Translate this page into:
Beauty from within: A comprehensive review on interplay between gut health and skin

*Corresponding author: Angel Sara Thangamuni, Faculty of Medicine, Tbilisi State Medical University, Tbilisi, Georgia. dr.angelsara@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Thangamuni A, Fathimathul Harshiba H, Muhammed Rafi N, Nitol A, Mohan J, Korrapati N. Beauty from within: A comprehensive review on interplay between gut health and skin. CosmoDerma. 2024;4:97. doi: 10.25259/CSDM_73_2024
Abstract
This article sheds light on the explicit relationship between intestinal health and its imminent effects on the skin. The gut-skin axis, a two-way communication mechanism, affects how healthy your skin overall is. Emerging studies suggest that a healthy gut microbiota can improve skin health drastically by lowering inflammation, boosting collagen formation, and mitigating various skin problems. With probiotics emerging as a promising approach, the study emphasizes the potential benefits of probiotic supplementation in enhancing gut and skin health. Understanding this complex relationship holds promise for innovative skincare and therapeutic therapies, thereby bridging the gap between internal well-being and external beauty.
Keywords
Gut-skin axis
Gut microbiota
Probiotics
Skin diseases
Inflammation
INTRODUCTION
Skin and gut function are closely linked, with the skin’s appearance and health being significantly influenced by the gut microbiome. Despite appearing separate organs, the skin and gut are essential for maintaining general health. Research shows the gut-skin axis, where gut microbiota (GM) affects the skin’s condition and appearance, emphasizing the reciprocal relationship between the skin and the gut.[1] The gut barrier, inflammatory mediators, and metabolites made by the gut microbiome are some of the mechanisms that mediate this relationship. These elements may have an impact on the onset and course of skin conditions such as psoriasis, eczema, and acne. In addition, modifications to the GM may cause systemic inflammation, which may worsen skin disorders. Novel therapeutic approaches have been made possible by our growing understanding of the gut-skin axis.[2] Beneficial bacteria or probiotics present in some foods and supplements have been shown to be a promising way to enhance gut health, which, in turn, may enhance skin health.[1] Studies have demonstrated the potential benefits of probiotic supplementation on gut health, including increased diversity of the gut microbiome and decreased inflammatory response in the body. Probiotics have proven to directly affect the skin microbiome and enhance skin conditions,[3] thereby piquing the interest in investigating its use as a therapeutic strategy for enhancing skin health.
METHODS
This is an overview of the prior research on the link between gut health and skin. In this article, the relevant literature on the gut-skin axis was researched through PubMed using keywords including “gut-skin axis,” “gut microbiota,” “probiotics,” “skin diseases,” and “inflammation.” The articles that were chosen were in the English language and were published between 2014 and 2024. The literature search was done between August 2023 and October 2023. The search included various pieces of literature, including research trials and review papers. This review will assist us in understanding the gut-skin connection, as well as in discovering new treatments for various conditions. The excluded articles were not relevant to gut skin or health.
RESULTS
The summary of various references used to put together this review article is shown in Table 1.
| S. No. | Title | Type of article | Reference link | Year of publication | Summary |
|---|---|---|---|---|---|
| 1. | Role of the microbiota in immunity and inflammation | Review Article | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4056765/ | 2014 | Microbiota play an important role in molding the host immune system and maintaining symbiotic interactions. It emphasizes the intricate link between microbiota and immune system, their coevolution, and their impact on host health and disease. |
| 2 . | Regulation of lung immunity and host defense by the intestinal microbiota | Review Article | https://pubmed.ncbi.nlm.nih.gov/26500629/ | 2015 | The article discusses the gastrointestinal microbiota’s role in regulating pulmonary immune responses and host defense against diseases such as influenza, pneumonia, and asthma, emphasizing the impact of intestinal dysbiosis on lung health and the significance of immunological regulatory networks in the lung. |
| 3. | Impacts of Gut bacteria on human health and diseases | Review Article | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4425030/ | 2015 | Gut bacteria are critical to human health because they provide necessary nutrients, and Vitamin K, and promote angiogenesis. However, antibiotic usage, sickness, stress, aging, a poor diet, and lifestyle changes can all alter their composition, potentially resulting in chronic disorders such as inflammatory bowel disease, obesity, cancer, or autism. |
| 4. | Reframing the teenage wasteland: Adolescent microbiota-gut-brain axis | Review Article | https://journals.sagepub.com/doi/full/10.1177/0706743716635536 | 2016 | Adolescence is a challenging stage filled with pressures, environmental stimuli, and physiological changes that can lead to psychiatric illnesses. According to research, pre-and probiotics may help prevent and treat many illnesses in young people, stressing the relevance of understanding the microbiota-gut-brain axis in overall health. |
| 5. | Sex differences in the gut microbiome–brain axis across the lifespan | Review Article | https://doi.org/10.1098/rstb.2015.0122 | 2016 | This paper talks about the differences in gut microbiome with respect to the different sexes while shedding light on the reasons behind it starting from the antenatal period to old age. |
| 6. | The GM in immune-mediated inflammatory diseases | Review Article | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4939298/ | 2016 | The paper explores the function of GM in IMIDs emphasizing the symbiotic interaction between humans and microorganisms. It also covers how the microbiome influences IMID, gut immune responses, and pain modulation in mice. |
| 7. | The gut-skin axis in health and disease: A paradigm with therapeutic implications | Review Article | https://pubmed.ncbi.nlm.nih.gov/27554239/ | 2016 | The article addresses the gut-skin axis, emphasizing similarities between the gut and skin. It suggests that gut illnesses are associated with skin comorbidities; however, this has not been fully investigated in mainstream gastroenterology or dermatological research. The review emphasizes understanding this relationship in terms of therapeutic implications. |
