Translate this page into:
An open-label, single-arm clinical study to evaluate efficacy and safety of Nyumi radiant skin gummies in healthy adult subjects with fine lines/wrinkles, hyperpigmentary spots, and under eyes dark circles

*Corresponding author: Ananya Agarwal, Department of Research and Development, Ikaria Wellness Pvt. Ltd., Mumbai, Maharashtra, India. ananya@nyumi.com
-
Received: ,
Accepted: ,
How to cite this article: Karmase A, Agarwal A, Patel B, Joshi P, Sethi S, Shrivastava A. An open-label, single-arm clinical study to evaluate efficacy and safety of Nyumi radiant skin gummies in healthy adult subjects with fine lines/wrinkles, hyperpigmentary spots, and under eyes dark circles. CosmoDerma. 2024;4:2. doi: 10.25259/CSDM_250_2023
Abstract
Objectives:
Flawless skin is certainly the most desired feature that plays a vital cosmetic role in human being. Apart from protecting our body from environmental factors, it also protects the body from water loss and infection-causing microorganisms. However, the skin condition changes due to various ageing factors. The present study was conducted to assess the safety and efficacy of multivitamin and nutraceutical ingredients-based gummies in adult human participants.
Material and Methods:
An open-label, single-arm clinical study was conducted on 36 enrolled healthy males and females (in ratio of 1:2) between 20 and 65 years of age to evaluate the efficacy and safety of the study product in healthy adult subjects with fine lines/wrinkles, hyperpigmentary spots, and under eyes dark circles. All eligible subjects consumed the test product for 75 days. They underwent clinical evaluation by dermatologist, instruments evaluation, and subjective evaluation.
Results:
Thirty two (n = 32) subjects completed all study visits. The study product, when consumed for 75 days, was found to be efficacious in reducing fine lines/wrinkles, under-eye dark circles, and pigmentation and also improving the overall skin condition as evidenced by dermatological, instrumental, and subjective assessments on day 25, day 50, and day 75. Regular consumption of gummies significantly reduced the appearance and severity of coarse wrinkling/lines and fine lines/wrinkles, improved skin laxity, reduced skin dryness by moisturizing the skin, lighten the pigmented area and under-eye dark circles by reducing the melanin concentration, increased the radiance/glow of the skin, improved the texture and firmness of the skin, reduced skin roughness, and also improved skin tone evenness. Moreover, no adverse event was recorded during the study conduction.
Conclusion:
The results of this clinical study suggest that consumption of two gummies together once daily for 75 days significantly improved skin radiance/glow, skin laxity, skin hydration, skin texture and firmness, skin tone evenness and reduced skin roughness, hence improving the overall skin condition. It was found to be efficacious in reducing the appearance and severity of coarse wrinkling/lines, fine lines/wrinkles, hyperpigmentary spots, and under-eye dark circles by reducing the melanin concentration as assessed by dermatologist, various instruments, and subjective assessment. The test product also proved to be safe based on no apparent or experienced discomfort, reactions or any kind of intolerance or adverse skin reactions or events during the trial.
Keywords
Nutraceuticals
Nyumi radiant skin gummies
Fine lines and wrinkles
Hyperpigmentary spots
Dark circles
INTRODUCTION
Skin is the largest organ that acts as a natural barrier protecting the body from the outer environment. Flawless skin is certainly the most universally desired human feature.[1] Apart from shielding our body from environmental factors, water loss, and infection-causing microorganisms, the skin also plays vital cosmetic role in human being.[2]
Some common skin conditions worldwide include acne, hyperpigmentation, dark complexion, skin dryness, poor skin radiance, fine lines, wrinkles, under-eye dark circles, eczema, etc. These skin conditions affect 1.9 billion people[3] and are the fourth-leading cause of the nonfatal disease burden across 188 low- and high-income countries.[4]
Skin hydration and moisturization both impact the quality of skin directly reflecting its appearance.[5] Skin radiance is also a vital aspect of a youthful, healthy-looking complexion. It can be determined by light reflectance from the surface of smooth skin. Skin radiance decreases with the age due to decreased epidermal cell turnover rate that results in deposition of dead keratinocytes in the outermost skin layer, i.e., stratum corneum. Therefore, the skin appearance becomes rough, dry, enlarged pores, and poor light reflexion.[1] Acne is a common complex condition involving hyperkeratinization, excessive sebum production, bacterial proliferation, and an inflammatory immune response.[6] Hyperpigmentation, in the form of post-inflammatory dark marks, melasma or solar lentigines, increases the perception of an aged appearance to a greater degree than wrinkles.[7] Subsurface pigmentation creates dull skin and a loss of radiance.
Aging is considered the consequence of both genetic and environmental influences. The former is characterized by wrinkling, loss of elasticity, laxity, and rough-textured appearance.[2] Intrinsic skin aging occurs as a result of chronological aging. Extrinsic skin aging, on the other hand, occurs due to lifestyle and environmental factors, viz., pollutants, sun exposure, stress, and inappropriate nutrient intake. The skin’s inherent anti-aging mechanisms diminish, defense mechanism weakens, critical processes slow down, and the rate of breakdown of key constituents increases with increasing age.[1]
The important components of an effective skincare routine include protection, prevention, cleansing, and moisturizing. Over the past three decades, the skincare industry has experienced a surge of new ingredients and delivery vehicles.[1] Oral supplements with added skin-care ingredients, are in great demand due to a variety of skin benefits. The mainspring of any therapy to improve skin condition is to achieve healthy, smooth, blemish-free, translucent, and resilient skin. Key active ingredients of the study product are well researched and have proven scientific background and efficacy. These gummies are formulated with multivitamin, minerals, and nutraceutical ingredients that improve the skin hydration, skin glow, texture, and skin firmness and reduces wrinkles, pigmentation, and dark circles. Through this study, we intend to study the cumulative effect of the test product on improvement in various skin parameters.
MATERIAL AND METHODS
Study design
An open-label, single-arm clinical study was conducted to evaluate the efficacy and safety of nutraceutical formulation in a gummy vehicle by oral route in healthy adult subjects with fine lines/wrinkles, hyperpigmentary spots, and under-eyes dark circles. The potential subjects were screened and enrolled in the study as per the inclusion and exclusion criteria only after obtaining written informed consent from the subjects. All eligible subjects consumed two gummies together once daily after dinner for 75 days. They underwent clinical evaluation by a dermatologist, instruments evaluation and subjective evaluation. Safety was assessed throughout the study by monitoring of adverse events (AEs).
Study consisted of total five visits
Visit 01: Screening visit (within 30 days before day 01)
Visit 02: Enrollment visit (day 01)
Visit 03: Evaluation phase (day 25 ± 02 days)
Visit 04: Evaluation phase (day 50 ± 02 days)
Visit 05: Evaluation phase and end of study (day 75 ± 02 days).
Study participants
Total 36 healthy males/non-pregnant and non-lactating females (in the ratio of 1:2) between 20 and 65 years (both inclusive at the time of consent) with fine lines/wrinkles, hyperpigmentary spots, and under-eyes dark circles were included in the study.
