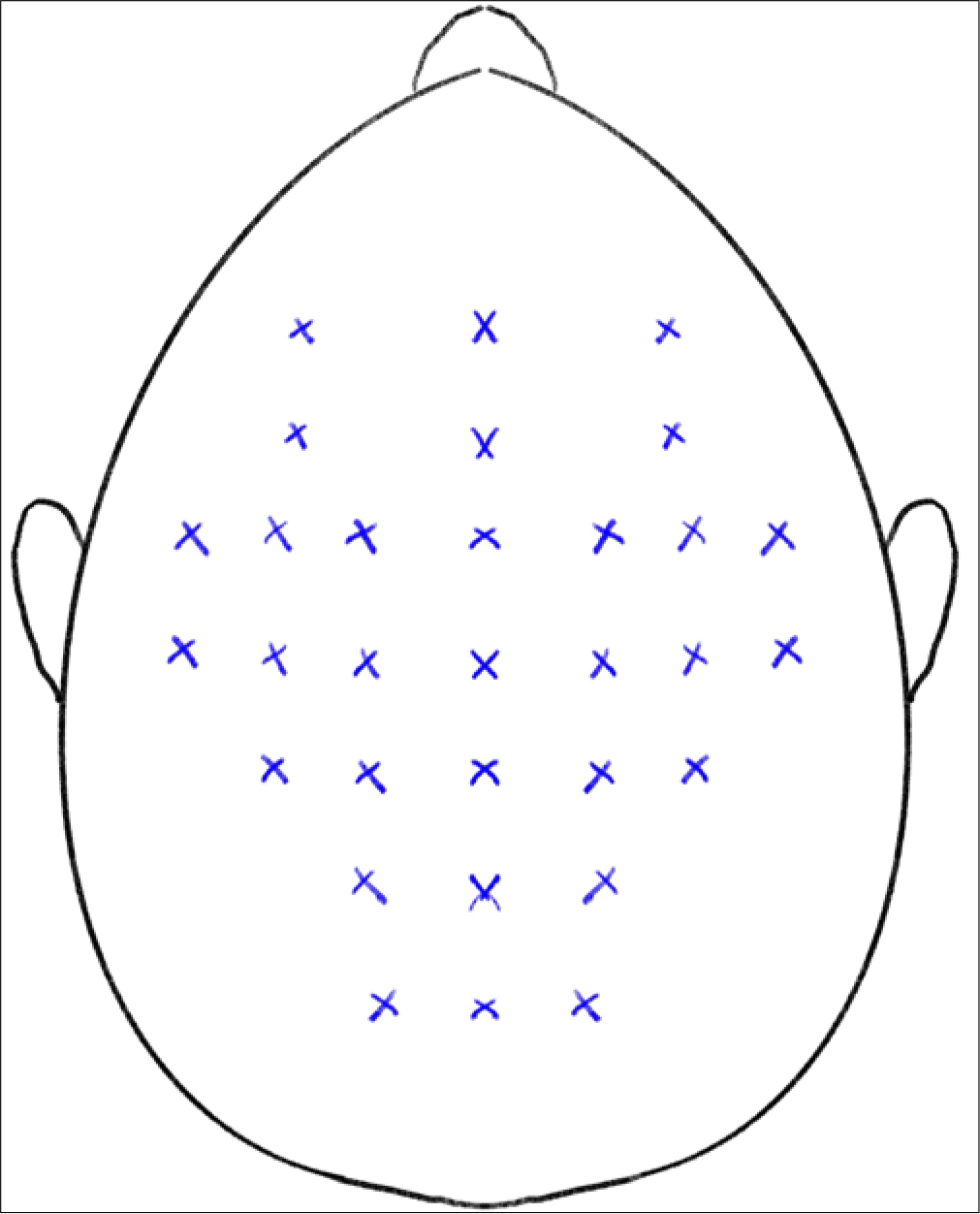
- Technique of BT injection for androgenetic alopecia.
Post-herpetic neuralgia: A dilution of 20 ml in 100 U is taken. Injections are given 1–2 cm apart at sites of tactile allodynia for a maximum of 200 U. The injections are subcutaneous or intradermal[28] [Figure 2].