| 8. | Gut microbiome and its role in obesity | Review Article | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5082693/ | 2016 | The human microbiota, including bacteria, fungi, and protozoa, is essential for health, particularly obesity and metabolic diseases. It helps with energy extraction, vitamin production, and immune system growth. Probiotics, prebiotics, synbiotics, and fecal transplants are among the tactics investigated in this research. |
| 9. | FMT: in perspective | Review Article | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4749851/#:~:text=Fecal%20microbiota%20transplantation%20(FMT)%20is,%20and%20confer%20a%20health%20benefit. | 2016 | FMT is reviewed for its role in restoring GM balance, treating gastrointestinal disorders, and potentially conferring health benefits. |
| 10. | Homeostatic immunity and the microbiota | Review Article | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5604871/ | 2017 | Microbiota shapes host immunity, explored in this review, emphasizing cellular mediators and emerging themes in the ongoing dialogue. |
| 11. | Skin-gut axis: the relationship between intestinal bacteria and skin health | Review Article | https://www.wjgnet.com/2218-6190/full/v6/i4/52.htm | 2017 | The article discusses the gastrointestinal microbiota’s role in regulating pulmonary immune responses and host defense against diseases such as influenza, pneumonia, and asthma, emphasizing the impact of intestinal dysbiosis on lung health and the significance of immunological regulatory networks in the lung. |
| 12. | GM’s effect on mental health: The gut-brain axis | Review Article | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5641835/ | 2017 | The article examines the gut-brain axis pathway and how it affects mental health, with a focus on fecal microbial indicators and psychobiological issues in obese people. It also delves into the gut microbiome’s role in psoriasis and its immunomodulatory effects, which may impact skeletal homeostasis and overall health. |
| 13. | Aspects of GM and immune system interactions in infectious diseases, immunopathology, and cancer | Review Article | https://pubmed.ncbi.nlm.nih.gov/30158926/ | 2018 | The microbiota found in the human gut is essential for immunological and overall health. Dysbiosis is linked to cancer, autoimmune diseases, inflammation, and infections. The article looks at probiotics as a way to rebalance, especially in cases of colon cancer, and examines other factors, such as nutrition and antibiotics. |
| 14. | The gut microbiome as a major regulator of the gut-skin axis | Review Article | https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2018.01459/full | 2018 | The relationship between GM and skin is underlined, as is their bidirectional impact on skin homeostasis and disorders such as acne and psoriasis. This article investigates the gut microbiome’s involvement in influencing immunological responses and the possible therapeutic effects of probiotic therapy. It also covers age-related alterations in cutaneous fibroblasts and matrix metalloproteinases. |
| 15. | The GM in the first decade of life | Review Article | https://www.cell.com/trends/microbiology/fulltext/S0966-842X(19)30214-8 | 2019 | Understanding the GM in children’s first decade is critical, particularly for pre-school and primary school-aged children up to the age of twelve. By the age of three, the infant microbiota matches that of adults, but discrepancies can have long-term consequences. |
| 16. | Functional role of probiotics and prebiotics on skin health and disease | Review Article | https://www.mdpi.com/2311-5637/5/2/41 | 2019 | The review article explores the functional roles of probiotics and prebiotics in promoting skin health and managing skin diseases. It discusses their mechanisms of action, potential benefits, and clinical implications based on existing research in the field. |
| 17. | Effects of prebiotics on skin conditions such as psoriasis | Research Trial | https://doi.org/10.21203/rs.3.rs-45348/v1 | 2020 | This paper suggests that probiotic supplementation for 8 weeks yielded positive effects on psoriasis symptoms, indicating the potential for gut microbiome modulation in managing skin conditions. |
| 18. | Crosstalk between GM and innate immunity and its implication in autoimmune diseases | Review Article | https://pubmed.ncbi.nlm.nih.gov/32153586/ | 2020 | The strong connection between GM and the innate immunity of human beings is explored in this study. This also contributes to the conversation between development of autoimmune disorders and its relation to presence of innate immunity related to the gut microbiome. |
| 19. | The gut microbiome alterations in allergic and inflammatory skin diseases-an update | Review Article | https://pubmed.ncbi.nlm.nih.gov/31520544/ | 2020 | The human microbiome, especially in the gut, is pivotal for immune balance, impacting overall health and contributing to conditions like allergic and inflammatory skin diseases. |
| 20. | How MHCII signaling promote benign host microbiota interaction | Review Article | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7323949/ | 2020 | Mucosal IgA deficiency, specific MHCII alleles, and hyper-polymorphic MHCII molecules impact gastrointestinal inflammation, suggesting potential disease biomarkers and therapeutic strategies. |
| 21. | Microbial community of human skin and its role vitiligo disease | Review Article | https://biomedgrid.com/fulltext/volume12/microbial-community-of-human-skin-and-its-role-vitiligo-disease.001705.php | 2021 | The skin’s microbiome forms a protective biofilm for maintaining skin health. However, dysbiosis, often linked to gut microbiome imbalance, can lead to skin conditions like vitiligo through immune dysregulation and melanocyte destruction. |
| 22. | Role of sex hormones and gender in the GM | Review Article | https://doi.org/10.5056/jnm20208 | 2021 | Sex hormones drastically affect the gut microbiome. The changes in sex hormones depending on the age, inflammatory conditions, medication, and probiotics also affect the gut micro biome. |
| 23. | Gut–skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions | Review Article | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7916842/ | 2021 | The microbiomes of the skin and gut influence conditions such as dermatitis, psoriasis, acne, dandruff, and cancer, in emphasizing the necessity |
| 24. | The role of SCFAs in the interplay between GM and diet in cardio-metabolic health | Review Article | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8007165/ | 2021 | The GM substantially impacts cardiometabolic health, with nutrition playing an important role. Diets high in fiber and omega-3 fatty acids (SCFAs) boost gut barrier integrity, glucose and lipid metabolism, immune system modulation, inflammatory response, and blood pressure, all of which help cardio-metabolic health. |
| 25. | The role of gut microbiome in inflammatory skin disorders: A systematic review | Review Article | https://pubmed.ncbi.nlm.nih.gov/35371420/ | 2021 | The review examines how changes in the gut microbiome may impact inflammatory skin disorders like eczema and psoriasis, suggesting potential implications for treatment strategies focusing on the gut-skin connection. |