Ethics
All the ethical policies have been adhered to while conducting this clinical study. Prior approval on study protocol (C3B02405) and informed consent form (ICF) was taken from the Institutional Ethics Committee before initiating the study. The trial was registered with Clinical Trial Registry of India (CTRI) (CTRI/2022/08/045104), and approval was obtained before the commencement of the study. The study was explained to all potential subjects, and the clinical study was initiated only after ICFs were signed. Details of the test product being evaluated were explained by the investigator, which included possible hazards, allergies, and potential expected reactions.
This clinical study was conducted in accordance with The code of ethics of the world medical association (Declaration of Helsinki), the International Council for Harmonization of technical requirements for pharmaceuticals for human use-good clinical practice (E6 R2), and applicable ethical guidelines for experiments involving humans.
Inclusion criteria
Healthy male and non-pregnant/non-lactating female subjects of 20–65 years in the ratio of 1:2 were included in this study. Females of childbearing potential must had a negative urine pregnancy test performed during screening. Key inclusion criteria included a fine line/wrinkles score of “2” (A few number of discreet fine lines) or greater surrounding the crow’s feet area as per physician global assessment (PGA), at least two hyperpigmentary spots on the face and grade 2 and grade 3 under-eyes dark circles as determined by six-point grading scale. Subject generally in good health, not under any dermatologic treatment/prescribed medications, willing to consume test product throughout the study period as instructed, willing to abstain from using any cosmetic product on face besides the provided products during the entire study course, can understand and provide written informed consent to participate in the study, and willing and able to follow the study protocol to participate in the study were some of the other inclusion criteria.
Exclusion criteria
Pregnant or breastfeeding or planning pregnancy during the study period was excluded from the study. Subjects having any active dermatological skin diseases (e.g., psoriasis, atopic dermatitis, rosacea, etc.) that might interfere with clinical assessments with any pigmentary disorder including freckles and melisma; having excessive hair, moles, open wounds, cuts, abrasions, irritation symptoms, tattoos, scars, sunburn, or any dermatological condition on the test site(s) that can interfere with the reading; receiving topical or systemic treatments; receiving medications (e.g., steroids or anti-histamines), which would compromise the study; chronic illness, which may influence the cutaneous state; and history of diabetes, acute cardiac and circulatory diseases, human immunodeficiency virus, and hepatitis. Any known allergy or sensitivity to cosmetic products and/or any ingredients of the test product; unwilling to avoid the unprotected sun or other ultraviolet (UV) radiation exposure during the study period (use of tanning beds was not acceptable); subjects working in harsh condition or are exposed to sun and pollution for most of the day (e.g., laborers); using other marketed anti-acne/ anti-aging/skin lightening products during the study period or in the past six weeks; subjects with a history of drug and alcohol abuse; participating in other similar cosmetic or therapeutic trial within last four weeks were some other key exclusion criteria.
Test product
The test product (Nyumi radiant skin gummies of Ikaria Wellness Pvt. Ltd.) is formulated with a blend of Indian and Western ingredients having a unique combination of Hyaluronic acid (HA), Curcumin, Multivitamin (Vitamin A, C, D2, E), Selenium, Zinc, etc. Two test gummies together once daily after dinner were provided to all study participants for 75 days. Test product compliance was checked during all study visits.
Efficacy endpoints
Primary endpoint(s)
The primary efficacy endpoints were assessment of the test product at day 01 (before application), day 25, day 50, and day 75 on fine lines/wrinkles, skin dryness, coarse wrinkling/lines, and laxity by PGA using Griffiths Scale[8] by dermatologist; effect of test product on fine wrinkling, coarse wrinkling, and laxity using severity scoring[9-11] by dermatologist; effect of test product on skin pigment using nine-point scale[12] by dermatologist; effect of test product on under-eyes dark circles using six-point grading scale[13,14] by dermatologist; and effect of test product on skin pigmentation using Mexameter® MX 18 (Courage-Khazaka Electronic, Köln, Germany).
Secondary endpoint(s)
Secondary efficacy endpoints include assessment of the test product at day 01 (before application), day 25, day 50, and day 75 on uneven skin tone using Felix von Luschan skin color chart;[15] effect of test product on skin radiance using modified Griffiths scale[8] by dermatologist; effect of test product on skin moisturization using Corneometer® CM 825 (Courage-Khazaka Electronic, Köln, Germany); effect of test product on skin gloss using Skin-Glossymeter GL 200 (Courage-Khazaka Electronic, Köln, Germany); effect of test product on skin firmness using Cutometer dual MPA 580 (Courage-Khazaka Electronic, Köln, Germany); effect of test product on wrinkles of crow’s feet area, pigmentation, skin texture, and under-eyes dark circles using 3D analysis system (Miravex Limited, Dublin, Ireland); subject satisfaction questionnaire after product usage at day 25, day 50, and day 75; and product response index (perception about product) after product usage at day 25, day 50, and day 75.
Subjects were monitored throughout the study for undesirable/AEs either by self-reporting or at the time of every study visit.
Statistical analysis
All statistical tests of hypothesis employed a level of significance of 0.05. If P-value was observed less than or equal to 0.05, test was considered statistically significant otherwise non-significant. The statistical analysis was done using SAS® statistical software (Version: 9.4 or higher; SAS Institute Inc., USA). Continuous variables were summarized using tables of descriptive statistics (i.e., Mean, Standard Deviation, Median, Minimum, and Maximum). Categorical variables were summarized using counts and percentages. For continuous variables, the within-treatment analyses were conducted to compare baseline to post-treatment data using a paired t-test. For categorical variables, the within-treatment analysis was done to compare baseline to post-treatment analysis using Wilcoxon signed-rank test.
RESULTS
Subject disposition and demography
Among 36 enrolled subjects, there were 24 (66.67%) females and 12 (33.33%) males. Age of the subjects ranged between 22 and 58 years with an average being 38.9 (±8.78) years. Thirtytwo (n = 32) subjects completed all study visits, and their data was analyzed. Four (n = 4) subjects were discontinued from the study due to lost to follow up.
Dermatological assessments
Assessment by PGA using Griffiths scale
On consuming the test product for 75 days, dermatological assessment of coarse wrinkling/lines showed significant reduction in its appearance by 14.1% on day 25 (P = 0.0078), 20.0% on day 50 (P = 0.0107), and 17.2% on day 75 (P = 0.0039); assessment of fine lines/wrinkles showed significant reduction in its appearance by 19.8% on day 25 (P = 0.0002), 46.1% on day 50 (P < 0.0001), and 63.0% on day 75 (P < 0.0001); assessment of skin laxity (under both eyes) showed significant improvement by 45.3% on day 25, 50.0% on day 50, and 68.8% on day 75 (all P < 0.0001); and assessment of skin dryness showed significant reduction by 23.4% on day 25, 28.3% on day 50, and 93.8% on day 75 (all P < 0.0001), as compared to baseline [Table 1 and Figure 1].
| Parameters | Visit 02 (day 01) |
Visit 03 (day 25) |
Visit 04 (day 50) |
Visit 05 (day 75) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (SD) | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | |
| Mean value | ||||||||||
| Coarse wrinkling/lines | 1.2* (±0.42) | 1.0* (±0.18) | −0.3 | −14.1 | 0.9* (±0.40) | −0.3 | −20.0 | 0.9* (±0.25) | −0.3 | −17.2 |
| Fine line/wrinkles | 2.0* (±0.18) | 1.6* (±0.49) | −0.4 | −19.8 | 1.1* (±0.31) | −0.9 | −46.1 | 0.8* (±0.44) | −1.3 | −63.0 |
| Skin laxity (under both eyes) score |
2.0* (±0.18) | 1.1* (±0.25) | −0.9 | −45.3 | 1.0* (±0.18) | −1.0 | −50.0 | 0.6* (±0.50) | −1.4 | −68.8 |
| Skin dryness score | 1.5* (±0.51) | 1.1* (±0.25) | −0.5 | −23.4 | 1.0* (±0.18) | −0.6 | −28.3 | 0.1* (±0.30) | −1.4 | −93.8 |
CFB: Change from baseline, SD: Standard deviation, PGA: Physician global assessment, *Wilcoxon signed-rank test for CFB

- (a-d) Physician global assessment using modified Griffiths scale.