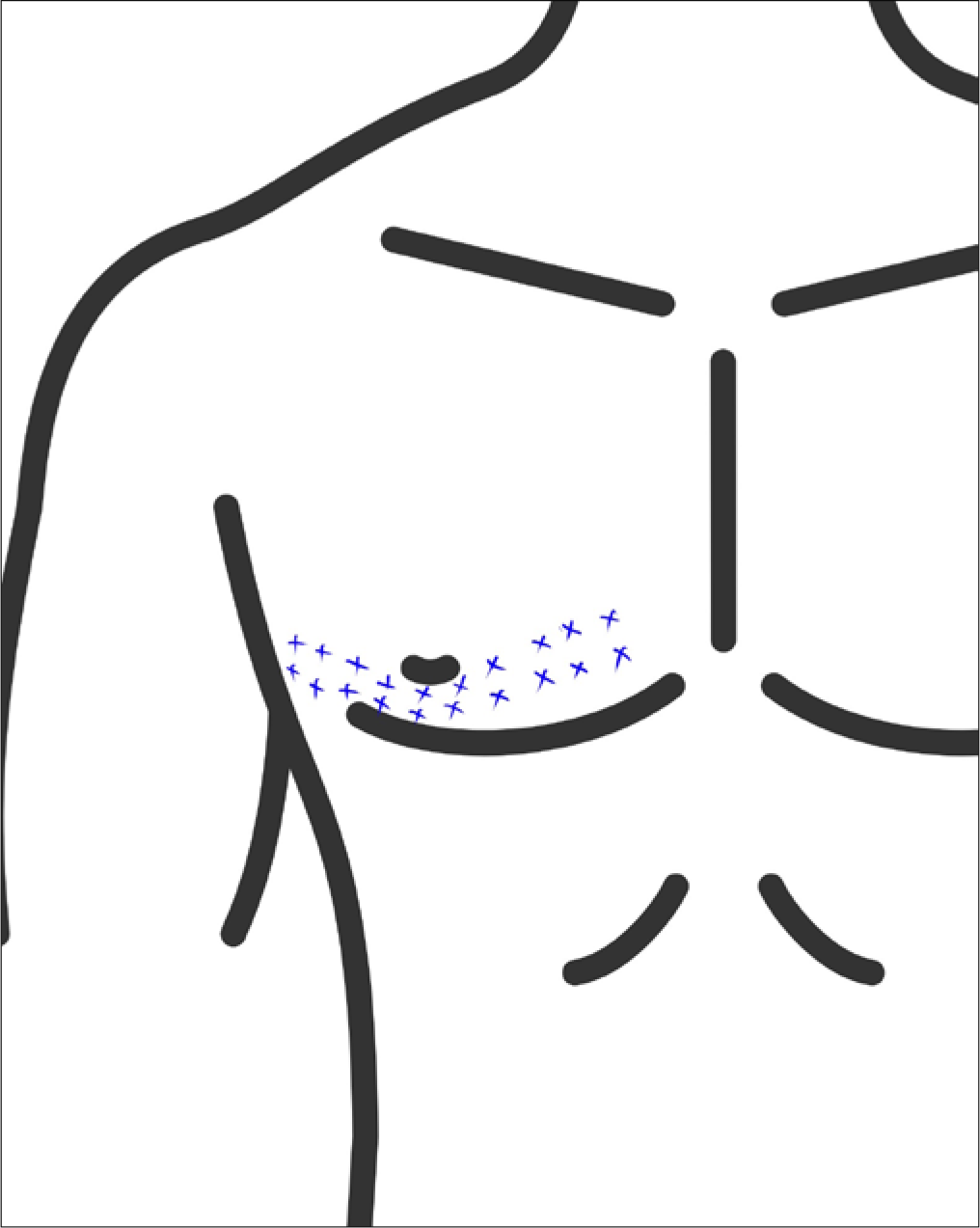
- Technique of BT injection for post herpetic neuralgia.