| 26. | Rosacea, germs, and bowels: A review on gastrointestinal comorbidities and gut-skin axis of rosacea | Review Article | https://pubmed.ncbi.nlm.nih.gov/33507499/ | 2021 | The review explores how gastrointestinal conditions and GM may influence rosacea, focusing on the gut-skin axis and its implications for treatment approaches. |
| 27. | The potential relevance of the microbiome to hair physiology and regeneration: the emerging role of metagenomics | Review Article | https://pubmed.ncbi.nlm.nih.gov/33652789/ | 2021 | The review explores how the scalp microbiome may affect hair health and growth, highlighting the role of metagenomics in understanding these interactions and potential implications for hair care. |
| 28. | Analysis of matched skin and gut microbiome of patients with vitiligo reveals deep skin dysbiosis: link with mitochondrial and immune changes | Review Article | https://pubmed.ncbi.nlm.nih.gov/33771527/ | 2021 | The review examines the skin and gut microbiomes of vitiligo patients, highlighting profound dysbiosis in the skin microbiome and discussing its potential connections with mitochondrial function and immune responses in the disease. |
| 29. | Gut-skin axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions | Review Article | https://doi.org/10.3390/microorganisms9020353 | 2021 | The article outlines recent research on gut microbiome dysbiosis, nutritional linkages and their interaction with skin disorders. It also elaborates on a list of prevalent skin conditions associated with dysbiosis in the skin microbiome. |
| 30. | Effects of CFS from probiotics on skin | Research trial | https://doi.org/10.3390/fermentation8070332 | 2022 | The study confirmed that cell-free supernatants from probiotics, specifically L. salivariusMG242 and L. fermentumMG901, improved skin moisture, barrier function, and exhibited antimicrobial effects against S. aureus and E. coli. |
| 31. | Gut microbiome and aging | Review Article | https://doi.org/10.1038/s41575-022-00605-x | 2022 | Age-related changes in the gut microbiome influence health outcomes, with potential for targeted interventions to restore healthier aging-associated microorganisms through personalized approaches, including dietary modifications and microbial restoration. |
| 32. | Impact of Gut micro biome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases | Review Article | https://doi.org/10.1080/19490976.2022.2096995 | 2022 | The gut skin axis has a strong immune based cross link, which may contribute to development on skin diseases when the gut microbiome is altered. We also see the effect of drugs on alteration on gut microbiome leading to skin diseases. |
| 33. | Issues for patchy tissues: defining roles for GALT in neurodevelopment and disease | Review Article | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10033573/ | 2022 | The article looks into the link between neurodevelopmental conditions like autism and gastrointestinal inflammation, focusing on the role of GALT in regulating gastrointestinal function and the microbiota-gut-brain axis. It also compares its structure and function in humans and mice. |
| 34. | Current status and future therapeutic options for FMT | Review Article | https://pubmed.ncbi.nlm.nih.gov/35056392/ | 2022 | The review discusses the current use and future potential of FMT as a therapeutic treatment, covering its effectiveness and safety across different medical conditions. |
| 35. | Gut dysbiosis and FMT in autoimmune diseases | Review Article | https://pubmed.ncbi.nlm.nih.gov/36142642/ | 2022 | The review examines how gut dysbiosis relates to autoimmune diseases and discusses the potential of FMT as a treatment option to restore microbiota balance and potentially influence disease outcomes. |
| 36. | Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe AD | Review Article | https://pubmed.ncbi.nlm.nih.gov/34931478/ | 2022 | This article studies the use of FMT to treat AD. Results indicate that FMT may help improve symptoms in adults with moderate-to-severe AD. |
| 37. | Exploring the relationship between the GM and aging: A possible age modulator | Review Article | https://pubmed.ncbi.nlm.nih.gov/37239571/ | 2023 | The review investigates how GM may influence aging, proposing it as a potential modulator of age-related changes and highlighting implications for future research in aging and health. |
| 38. | The role of probiotics in skin health and related gut-skin axis: A review | Review Article | https://pubmed.ncbi.nlm.nih.gov/37513540/ | 2023 | The review article examines the role of probiotics in promoting skin health through the gut-skin axis. It discusses how probiotics influence skin conditions and overall health by modulating GM, immune responses, and inflammation. |
| 39. | Postbiotics in human health: a narrative review | Review Article | https://pubmed.ncbi.nlm.nih.gov/36678162/ | 2023 | The review explores the health benefits of postbiotics, focusing on their therapeutic effects and mechanisms, including immune modulation and gut health improvement. |
| 40. | Probiotics and postbiotics play a role in maintaining dermal health | Review Article | https://pubmed.ncbi.nlm.nih.gov/37051640/ | 2023 | The review article discusses the roles of probiotics and postbiotics in preserving skin health. It explores their mechanisms and benefits in maintaining dermal health, highlighting their potential therapeutic applications based on current research. |
| 41. | FMT affects the recovery of AD-skin lesions and enhances GM homeostasis | Review Article | https://pubmed.ncbi.nlm.nih.gov/36924566/ | 2023 | This study investigates the effects of FMT on AD in mice. FMT improved skin lesions and balanced GM, suggesting it could be beneficial for AD treatment. |
| 42. | Role of nutribiotics in skin care | Review Article | https://www.mdpi.com/2076-3417/14/8/3505#:~:text=Clinical%20studies%20show%20that%20nutribiotics,%20barrier%20and%20promoting%20wound%20healing. | 2024 | The review explores how nutribiotics, such as vitamins and antioxidants, benefit skin health by strengthening the skin barrier and aiding in wound healing, supported by clinical evidence. |
| 43. | FMT for the treatment of generalized eczema occurring after COVID-19 vaccination | Case Report | https://pubmed.ncbi.nlm.nih.gov/38292322/ | 2024 | The study reports using FMT to treat eczema that appeared after COVID-19 vaccination, detailing the patient’s response to the treatment. |
GM: Gut microbiota, GALT: Gut-associated lymphoid tissue, IMIDs: Immune-mediated inflammatory disorders, FMT: Fecal microbiota transplantation, SCFAs: Short-chain fatty acids, AD: Atopic dermatitis, IgA: immunoglobulin A, L. salivarius: Ligilactobacillus salivarius, L. fermentum: Limosilactobacillus fermentum, S. aureus: Staphylococcus aureus, E. coli: Escherichia coli, CFS: Cell-free supernatant.