Assessment of fine wrinkling, coarse wrinkling, and laxity using severity scoring
Dermatological assessment of coarse wrinkling showed significant reduction in the severity of coarse wrinkling by 17.7% on day 25 (P = 0.0002), 24.4% on day 50 (P < 0.0001), and 44.3% on day 75 (P < 0.0001); assessment of fine wrinkling showed significant reduction in the severity of fine wrinkling by 23.3% on day 25, 50.5% on day 50, and 76.7% on day 75 (all P < 0.0001); and assessment of skin laxity showed significant reduction in the severity of skin laxity by 23.3% on day 25, 49.8% on day 50, and 70.6% on day 75 (all P < 0.0001), as compared to baseline [Table 2 and Figure 2].
| Parameters | Visit 02 (day 01) |
Visit 03 (day 25) |
Visit 04 (day 50) |
Visit 05 (day 75) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (SD) | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | |
| Mean value | ||||||||||
| Coarse Wrinkling Score | 2.1* (±0.55) | 1.7* (±0.47) | −0.4 | −17.7 | 1.6* (±0.63) | −0.6 | −24.4 | 1.1* (±0.34) | −1.0 | −44.3 |
| Fine Wrinkling Score | 4.0* (±0.18) | 3.1* (±0.73) | −0.9 | −23.3 | 2.0* (±0.45) | −2.0 | −50.5 | 0.9* (±0.25) | −3.1 | −76.7 |
| Skin Laxity (under both eyes) Score |
3.4* (±0.80) | 2.6* (±0.56) | −0.9 | −23.3 | 1.7* (±0.61) | −1.8 | −49.8 | 0.9* (±0.30) | −2.5 | −70.6 |
CFB: Change from baseline, SD: Standard deviation. *Wilcoxon signed-rank test for CFB
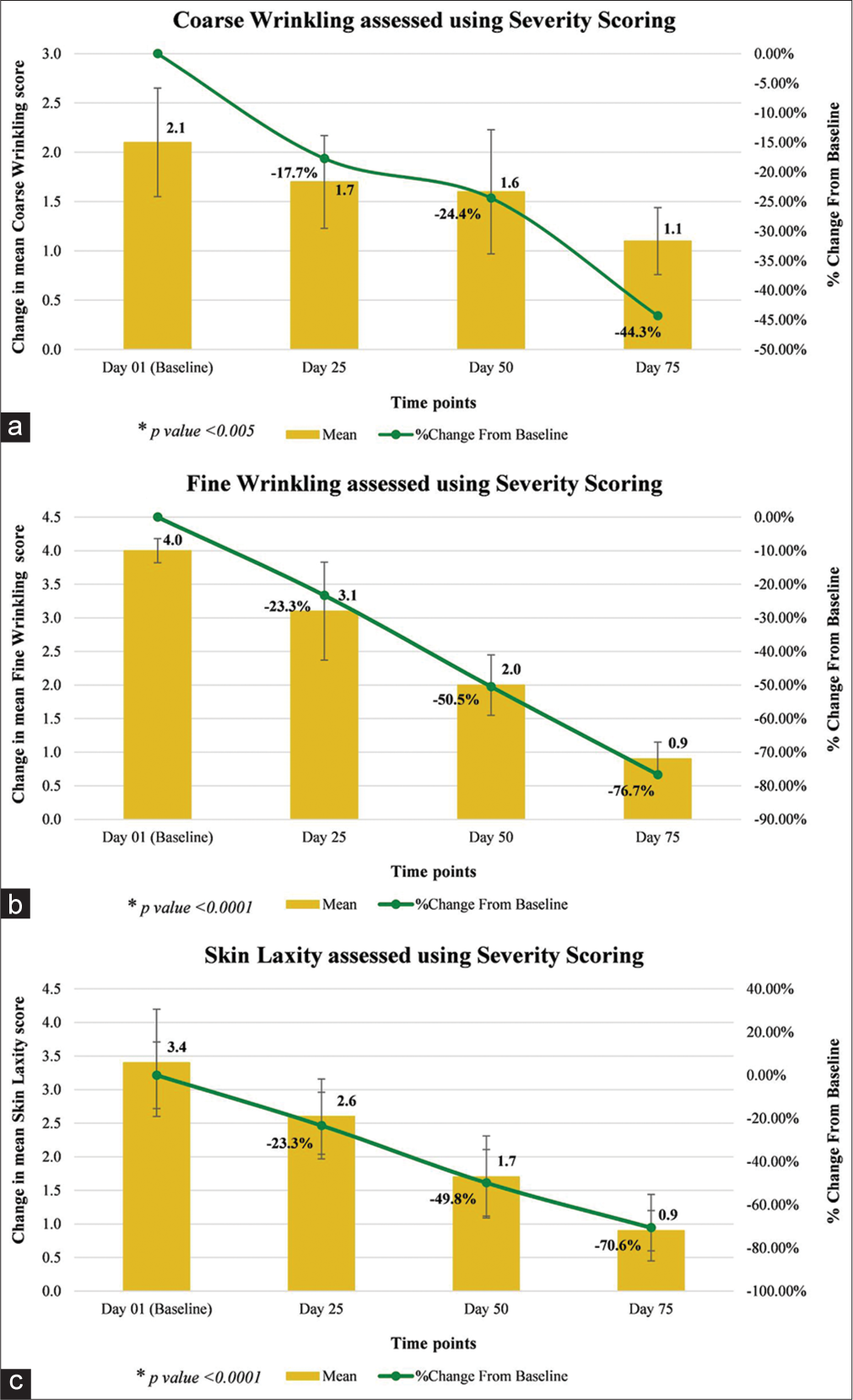
- (a-c) Wrinkles and skin laxity using severity scoring scale.