Palmar hyperhidrosis: About 100 U are diluted with 5 ml of preservative-free saline. The injections are superficially injected in a grid-like fashion intradermally with a maximum dose of 100 U per palm.[29] Nerve blocks may be given to alleviate the pain. The area of hyperhidrosis may be delineated with a starch iodine test. Care must be taken to include the medial and lateral aspects of the fingers [Figure 3].
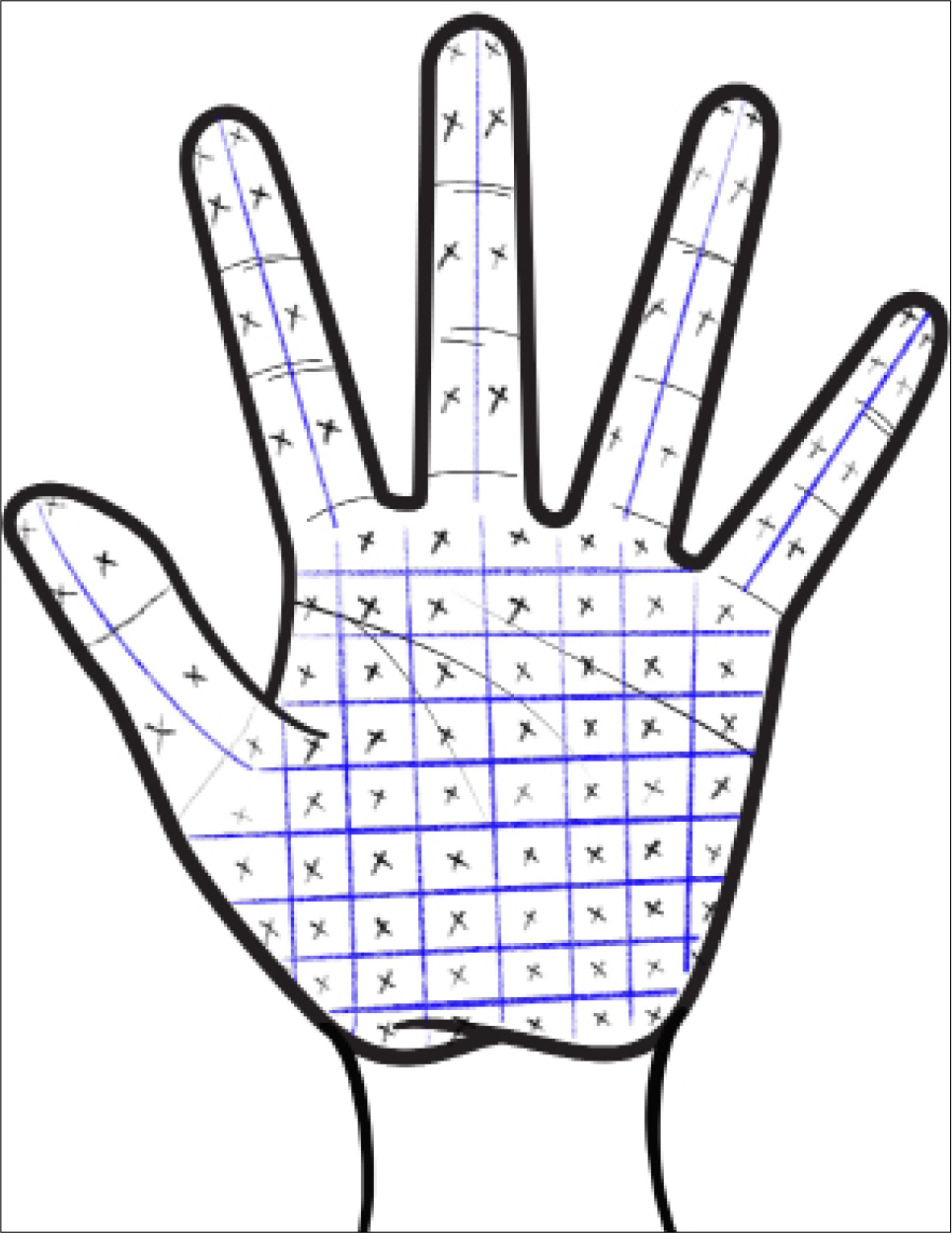
- Technique of BT injection for palmar hyperhidrosis.