DISCUSSION
Prevalence
The gut microbiome is a major contributor to age-related health in all age groups. The gut microbiome relays environmental signals and indicates the risks of disease – these changes along with host age.[4]
As individuals age, they undergo physical and intellectual changes, often referred to as “healthy aging.” This process is influenced by genetic, environmental, and lifestyle changes. The gut microbiome, which changes reciprocally with age, is influenced by factors such as physiological deterioration, lifestyle changes, medication, and diet.[4]
The GM develops with the host from birth, crucial for long-term health by regulating immune system development, nutrient absorption, metabolism, and preventing pathogen colonization. Changes in its composition are linked to health issues such as obesity, asthma, metabolic syndromes, and chronic inflammatory diseases.[5]
During childhood, we see less diversity in microbial species than in adults, but the intestinal microbiota develops and slowly changes, with decreases in the numbers of aerobes and facultative anaerobes such as Bifidobacterium and Clostridium genera, as well as concurrent increases in anaerobic species.[6] Moreover, diet, sleep patterns, stress, alcohol and drug usage, and prescription antibiotic use all affect the GM.[6]
Studies suggest that GM can have a long-term impact on brain stress circuitry during a small window of life, up to adolescence. This is particularly intriguing as dysregulation of stress-responsive brain circuitry is linked to common psychiatric illnesses in adolescence, suggesting that GM changes can affect these circuits.[6]
Sex, beyond age, significantly divides the gut microbiome. Males and females differ anatomically, physiologically, and behaviorally, influenced by genetic, hormonal, and epigenetic factors across life stages. The gut microbiome helps maintain homeostasis and further distinguishes between the sexes.[7]
The nutritional and energetic demands vary in males and females, which requires the maintenance of the different gut microbiomes through physiological and behavioral adaptations.[7] Gender and sex hormones play a role in this after puberty. The bacteria-to-human cell ratio is higher in females than in males (1:3 in males and 2:2 in females).[8] Tiny alterations in a single bacterial species within the gut microbiome can cause skin inflammation. These may lead to acne, alopecia areata, atopic dermatitis, and other skin diseases.[9]
Pathophysiology
Immunological components that are present between the gut and the skin facilitate communication between the two organ systems.[9] The microbiota and the immune system are vital for preserving health. Commensal bacteria in the gut and on the skin influence immune responses, maintaining barrier immunity and host homeostasis while reducing inflammation and microbial translocation.[10] The mucosal firewall, consisting of gut epithelial cells, mucous layer, T-cells, immunoglobulin A (IgA), and dendritic cells, prevents commensal bacteria from entering gut-associated lymphoid tissues (GALTs), which act as a defense barrier between the host and the environment.[11] GALT development is influenced by GM, including intestinal crypt cells, Peyer’s patches, isolated lymphoid follicles, appendix, and mesenteric lymph nodes, governed by the interaction between gut microbes and lymphoid tissue inducer cells.[12] The complex relationship between the stomach and skin, which affects general homeostasis, is described by the gut-skin axis [Figure 1].[13] The immune system is influenced by the GM, which controls both local and systemic inflammation.[14] Microbial communities convert complex polysaccharides into vitamins and short-chain fatty acids (SCFAs), such as butyrate, to improve the intestinal barrier and preserve its integrity.[15] Gut mucosal defense, led by GALT’s innate immune cells, stops microbial relocation. Pathogenic microbe translocation is restricted by AMPs, macrophages, and CD103+ CD11b+ DCs, which present commensal antigens for differentiation.[16] Consequently, this preserves the integrity of the intestinal barrier. Controlling inflammatory responses to gut microorganisms is one of IgA’s functions.[17] Lymphocytes that are specific to certain commensal bacteria gather in the lamina propria and Peyer’s patches, modifying the gut microbial profile in the direction of homeostasis.[9] Further research is necessary to fully understand the relationship between skin health and the immunological responses triggered by the gut microbiome.[18]
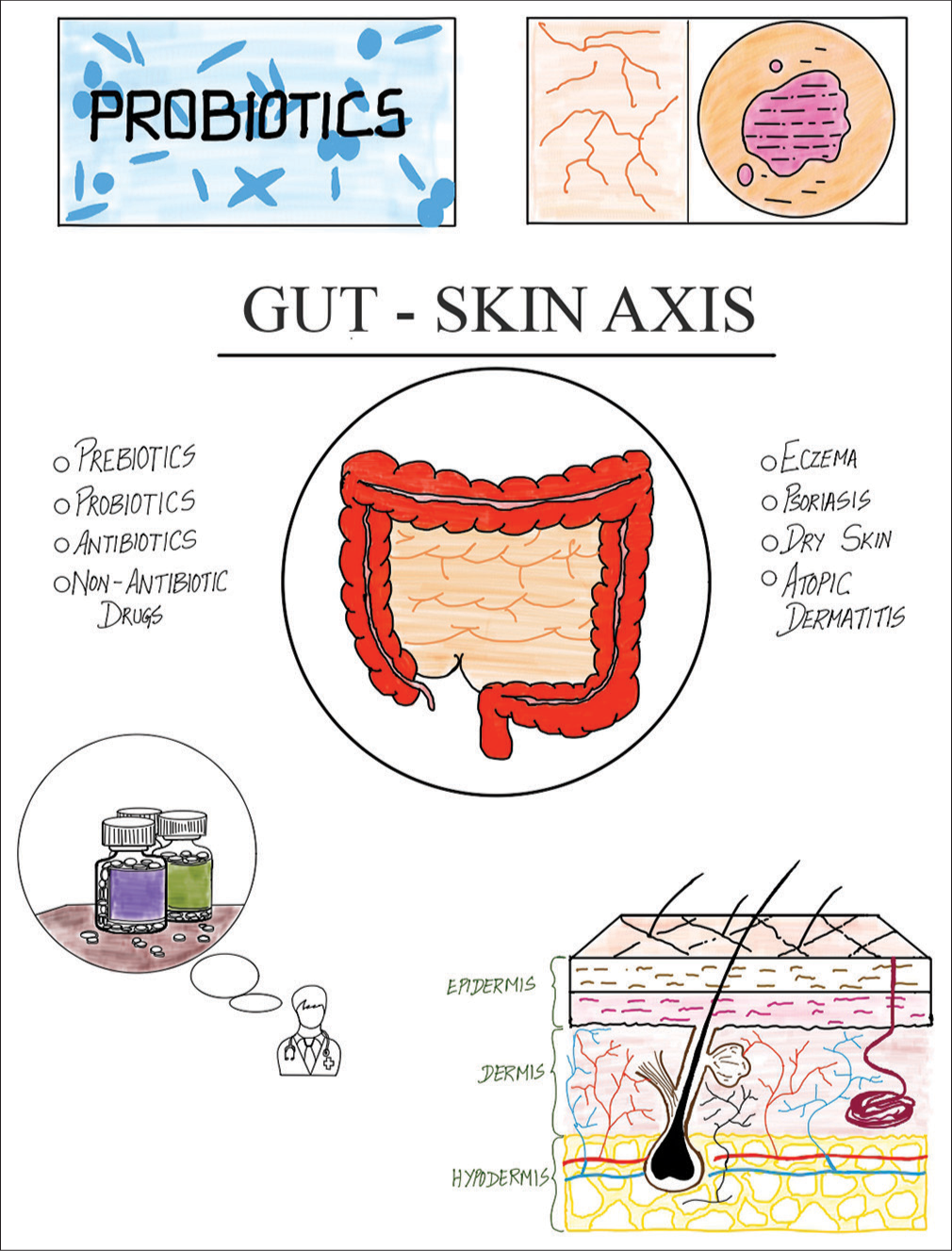
- Overview of the gut-skin axis illustrated by Thangamuni A. and Mohan J.
ROLE OF THE GUT MICROBIOME IN SKIN HOMEOSTASIS