Dermatological assessments using scoring scales
Dermatological assessment of skin pigment showed significant reduction in its appearance by 18.8% on day 25 (P = 0.0002), 46.7% on day 50 (P < 0.0001), and 56.3% on day 75 (P < 0.0001); assessment of under-eyes dark circle showed significant reduction in its appearance by 20.3% on day 25, 23.3% on day 50, and 58.9% on day 75 (all P < 0.0001); and assessment of uneven skin tone showed significant improvement by 7.3% on day 25, 8.7% on day 50, and 10.3% on day 75 (all P < 0.0001); also there was significant improvement in skin radiance by 29.0% on day 25, 43.5% on day 50, and 69.9% on day 75 (all P < 0.0001), as compared to baseline [Table 3 and Figure 3].
| Parameters | Visit 02 (day 01) |
Visit 03 (day 25) |
Visit 04 (day 50) |
Visit 05 (day 75) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (SD) | Post baseline (SD) |
CFB | %CFB | Post baseline (SD) |
CFB | %CFB | Post baseline (SD) |
CFB | %CFB | |
| Mean value | ||||||||||
| Skin pigment using 9-point scale | 2.1* (±0.30) | 1.7* (±0.47) | −0.4 | −18.8 | 1.1* (±0.31) | −1.0 | −46.7 | 0.9* (±0.30) | −1.2 | −56.3 |
| Under-eyes dark circles using 6-point grading scale | 2.6* (±0.50) | 2.0* (±0.18) | −0.6 | −20.3 | 1.9* (±0.31) | −0.7 | −23.3 | 1.0* (±0.25) | −1.6 | −58.9 |
| Uneven skin tone using Felix Von Luschan Skin colour chart | 25.1#(±0.84) | 23.2#(±0.94) | −1.8 | −7.3 | 22.9#(±0.82) | −2.2 | −8.7 | 22.5#(±0.57) | −2.6 | −10.3 |
| Skin radiance using Modified Griffiths Scale | 5.8* (±0.57) | 4.1* (±0.44) | −1.7 | −29.0 | 3.2* (±0.50) | −2.5 | −43.5 | 1.7* (±0.58) | −4.0 | −69.9 |
CFB: Change from baseline, SD: Standard deviation, *Wilcoxon Signed Rank test for CFB, #Paired t-test for CFB
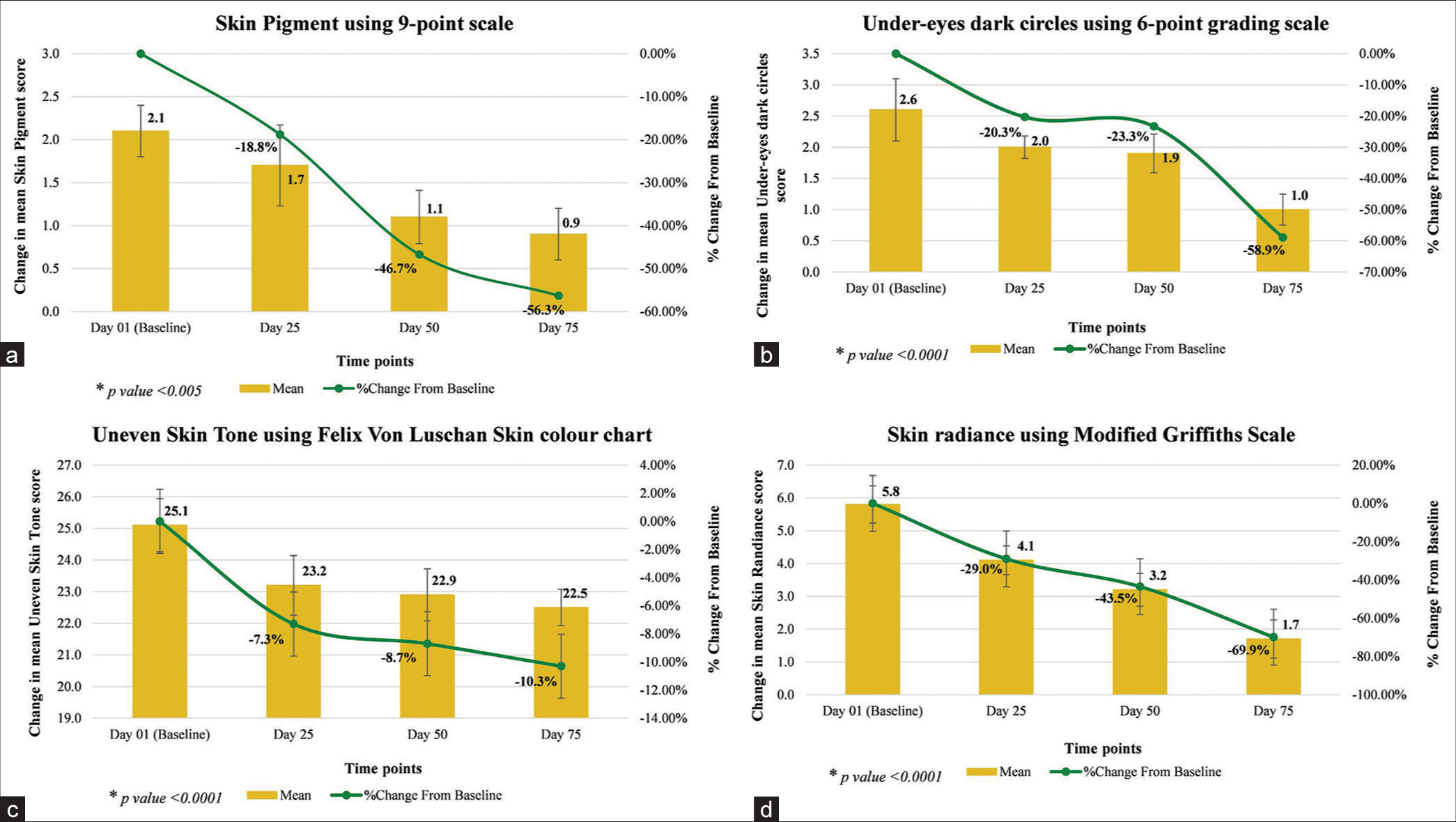
- (a-d) Dermatological assessments.
Instrumental assessments
Instrument analysis
Instrumental assessment of skin pigmentation showed significant reduction by 6.63% on day 25, 14.03% on day 50, and 22.09% on day 75; assessment of hydration level of the skin surface (stratum corneum) showed significant improvement by 46.47% on day 25, 77.03% on day 50, and 99.99% on day 75; assessment of skin gloss showed significant improvement by 1.18-fold on day 25, 2.11-folds on day 50, and 2.59-folds on day 75; and assessment of skin firmness showed significant improvement by 3.94% on day 25, 9.95% on day 50, and 16.36% on day 75 (all P < 0.0001), as compared to baseline [Table 4 and Figure 4].