Axillary hyperhidrosis: The dilution is the same as axillary hyperhidrosis. The total number of units to be injected is 50 for each axilla. The injection technique is the same as for palmar hyperhidrosis[29] [Figure 4].
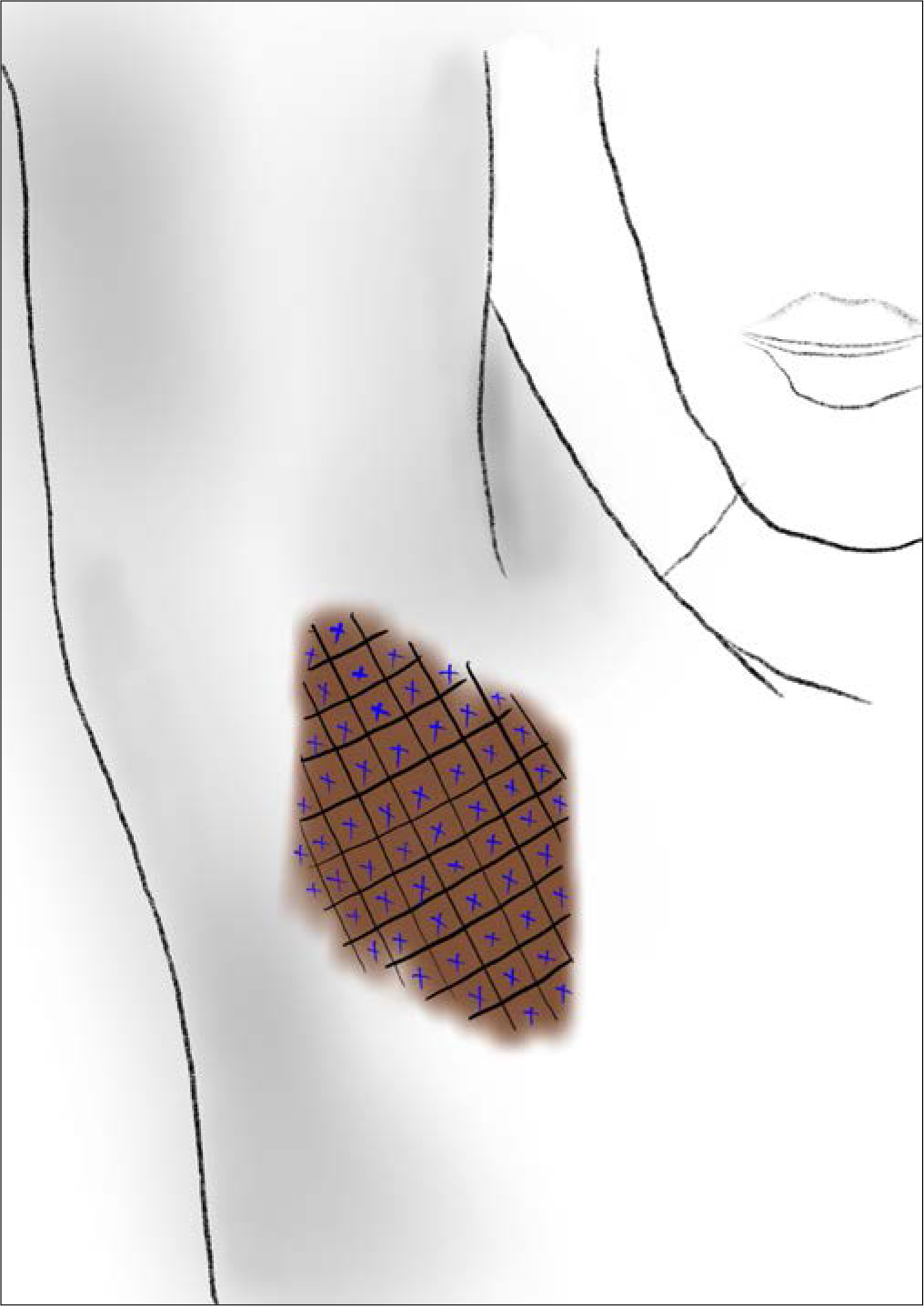
- Technique of BT injection for axillary hyperhidrosis.