Skin balance is crucial for protection, temperature regulation, and moisture retention. Kernelization, the turnover of skin cells, is essential for this balance. Genetic activities control this process, resulting in a keratinized cell structure held together by lipid layers. This keeps the skin barrier intact, prevents moisture loss, and shields against external substances.[19] Specific gut bacteria and their metabolites, including retinoic acid, Bacteroides fragilis polysaccharide A, Faecalibacterium prausnitzii, and certain bacteria from Clostridium clusters IV and XI, promote the growth of regulatory T-cells. These T-cells, a type of lymphocyte, play a crucial role in preventing inflammation.[20] Segmented filamentous bacteria spur proinflammatory Th17 and Th1 cells. SCFAs, like butyrate, curb immune responses by limiting inflammatory cell functions and regulating histone deacetylase and nuclear factor-kappa B signaling pathways. This promotes the proliferation of regulatory cells essential for skin health, including hair follicle stem cell regulation and wound healing.[21] Moreover, recent findings suggest that the gut microbiome could have a more direct influence on skin health, disease, and immune function by facilitating the spread of gut bacteria and their byproducts to the skin.[22] The gut and skin microbiomes are interconnected, with SCFAs like propionate, acetate, and butyrate playing a crucial role in influencing skin microbiome composition and immune defenses. Propionibacterium, a genus producing SCFAs, has potent antimicrobial properties against USA300, a common methicillin-resistant Staphylococcus aureus strain.[21]
Acne vulgaris
Acne vulgaris is a common facial skin condition that significantly impacts appearance and self-confidence, characterized by comedones, papules, pustules, and nodules. Its development involves excessive sebum production, disturbances in the skin microbiome, and activation of immune responses by Propionibacterium acnes bacteria. Studies suggest acne patients often show changes in GM, such as decreased Firmicutes and increased Bacteroidetes, influenced by dietary factors like high glycemic index foods. However, conflicting findings regarding Firmicutes levels before acne treatments highlight the complex relationship between gut microbes and acne pathogenesis.[23]
Rosacea
Rosacea, a common chronic inflammatory condition primarily affecting the central face, presents with symptoms such as flushing, persistent redness, papules, pustules, telangiectasia, and sensations of burning or stinging, along with dryness, rough texture, facial swelling, phymatous changes, and eye irritation.[24]
It is associated with various gastrointestinal conditions, influenced by genetics, microbiota, and immune responses. Genetic predisposition may link rosacea with gastrointestinal diseases. Changes in microbiota and immune function play key roles in this relationship, impacting both skin and systemic health. Dysbiosis and small intestinal bacterial overgrowth (SIBO) disrupt immune responses by altering SCFAs and intestinal microbes, potentially affecting skin microbiota and immune responses.[24]
While evidence is still inconclusive, treatments targeting SIBO and Helicobacter pylori have shown promise in managing rosacea effectively in some studies. Recognizing gastrointestinal issues in rosacea patients suggests new treatment approaches, given the limitations of conventional therapies prone to frequent relapses.[24]
Hair disorders
Human skin and hair follicles harbor diverse microbial communities that are essential for regulating immune responses and maintaining skin health. These microbes interact closely with immune cells, influencing the balance within the skin and its inflammatory responses.[25] Changes in the microbiome of hair follicles have been associated with various inflammatory skin conditions, indicating their potential role in the development and progression of these diseases. While the hair follicle infundibulum is a site of active immune interaction, the bulb and bulge regions are considered immune-privileged areas critical for hair growth. Disruption of this immune privilege can lead to inflammatory hair disorders such as alopecia areata and cicatricial alopecia, which are characterized by inflammation and damage to the follicles.[25]
Aging
A recent focus on the GM, aging, and longevity highlights its crucial role in how we age. Factors influencing GM diversity include biological factors, habits, environment, and age itself. Differences in GM composition between centenarians and younger older adults suggest GM is a promising target for strategies that slow down aging.[26]
Studies have looked into interventions such as probiotics, prebiotics, symbiotics, physical activity, and the Mediterranean diet to change GM and potentially delay aging and its problems.[26] These interventions improve colon health, immune function, lipid profile, cognition, muscle health, and reduce inflammation, oxidative stress, and harmful bacteria. The microbiota’s ability to slow aging is mainly due to their anti-inflammatory and antioxidant effects. More research is needed to develop effective ways to promote healthy aging and longer life through managing GM.[26]
Atopic dermatitis (AD)
The AD is a persistent rash that often initiates the atopic march. Current treatments focus on managing symptoms rather than curing the condition due to its chronic nature. The appearance of AD varies depending on disease stage, patient age, skin color, and infections, presenting as blisters (acute), thickened skin (chronic), or a combination (subacute). Infants typically experience lesions on the face, neck, and limbs’ extensor surfaces, while children and adults commonly have them on flexor surfaces.[23]