| Parameters | Visit 02 (day 01) |
Visit 03 (day 25) |
Visit 04 (day 50) |
Visit 05 (day 75) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (SD) | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | |
| Mean value | ||||||||||
| Skin pigmentation using Mexameter® MX 18 (a.u.) | 514.17# (±74.81) | 480.62# (±73.85) | −33.55 | −6.63 | 442.22# (±69.72) | −71.45 | −14.03 | 402.00# (±69.85) | −112.17 | −22.09 |
| Skin Moisturization using Corneometer® CM 825 (a.u.) | 27.83# (±5.22) | 39.98# (±4.16) | 12.15 | 46.47 | 48.49# (±2.98) | 20.35 | 77.03 | 54.02# (±2.89) | 26.19 | 99.99 |
| Skin gloss using Skin- Glossymeter GL 200 (GU) | 2.59# (±0.76) | 5.45# (±0.97) | 2.86 | 118.28 | 7.56# (±0.91) | 4.99 | 211.15 | 8.78# (±1.51) | 6.19 | 259.57 |
| Skin firmness using Cutometer dual MPA 580 (mm) | 0.40# (±0.08) | 0.39# (±0.08) | −0.01 | −3.94 | 0.37# (±0.08) | −0.04 | −9.95 | 0.34# (±0.08) | −0.06 | −16.36 |
CFB: Change from baseline, SD: Standard deviation, #Paired t-test for CFB
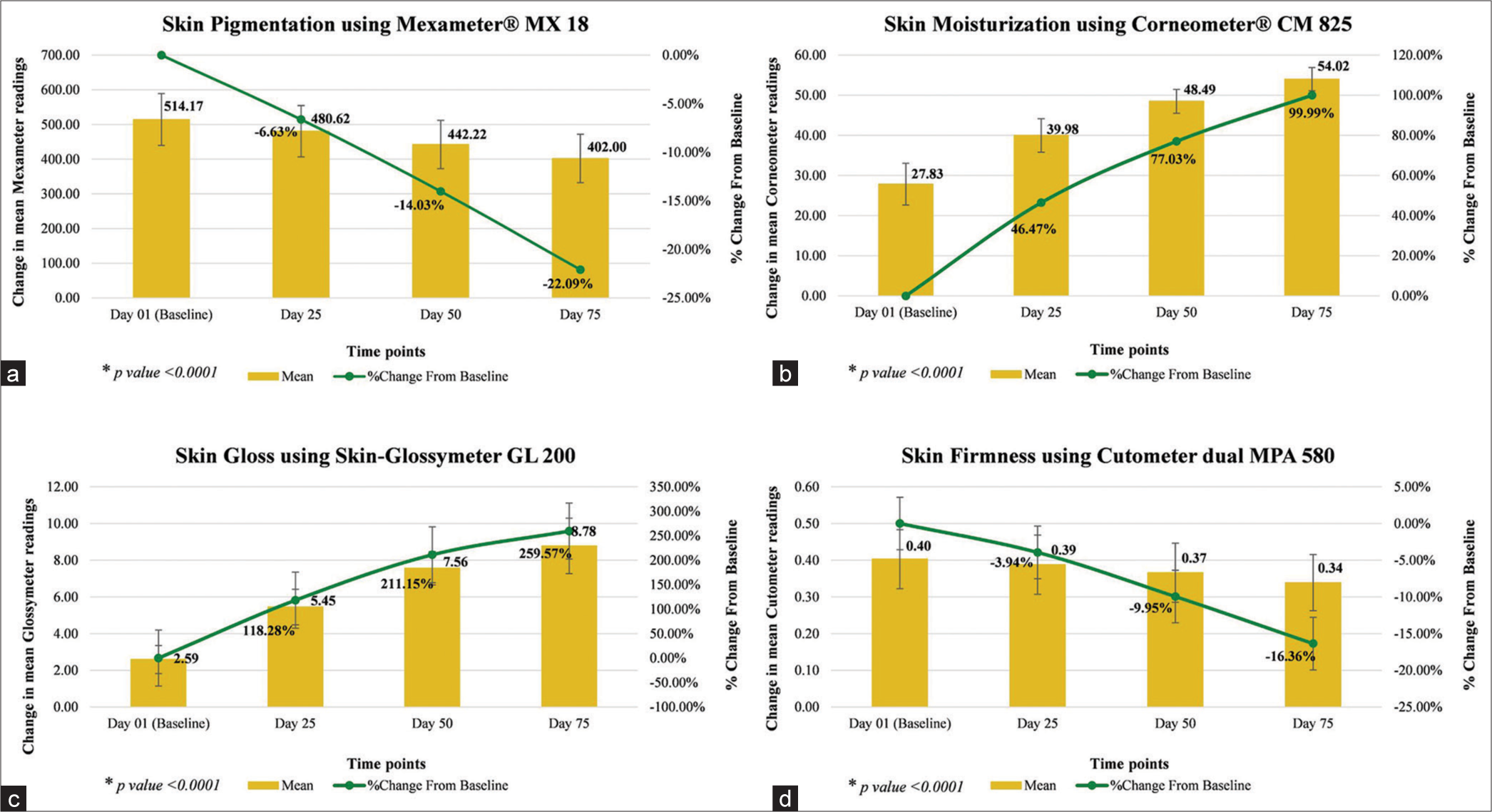
- (a-d) Instrumental assessments.
Assessment of wrinkles, pigmentation, skin texture, and under-eyes dark circles using 3D analysis system
Instrumental assessment of number of fine lines/wrinkles showed significant reduction by 17.2% on day 25, 33.0% on day 50, and 48.9% on day 75; assessment of length of fine lines/wrinkles showed significant reduction by 7.60% on day 25, 13.52% on day 50, and 19.64% on day 75; assessment of average width of fine lines/wrinkles showed significant reduction by 4.43% on day 25, 12.45% on day 50, and 24.81% on day 75; assessment of average depth of fine lines/wrinkles showed significant reduction by 8.23% on day 25, 18.85% on day 50, and 39.01% on day 75; assessment of color of pigmentation showed significant improvement by 4.16% on day 25, 8.23% on day 50, and 12.79% on day 75; assessment of average concentration of melanin in pigmented area showed significant reduction by 3.14% on day 25, 6.80% on day 50, and 9.99% on day 75; assessment of skin roughness showed significant reduction by 15.06% on day 25, 28.17% on day 50, and 43.55% on day 75; assessment of color of under-eyes dark circle showed significant improvement by 4.54% on day 25, 9.39% on day 50, and 18.10% on day 75; and assessment of average concentration of melanin in under-eyes dark circles showed significant reduction by 3.27% on day 25, 6.65% on day 50, and 10.03% on day 75 (all P < 0.0001), as compared to baseline [Table 5, Figures 5-7a-c].