Scars: A dilution of 1.25 ml saline in 100 U is taken. The injections are given intradermally 1 cm apart 0.5 mm away from the scar along the entire scar[15] [Figure 5].
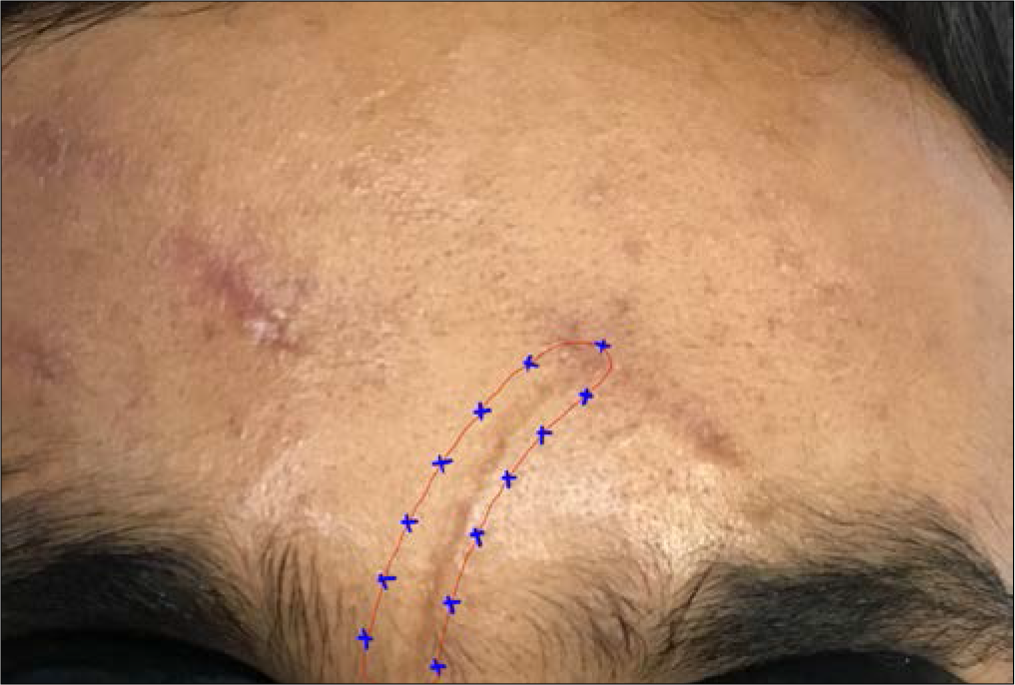
- Technique of BT injection for scar revision.
Keloids: Intralesional BT can be injected into keloids up to 100 U 1 cm apart. Combinations with 5 fluorouracil and triamcinolone 20 mg/ml may be used.[30]
CONCLUSION
BT is increasingly being used for a lot of indications other than cosmetic ones. Its use as a therapeutic modality for non-aesthetic conditions in dermatology must be encouraged and the same should be included in the training curriculum for dermatology residents to better equip them with this modality of treating various indications.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Cosmetic Injectables -Dermal Fillers and Botulinum toxin (1st ed). Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2020.
- [Google Scholar]
- Where and how to use botulinum toxin on the face and neck-Indications and techniques. Cosmoderma. 2021;1:17.
- [CrossRef] [Google Scholar]
- History of botulinum toxin treatment in movement isorders. Tremor Hyperkinetic Mov (N Y). 2016;6:394.
- [CrossRef] [Google Scholar]
- The therapeutic usage of botulinum toxin (Botox) in non-cosmetic head and neck conditions-An evidence based review. Saudi Pharm J SPJ Off Publ Saudi Pharm Soc. 2017;25:18-24.
- [CrossRef] [PubMed] [Google Scholar]
- OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793-803.