Although AD primarily affects the skin, its pathology involves complex immunological changes affecting organs such as the gut and lungs. Dysbiosis of the microbiota is linked to AD, with evidence suggesting that GM play a crucial role in regulating immune responses and influencing allergic diseases. Reduced microbial diversity early in life may hinder immune development, favoring Th2-driven allergic responses. Increased levels of certain gut bacteria, such as Bacteroides spp., are associated with food allergies and other atopic conditions, potentially triggering inflammation and altering gut permeability.[23]
Vitiligo
Researchers investigated the skin and GM in vitiligo patients compared to healthy controls. Results showed reduced microbial diversity in the stools of vitiligo patients, with significant differences in microbiota composition between lesional and non-lesional skin areas.[27] Lesional skin biopsies reveal depletion of protective bacteria and enrichment of pathogenic species, with elevated mitochondrial deoxyribonucleic acid levels and immune markers in the blood, suggesting a link between skin dysbiosis, mitochondrial damage, and immune dysfunction in vitiligo, highlighting microbiota as potential targets for therapeutic interventions.[27]
Ill effects of having an unhealthy gut environment and how it affects general health and skin in particular
There are many adverse physical effects of having an unhealthy gut. These include digestive symptoms such as gas, bloating, constipation, diarrhea, and heartburn. Increased infections such as the common cold, as well as sleep disturbances and fatigue, are also seen in a person with an unhealthy gut.[28]
The gut microbiome plays an important role in a wide array of skin disorders. It is an essential immune system regulator. Dysbiosis in the gut is associated with an altered immune response, allowing the development of many skin diseases such as AD, psoriasis, acne vulgaris, dandruff, and even skin cancer.[13]
Gastrointestinal disorders can present with dermatological skin findings. Inflammatory bowel disease (IBD) is linked to skin manifestations such as pyoderma gangrenosum, and erythema nodosum. Celiac disease is associated with skin manifestations such as dermatitis herpetiformis, alopecia, vitiligo, and oral mucosal lesions. Psoriasis is more commonly found in patients with Crohn’s disease than in healthy people.[29]
Not only does an unhealthy gut affect physical well-being but also the mental state of a person. High stress, low mood, and anxiety can be observed due to this.[30]
Research in humans has also shown a link between the bacteria in the gut and obesity. When there is less variety in the types of bacteria found in fecal samples from overweight or obese individuals, it is connected to increased body fat, cholesterol problems, difficulties in controlling blood sugar levels, and higher levels of mild inflammation.[31]
ROLE OF PREBIOTICS, PROBIOTICS, AND POSTBIOTICS IN SKIN DISORDERS
Prebiotics
Prebiotics are fermented components that enhance GM composition and activity for the host. They have low-dosage action, no side effects, and remain in the intestines. Common prebiotics include oligosaccharides (such as glycans), fructans (inulin-type), sugar alcohols, and complex polysaccharides. They aid in the treatment of disorders such as acute contact dermatitis (ACD), acne, and photoaging by stimulating probiotic development.[32]
Probiotics
Specific probiotic bacteria have been demonstrated to prevent ACD and moderate its symptoms. They appear to do so by altering several biological processes not only in ACD but also in a variety of other skin diseases (e.g., acne, psoriasis, photoaging, and wounds).[32]
The skincare sector has observed a rise in topical products containing Lactobacillus bulgaricus to treat acne and seborrhea. However, other concerns persist, such as manufacturers using antiseptics to restrict microbial growth, which may impair the survival of probiotic strains and modify the microbiota of receptors because external goods cannot be made under sterile conditions.[33]
The different effects of probiotics on the skin
Skin whitening
Probiotics inhibit tyrosinase, which reduces melanin and brightens skin. Strains such as Bifidobacterium and Lactobacillus do this through antioxidant activity.[33]
Skin moisturization
Probiotics increase hydration by decreasing water loss and strengthening the skin barrier. Lactobacillus strains increase ceramide and hyaluronic acid levels.[33]
Skin barrier integrity
Lactobacillus rhamnosus improves the epidermal barrier by up-regulating essential proteins, resulting in improved structure and decreased permeability.[33]
Anti-aging
Probiotics slow cell death and extend the cell cycle. Strains such as Sphingomonas and Lactobacillus plantarum defend against aging and ultraviolet damage.[33]
Anti-wrinkle