| Parameters | Visit 02 (day 01) | Visit 03 (day 25) | Visit 04 (day 50) | Visit 05 (day 75) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (SD) | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | |
| Mean value | ||||||||||
| Fine lines/wrinkles of crow’s feet area | ||||||||||
| Count of fine lines/wrinkles | 50.6# (±12.18) | 41.9# (±10.18) | −8.7 | −17.2 | 34.6# (±8.74) | −16.9 | −33.0 | 26.0# (±7.65) | −24.6 | −48.9 |
| Average width of fine line/wrinkles (mm) | 0.64# (±0.04) | 0.61# (±0.05) | −0.03 | −4.43 | 0.56# (±0.06) | −0.08 | −12.45 | 0.48# (±0.12) | −0.16 | −24.81 |
| Length of fine line/wrinkles (mm) |
281.73# (±53.35) | 261.74# (±83.80) | −19.99 | −7.60 | 249.53# (±84.21) | −35.76 | −13.52 | 230.42# (±82.64) | −51.31 | −19.64 |
| Average depth of fine line/wrinkles (mm) |
0.0204# (±0.0042) | 0.0188# (±0.0048) | −0.0016 | −8.23 | 0.0169# (±0.0056) | −0.0036 | −18.85 | 0.0127# (±0.0063) | −0.0077 | −39.01 |
| Skin pigmentation | ||||||||||
| Color (L*) of pigmented area | 49.02# (±4.43) | 51.02# (±4.20) | 2.00 | 4.16 | 52.95# (±4.24) | 3.97 | 8.23 | 55.21# (±4.08) | 6.19 | 12.79 |
| Average concentration of melanin in pigmented area (a.u.) | 64.96# (±6.06) | 62.91# (±5.90) | −2.04 | −3.14 | 60.91# (±5.74) | −4.44 | −6.80 | 58.48# (±5.72) | −6.48 | −9.99 |
| Skin texture | ||||||||||
| Roughness (a.u.) | 13.64# (±2.84) | 11.64# (±2.74) | −2.00 | −15.06 | 10.02# (±2.59) | −3.78 | −28.17 | 7.88# (±2.57) | −5.76 | −43.55 |
| Under-eye dark circles | ||||||||||
| Color (L*) of dark circles | 44.30# (±3.55) | 46.29# (±3.56) | 1.99 | 4.54 | 48.17# (±3.42) | 4.10 | 9.39 | 52.16# (±9.04) | 7.86 | 18.10 |
| Average concentration of melanin (a.u.) | 66.89# (±5.19) | 64.72# (±5.34) | −2.17 | −3.27 | 62.91# (±5.14) | −4.46 | −6.65 | 60.21# (±5.27) | −6.68 | −10.03 |
CFB: Change from baseline, SD: Standard deviation. #Paired t-test for CFB
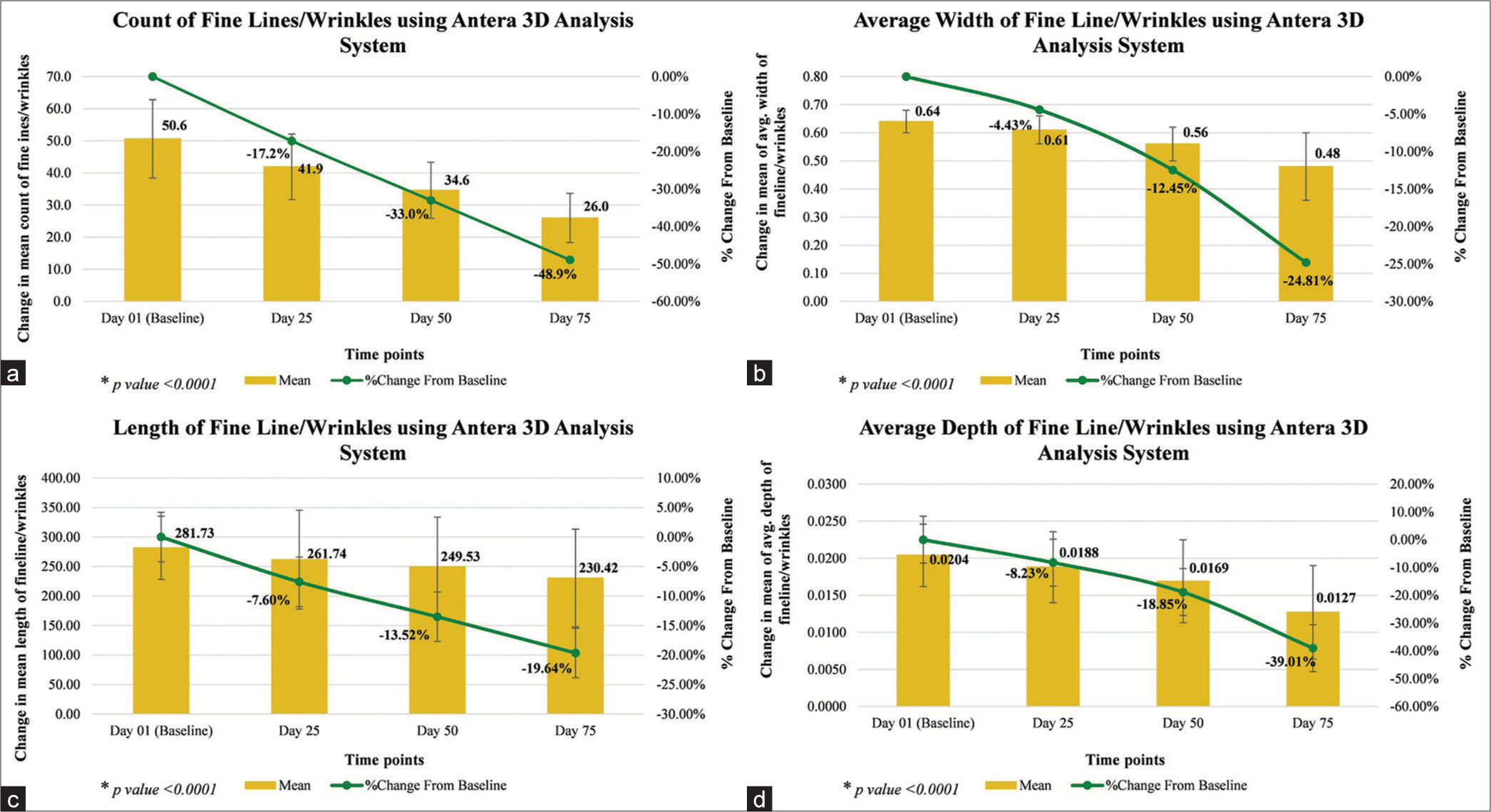
- (a-d) Fine lines/wrinkles assessment by Antera 3D.

- (a-d) Pigmentation and dark circles assessment by Antera 3D.