- [CrossRef] [PubMed] [Google Scholar]
- Nonaesthetic applications for botulinum toxin in plastic surgery. Plast Reconstr Surg. 2020;146:157-70.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of axillary hyperhidrosis. J Cosmet Dermatol. 2022;21:62-70.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin in treating Hailey-Hailey disease: A systematic review. J Cosmet Dermatol. 2021;20:1396-402.
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant botulinum toxin A in dyshidrotic hand eczema: a controlled prospective pilot study with left-right comparison. J Eur Acad Dermatol Venereol. 2002;16:40-2.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin Type B for hidradenitis suppurativa: A randomised, double-blind, placebo-controlled pilot study. Am J Clin Dermatol. 2020;21:741-8.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin treatment for facial palsy: A systematic review. J Plast Reconstr Aesthetic Surg JPRAS. 2017;70:833-41.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin A treatment for post-herpetic neuralgia: A systematic review and meta-analysis. Exp Ther Med. 2020;19:1058-64.
- [CrossRef] [Google Scholar]
- A pilot study to evaluate effectiveness of botulinum toxin in treatment of androgenetic alopecia in males. J Cutan Aesthetic Surg. 2017;10:163-7.
- [CrossRef] [PubMed] [Google Scholar]
- Refractory trichodynia treated using Onabotulinumtoxin-A. Pain Med. 2021;22:759-60.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of botulinum toxin injection dose on the appearance of surgical scar. Sci Rep. 2021;11:13670.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin-Know the product before injecting. Cosmoderma. 2021;1:56.
- [CrossRef] [Google Scholar]
- The botulinum toxin as a therapeutic agent: Molecular and pharmacological insights. Res Rep Biochem. 2015;2015:173-83.
- [CrossRef] [Google Scholar]
- Botulinumtoxin: Non-cosmetic indications and possible mechanisms of action. J Cutan Aesthetic Surg. 2008;1:3-6.
- [CrossRef] [PubMed] [Google Scholar]
- 2004. Supplements to Clinical Neurophysiology [Internet]. [cited 2022 Jan 16].159-66 Available fromhttps://linkinghub.elsevier.com/retrieve/pii/S1567424X09703538
- [CrossRef] [Google Scholar]
- Botulinum toxin abolishes sweating via impaired sweat gland responsiveness to exogenous acetylcholine. Br J Dermatol. 2009;161:757-61.
- [CrossRef] [PubMed] [Google Scholar]
- Biology of sweat glands and their disorders. I Normal sweat gland function. J Am Acad Dermatol. 1989;20:537-63.
- [CrossRef] [Google Scholar]
- Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci U S A. 2002;99:511-6.
- [CrossRef] [PubMed] [Google Scholar]
- Presynaptic effects of botulinum toxin type A on the neuronally evoked response of albino and pigmented rabbit iris sphincter and dilator muscles. Jpn J Ophthalmol. 2000;44:106-9.
- [CrossRef] [Google Scholar]
- Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005;18:292-303.
- [CrossRef] [PubMed] [Google Scholar]
- Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS Lett. 1999;456:137-42.
- [CrossRef] [Google Scholar]
- Treatment of Alopecia Areata of the Scalp With Intradermal Injections of Botulinum Toxin - Full Text View - ClinicalTrials.gov [Internet] 2022. Clinicaltrials.gov. [cited 16 January 2022]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT00408798
- [Google Scholar]
- The use of botulinum toxin in keloid scar management: A literature review. Scars Burns Heal. 2020;6:2059513120926628.
- [CrossRef] [PubMed] [Google Scholar]
- Subcutaneous injection of botulinum toxin A is beneficial in postherpetic neuralgia. Pain Med. 2010;11:1827-33.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of hyperhidrosis with botulinum toxin Aesthet Surg J. . 2012;32:238-44.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional triamcinolone alone or in combination with botulinium toxin A is ineffective for the treatment of formed keloid scar: A double blind controlled pilot study. Dermatol Ther. 2019;32:e12781.
- [CrossRef] [PubMed] [Google Scholar]