Probiotics reduce wrinkles by blocking matrix metalloproteinase-1, which slows collagen degradation. Lactobacillus strains preserve skin suppleness through antioxidant activity.[33]
Combining probiotics and prebiotics such as Bifidobacterium and Glucooligosaccharides can reduce transepidermal water loss and prevent erythema, treating skin issues such as AD, eczema, and photoaging. Probiotics produce metabolites like sodium butyrate for psoriasis, while postbiotics like SCFA have anti-inflammatory properties. Skin diseases such as AD, psoriasis, acne, and rosacea are linked to gut dysbiosis through the gut-skin axis.[32]
Prebiotics, probiotics, and synbiotics can prevent and treat inflammatory skin conditions such as AD and acne. Oral probiotics can affect the gut flora, aiding in skin diseases such as AD, acne, and rosacea. Probiotic use during pregnancy and early childhood can reduce AD severity. Probiotics have also been studied for the treatment of allergy conditions.[34]
Postbiotics
“Postbiotic” refers to the “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host.”[35] Typically, the forms could be a diverse range of cellular structures and metabolites, including teichoic acids, exopolysaccharides, peptidoglycan, bacteriocins, and so on. Postbiotics’ efficacy is driven by three underlying mechanisms: Pathogen protection, epithelial barrier enhancement, and inflammatory and immune response control.[35] They are currently used not only in the fermented food sector but also as a promising therapy method for a variety of health diseases, including gastrointestinal disorders such as bloating and diarrhea.[35]
Advantages of postbiotics over probiotics
The use of non-viable postbiotics as a safer alternative has grown in favor as safety concerns about the use of live strains have emerged in specific patient populations, such as immunodeficient people, babies, and vulnerable patients. They could greatly lower the consumer risk of microbial transmission and infection.[35]
Postbiotics, unlike probiotics, contain no living organisms. Postbiotics-based products would be long-lasting and incredibly simple to standardize, making them easier to store, have a longer shelf life, and improve logistics in adverse environmental conditions.[35]
Moreover, probiotics and postbiotics have been shown to boost the immune system, increase the creation of skin barrier components, and reduce skin inflammation, all of which are essential for maintaining healthy skin.[36]
ROLE OF FECAL MICROBIOTA TRANSPLANTATION (FMT) IN SKIN DISORDERS
FMT involves introducing a donor’s fecal matter into a recipient’s intestines to modify their microbial composition and provide health benefits.[37] It is used to treat Clostridium difficile infection, IBD metabolic syndrome, obesity, and other conditions. However, C. difficile infection is the only condition for which FMT is officially approved.[37-39]
The process starts with selecting a donor without a family history of autoimmune, metabolic, or malignant diseases and screening them for pathogens. The feces are then mixed with water or saline and filtered to remove particulate matter. The resulting solution can be administered through a nasogastric tube, nasojejunal tube, esophagogastroduodenoscopy, colonoscopy, or retention enema. Targeting GM to modulate autoimmune disorders can involve antibiotics, probiotics, prebiotics, synbiotics, or FMT.[37]
A 2023 study found that a 78-year-old male with generalized eczema experienced recurrent flare-ups after receiving the COVID-19 vaccine, but after three FMT sessions, he had no further flare-ups.[40] FMT has also been shown to aid in skin lesion recovery, GM homeostasis, and skin epidermal thickness reduction.[41]
Both the skin and intestine serve as crucial immunological barriers and play a role in immune regulation. The intestinal microbiota influences the skin through their effect on systemic immunity, suggesting that altering GM could improve skin conditions.[42]
CONCLUSION
Wrapping up the discussion, the gut-skin axis is crucial in preserving the health of the skin. Supplementing with probiotics has shown the potential to enhance the quality of various skin conditions by promoting gut health. The importance of a robust and healthy GM early in life is highlighted by age-related alterations in the gut microbiome. Understanding and harnessing this gut-skin connection aids in discovering and establishing innovative skincare approaches that promote total well-being.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Microbial community of human skin and its role vitiligo disease. Am J Biomed Sci Res. 2021;12:16.
- [CrossRef] [Google Scholar]
- Skin-gut axis in psoriasis setting and plan for action: The effect of probiotics supplementation on clinical outcomes, metabolic endotoxemia, inflammation, and cardiovascular risk in patients with psoriasis. Research Square 2020 Available from: https://www.researchsquare.com/article/rs-45348/v1 [Last accessed on 2024 May 31]
- [CrossRef] [Google Scholar]
- Improvements in human keratinocytes and antimicrobial effect mediated by cell-free supernatants derived from probiotics. Fermentation. 2022;8:332.