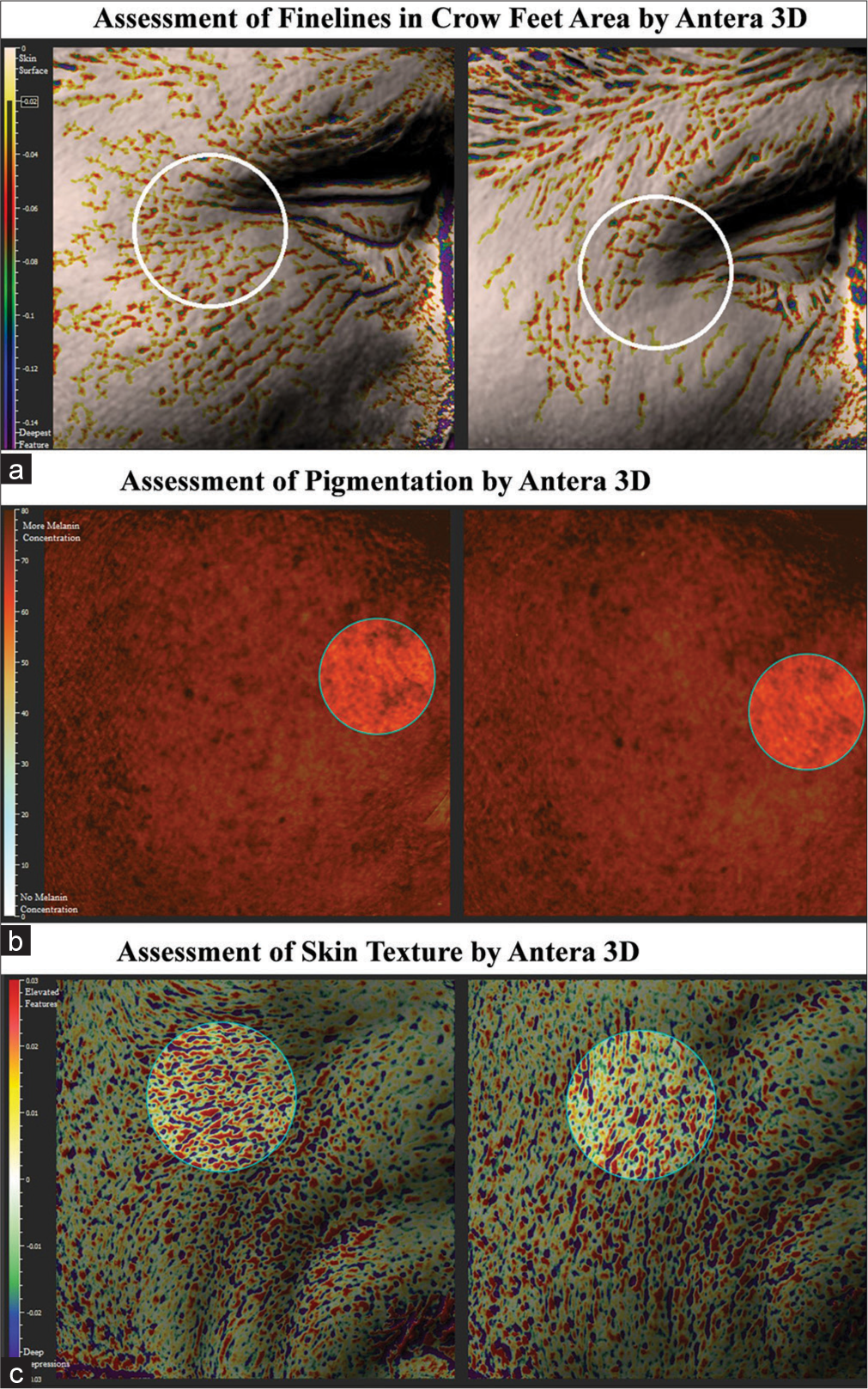
- (a-c) Image analysis by Antera 3D.
Subjective assessments
After consuming the test product for 25, 50, and 75 days, all study subjects (100%) were agreed to strongly agreed that there is reduction in fine lines/wrinkles, area and visibility of pigmentation/dark spots, and appearance of under-eye dark circle; and there is improvement in skin moisturization, skin glow, skin firmness, skin texture, and appearance on the skin. All participants (100%) also agreed that the skin tone has become even, and the skin feels soft and smooth. This clinically indicates that the study product reduces fine lines/wrinkles, area and visibility of pigmentation/dark spots, and appearance of under-eye dark circle; improves skin moisturization, skin glow, skin firmness, skin texture, and appearance on the skin; makes skin tone even; and makes the skin feel soft and smooth, as assessed by subject satisfaction questionnaire.
Safety assessment
No apparent AEs were observed.
DISCUSSION
Skin aging in humans is a composite biological mechanism. The former is influenced by various intrinsic and extrinsic factors.[16] The intrinsic factors include genetics, cellular metabolism, hormone, and other metabolic processes. The extrinsic factors, on the other hand, can be listed as chronic light exposure, pollution, ionizing radiation, chemicals, toxins, etc.[17] Long-term exposure to UV radiation of the Sun is the key factor of extrinsic skin aging, also referred to as photoaging.[18] Skin aging can be determined by a few characteristic features, viz., wrinkling, loss of elasticity, laxity, and rough-textured appearance.[2] Some external factors such as sunlight, smoking, environmental pollution, alcohol abuse, and nutrient deficiency can accelerate the process leading to an age-dependent collagen loss in the skin.[19,20] This results in diminished skin elasticity that leads to the emergence of lines and wrinkles.
As the skin reflects visible signs of aging, most people, usually females, spend a considerable amount on cosmetics and pharmaceutical products to prevent or reverse signs and symptoms of skin aging.[21] The core objective of any skin anti-aging treatment is to attain a healthy, smooth, blemish-free, translucent, and resilient skin.[22]
The present study was conducted on 36 healthy men and women volunteers. The test product is formulated with a blend of Indian and Western ingredients having a unique combination of HA, Curcumin, Multivitamin (Vitamin A, C, D2, E), Selenium, Zinc, etc. Each participant consumed two gummies together once daily after dinner for 75 days. It can be figured out from the results of dermatologist and instrumental evaluation that the study product was effective in reducing the appearance of coarse wrinkling/lines and fine lines/wrinkles and skin dryness along with improving the under-eye skin laxity as assessed by dermatologist using PGA. They were found efficacious in reducing the severity of coarse wrinkling, fine wrinkling, and skin laxity as assessed by dermatologist using a severity scoring scale.
The outcome of several previous studies has shown that age-dependent reduction in collagen synthesis can be reversed by oral administration of specific bioactive collagen peptides.[19] Antioxidants such as vitamin C and vitamin E or antioxidative enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and coenzyme Q10 can be utilized as reducing agents that can relieve skin aging by neutralizing reactive oxygen species.[23] Some plant species can also be used as a natural source of antioxidants, which prevent skin aging.[24]
Disorders of hyperpigmentation or dark spots are also very common among people nowadays. Lentigines, post-inflammatory hyperpigmentation, dark eye circles, and melasma are most common among them. Pigmentary disorders cause psychological distress and negatively impact the quality of life of an individual. Botanical and natural ingredients have become popular as depigmenting products and provide good alternative treatment.[25] Natural and alternative system helps in a way to treat various skin conditions and ailments. Nutraceutical industry is mostly consumer driven and has become most popular during the recent past due to enhanced awareness of potential health benefits and to fulfill the need of improved wellness.
In the present study, the study product significantly reduced the appearance of skin pigmentation as assessed by dermatologist using a nine-point scale, under-eyes dark circles as assessed by a dermatologist using a six-point grading scale, found effective in making the skin tone even as assessed by a dermatologist using Felix Von Luschan skin color chart and also in improving skin radiance as assessed by dermatologist using Modified Griffiths Scale. The gummies reduced skin pigmentation, improved skin hydration, and improved skin gloss and skin firmness as assessed by Mexameter, Corneometer, Skin Glossymeter, and Cutometer, respectively. The skin condition was improved by consuming the gummies as evidenced by analysis from Antera 3D system where it was found effective in reducing the appearance of fine lines/wrinkles of crow’s feet area, appearance of pigmentation, skin roughness, appearance of under eyes dark circles, and effective in improving the skin texture.