- [CrossRef] [Google Scholar]
- The gut microbiome as a modulator of healthy ageing. Nat Rev Gastroenterol Hepatol. 2022;19:565-84.
- [CrossRef] [PubMed] [Google Scholar]
- The gut microbiota in the first decade of life. Trends Microbiol. 2019;27:997-1010.
- [CrossRef] [PubMed] [Google Scholar]
- Reframing the teenage wasteland: Adolescent microbiota-gut-brain axis. Can J Psychiatry. 2016;61:214-21.
- [CrossRef] [PubMed] [Google Scholar]
- Sex differences in the gut microbiome-brain axis across the lifespan. Philos Trans R Soc B Biol Sci. 2016;371:20150122.
- [CrossRef] [PubMed] [Google Scholar]
- Roles of sex hormones and gender in the gut microbiota. J Neurogastroenterol Motil. 2021;27:314-25.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995.
- [CrossRef] [PubMed] [Google Scholar]
- Role of the microbiota in immunity and inflammation. Cell. 2014;157:121-41.
- [CrossRef] [PubMed] [Google Scholar]
- Crosstalk between gut microbiota and innate immunity and its implication in autoimmune diseases. Front Immunol. 2020;11:282.
- [CrossRef] [PubMed] [Google Scholar]
- Issues for patchy tissues: Defining roles for gut-associated lymphoid tissue in neurodevelopment and disease. J Neural Transm (Vienna). 2023;130:269-80.
- [CrossRef] [PubMed] [Google Scholar]
- Gut-skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021;9:353.
- [CrossRef] [PubMed] [Google Scholar]
- The gut microbiome alterations in allergic and inflammatory skin diseases-an update. J Eur Acad Dermatol Venereol. 2019;34:455-64.
- [CrossRef] [PubMed] [Google Scholar]
- The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021;13:1897212.
- [CrossRef] [PubMed] [Google Scholar]
- Homeostatic immunity and the microbiota. Immunity. 2017;46:562-76.
- [CrossRef] [PubMed] [Google Scholar]
- How MHCII signaling promotes benign host-microbiota interactions. PLOS Pathog. 2020;16:e1008558.
- [CrossRef] [PubMed] [Google Scholar]
- Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol. 2018;9:1830.
- [CrossRef] [PubMed] [Google Scholar]
- The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
- [CrossRef] [PubMed] [Google Scholar]
- The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol. 2016;7:1081.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol. 2015;6:1085.
- [CrossRef] [PubMed] [Google Scholar]
- The gut-skin axis in health and disease: A paradigm with therapeutic implications. BioEssays. 2016;38:1167-76.
- [CrossRef] [PubMed] [Google Scholar]
- The role of gut microbiome in inflammatory skin disorders: A systematic review. Dermatol Reports. 2021;14:9188.
- [CrossRef] [PubMed] [Google Scholar]
- Rosacea, germs, and bowels: A review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-24.
- [CrossRef] [PubMed] [Google Scholar]
- The potential relevance of the microbiome to hair physiology and regeneration: The emerging role of metagenomics. Biomedicines. 2021;9:236.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring the relationship between the gut microbiota and ageing: A possible age modulator. Int J Environ Res Public Health. 2023;20:5845.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of matched skin and gut microbiome of patients with vitiligo reveals deep skin dysbiosis: Link with mitochondrial and immune changes. J Invest Dermatol. 2021;141:2280-90.
- [CrossRef] [PubMed] [Google Scholar]
- Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16:7493-519.
- [CrossRef] [PubMed] [Google Scholar]
- Skin-gut axis: The relationship between intestinal bacteria and skin health. World J Dermatol. 2017;6:52-8.
- [CrossRef] [Google Scholar]
- Gut microbiota's effect on mental health: The gut-brain axis. Clin Pract. 2017;7:987.
- [CrossRef] [PubMed] [Google Scholar]
- The gut microbiome and its role in obesity. Nutr Today. 2016;51:167-74.
- [CrossRef] [PubMed] [Google Scholar]
- Functional role of probiotics and prebiotics on skin health and disease. Fermentation. 2019;5:41.
- [CrossRef] [Google Scholar]
- The role of probiotics in skin health and related gut-skin axis: A review. Nutrients. 2023;15:3123.
- [CrossRef] [PubMed] [Google Scholar]
- Postbiotics in human health: A narrative review. Nutrients. 2023;15:291.
- [CrossRef] [PubMed] [Google Scholar]
- Probiotics and postbiotics play a role in maintaining dermal health. Food Funct. 2023;14:3966-81.
- [CrossRef] [PubMed] [Google Scholar]
- Fecal microbiota transplantation: In perspective. Therap Adv Gastroenterol. 2016;9:229-39.
- [CrossRef] [PubMed] [Google Scholar]
- Current status and future therapeutic options for fecal microbiota transplantation. Medicina. 2022;58:84.
- [CrossRef] [PubMed] [Google Scholar]
- Gut dysbiosis and fecal microbiota transplantation in autoimmune diseases. Int J Mol Sci. 2022;23:10729.
- [CrossRef] [PubMed] [Google Scholar]
- Case report: Fecal microbiota transplantation for the treatment of generalized eczema occurring after COVID-19 vaccination. Clin Cosmet Investig Dermatol. 2024;17:229-35.
- [CrossRef] [PubMed] [Google Scholar]
- Fecal microbiota transplantation affects the recovery of AD-skin lesions and enhances gut microbiota homeostasis. Int Immunopharmacol. 2023;118:110005.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe atopic dermatitis. Immun Inflamm Dis. 2022;10:e570.
- [CrossRef] [PubMed] [Google Scholar]