Skin aging is often associated with loss of micronutrients and moisture content in the skin. The key molecule supposed to be involved in binding and retaining water molecules in the skin is HA.[26] It can be demonstrated from the literature survey that HA-based formulations exhibit noteworthy anti-wrinkle, anti-nasolabial fold, anti-aging, space-filling, and face rejuvenating properties. The possible mechanism of action includes soft-tissue augmentation, improved skin hydration, collagen and elastin stimulation, and face volume restoration.[27] Another active ingredient of gummies is curcumin, which is a compound isolated from turmeric that possesses antioxidant and anti-inflammatory effect. Curcumin is appropriate for treating skin conditions that are characterized by derangement of the inflammatory response. It is evident from several clinical studies that curcumin may act as an effective agent in the treatment of several skin conditions including skin aging, fine lines, wrinkles, and hyperpigmented macules.[28]
The importance of both macro and micronutrients such as certain vitamins and minerals is vital for skin health.[29] An impaired nutritional status alters the structural integrity and biological function of the skin resulting in an abnormal skin barrier.[30] Literature suggests that Vitamin A prevents UV irradiation-mediated skin damage; Vitamin C Suppresses UV irradiation-triggered production of free radicals, protecting cells from oxidative stress; Vitamin D modulates inflammation, angiogenesis; Zinc protects from photo damage; and Selenium protect the skin from UV irradiation-induced oxidative stress; hence, all the micronutrients of the gummies have proven efficacy in maintaining skin health.
It is therefore evident from the above discussion that all the ingredients of the study product have proven efficacy on the indications associated with skin expressions. The synergistic effect of active ingredients shows that the gummies effectively improve skin quality in healthy adult volunteers after consuming the recommended dosage for 75 days as evaluated by dermatological, instrumental, and subjective assessments.
CONCLUSION
In this open-label, single-arm clinical study, the study product was evaluated for safety and effectiveness. The gummies were found to be safe based on no apparent or experienced discomfort, reactions or any kind of intolerance or adverse skin reactions or events evidenced in the trial.
The study product was found to be efficacious in reducing fine lines/wrinkles, under-eye dark circles, and pigmentation, and also improving the overall skin condition as evidenced in this trial. Regular consumption of two gummies together once a day significantly reduced the appearance and severity of coarse wrinkling/lines and fine lines/wrinkles; improved the skin laxity; reduced skin dryness by moisturizing the skin; lightened the pigmented area and under-eye dark circles by reducing the melanin concentration; increased the radiance/glow of the skin; improved the texture and firmness of the skin by reducing skin roughness; and also improved skin tone evenness. The gummies were sensorially accepted by all the subjects.
Acknowledgment
The authors are grateful to the study participants and sincerely acknowledge the contribution of CRO staff for their effortless contribution in completing this study.
Ethical approval
The research/study is approved by the OM - Institutional Ethics Committee, Protocol no. C3B02405 on 13th August 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Ikaria Wellness Pvt. Ltd.
Conflicts of interest
Though the authors 1 and 2 are employees of Ikaria Wellness Pvt. Ltd., we confirm that there is no conflict of interest among the authors.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Skincare bootcamp: The evolving role of skincare. Plast Reconstr Surg Glob Open. 2016;4(12 Suppl):e1152.
- [CrossRef] [PubMed] [Google Scholar]
- Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018;27:729-38.
- [CrossRef] [PubMed] [Google Scholar]
- A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-8.
- [CrossRef] [PubMed] [Google Scholar]
- A machine learning-based, decision support, mobile phone application for diagnosis of common dermatological diseases. J Eur Acad Dermatol Venereol. 2020;35:536-45.
- [CrossRef] [PubMed] [Google Scholar]
- Novel interpenetrating polymer network provides significant and long-lasting improvements in hydration to the skin from different body areas. J Cosmet Dermatol. 2020;19:1246-53.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-73.e33.
- [CrossRef] [PubMed] [Google Scholar]
- A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol. 1992;128:347-51.
- [CrossRef] [PubMed] [Google Scholar]
- Topical tretinoin for treatment of photodamaged skin. Arch Dermatol. 1991;127:659-65.
- [CrossRef] [PubMed] [Google Scholar]
- Tretinoin emollient cream: A new therapy for photodamaged skin. J Am Acad Dermatol. 1992;26:215-24.
- [CrossRef] [PubMed] [Google Scholar]
- Skin ageing and wrinkles: Clinical and photographic scoring. J Cosmet Dermatol. 2004;3:23-5.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of topical depigmenting agent in healthy human fair skin female volunteers: A single-arm study. J Cosmet Dermatol. 2017;17:830-9.
- [CrossRef] [PubMed] [Google Scholar]
- Classification by causes of dark circles and appropriate evaluation method of Dark Circles. Skin Res Technol. 2015;22:276-83.
- [CrossRef] [PubMed] [Google Scholar]
- Reduced appearance of under-eye bags with twice-daily application of epidermal growth factor (EGF) serum: A pilot study. J Drugs Dermatol. 2015;14:405-10.
- [Google Scholar]
- File: Felix von Luschan skin color chart. 2006. St. Petersburg, FL, USA: Wikipedia Foundation, Inc.; Available from: http://en.wikipedia.org/wiki/file:%20felix_von_luschan_skin_color_chart.jpg [Last accessed on 2023 Feb 30]
- [Google Scholar]
- Human models of aging and longevity. Expert Opin Biol Ther. 2008;8:1393-405.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular-level insights into aging processes of skin elastin. Biochimie 2016128-9, 163-73
- [CrossRef] [PubMed] [Google Scholar]
- A collagen supplement improves skin hydration, elasticity, roughness, and density: Results of a randomized, placebo-controlled, blind study. Nutrients. 2019;11:2494.
- [CrossRef] [PubMed] [Google Scholar]
- The dynamic anatomy and patterning of skin. Exp Dermatol. 2016;25:92-8.
- [CrossRef] [PubMed] [Google Scholar]
- Analyses of changes on skin by aging. Skin Res Technol. 2016;23:48-60.
- [CrossRef] [PubMed] [Google Scholar]
- Antiaging therapies. Indian J Dermatol Venereol Leprol. 2006;72:183-6.
- [CrossRef] [PubMed] [Google Scholar]
- Role of antioxidants in the skin: Anti-aging effects. J Dermatol Sci. 2010;58:85-90.
- [CrossRef] [PubMed] [Google Scholar]
- Anticedants and natural prevention of environmental toxicants induced accelerated aging of skin. Environ Toxicol Pharmacol. 2015;39:384-91.
- [CrossRef] [PubMed] [Google Scholar]
- Are natural ingredients effective in the management of hyperpigmentation? A systematic review. J Clin Aesthet Dermatol. 2018;11:28-37.
- [Google Scholar]
- Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 2018;120:1682-95.
- [CrossRef] [PubMed] [Google Scholar]
- Potential of curcumin in skin disorders. Nutrients. 2019;11:2169.
- [CrossRef] [PubMed] [Google Scholar]
- Human skin condition and its associations with nutrient concentrations in serum and diet. Am J Clin Nutr. 2003;77:348-55.
- [CrossRef] [PubMed] [Google Scholar]
- Role of micronutrients in skin health and function. Biomol Ther (Seoul). 2015;23:207-17.
- [CrossRef] [PubMed] [Google Scholar]






