Translate this page into:
A study to evaluate the effectiveness of Xyzal UC cream in participants with urticaria

*Corresponding author: Devesh Kumar Joshi, Department of Medical Affairs, Dr Reddy’s Laboratories Ltd., Hyderabad, Telangana, India. deveshkj@drreddys.com
-
Received: ,
Accepted: ,
How to cite this article: Jain AK, Joshi DK, Bhagat SV, Sanghavi A, Gala M, Muchhala SS, et al. A study to evaluate the effectiveness of Xyzal UC cream in participants with urticarial. CosmoDerma. 2024;4:84. doi: 10.25259/CSDM_100_2024
Abstract
Objectives:
This study aimed to assess the effectiveness and acceptability of Xyzal UC cream, containing the active component Tazman pepper (Tasmanian pepper berry), in alleviating the symptoms of urticaria.
Materials and Methods:
A prospective, single-center trial was conducted, involving 30 volunteers aged between 18 and 55 years with mild-to-moderate urticaria. Participants were chosen based on specific inclusion and exclusion criteria, which included a recent history of urticaria episodes supported by photographic evidence. The participants were instructed to apply a sufficient quantity of Xyzal UC cream on affected areas during urticaria episodes. Primary endpoints included grading for urticarial symptoms (redness, swelling, and itching) over 90 min and instant cooling and soothing effects of the cream over 15 min post-application using a Likert scale questionnaire. The secondary endpoint was to assess the tolerability of the test product. Photographs of the same site were taken at baseline (before product application), post-application, and after 90 min (or earlier, whenever resolved) by the participants.
Results:
The average age of participants as per the demographic data was found to be 33.33 years. The study found significant improvements in urticaria symptoms post-application of Xyzal UC cream. At 15 min post-application, 60% of participants agreed that redness had reduced, increasing to 93.3% of the total number of participants at 90 min. Similarly, 66.6% agreed to strongly agreed to a reduction in swelling at 15 min, with this figure rising to 93.3% at 90 min. The itching was notably reduced, with 83.4% of participants agreeing to strongly agreeing for relief from itching at 15 min, and 96.7% at 90 min. Participants also experienced a strong cooling and soothing effect immediately within 1 min post-application with 100% patient agreement. No adverse events or serious adverse events were reported, indicating a good tolerability profile for the cream.
Conclusion:
Xyzal UC cream exhibited substantial efficacy in promptly alleviating urticaria symptoms, including redness, swelling, and itching, with high levels of participant satisfaction and a positive tolerance profile. The instant cooling and soothing properties of the cream offer immediate relief, positioning it as a promising substitute for conventional therapies often associated with adverse effects. These findings highlight the potential of Xyzal UC cream as a viable and patient-compliant choice for managing mild-to-moderate urticaria.
Keywords
Urticaria
Topical formulation
Tazman pepper
Siligel
Soothing effect
INTRODUCTION
Urticaria, commonly referred to as hives, is a prevalent and diverse heterogeneous skin condition, marked by the sudden emergence of red, itchy wheals, which can sometimes be accompanied by angioedema.[1,2] These lesions, which can range greatly in size, may appear anywhere on the body, with a duration of usually 1–24 h,[1] often causing considerable discomfort and distress.[2] The condition arises from the activation and degranulation of subepidermal mast cells, leading to the release of histamine and other mediators. This process triggers sensory nerve activation, vasodilation, plasma leakage, and cellular infiltration, leading to subcutaneous (angioedema) or intracutaneous (wheal) edema.[3] Urticaria affects approximately 20% of the population at some point in their lives, with chronic forms impacting the quality of life through persistent symptoms and recurrent episodes.[2,4] In 2017, around 86 million individuals worldwide were affected by urticaria, representing roughly 1.1% of the global population.[5] Various factors such as infections, medications, dietary items, psychological triggers, and respiratory allergens are implicated in the cause, although at times, the origin can be idiopathic.[6]
Current treatment strategies for urticaria primarily include antihistamines and short-term systemic corticosteroids, with options such as omalizumab, cyclosporine, and leukotriene receptor antagonists used for resistant cases.[6] First-generation H1 antihistamines are fundamental therapies for the management of urticaria.[7] While effective, these do not have instant onset of action[8] and are often associated with notable sedative effects.[9] The unmet need for rapid action is significant, as itching and swelling can severely impact patients’ quality of life by disrupting sleep, work, and social interactions.[10] Instant cooling and soothing effects are crucial for urticaria patients because they mask the sensation of itch, thus reducing discomfort.[11] Cooling the skin constricts blood vessels, minimizing the release of histamine and other inflammatory mediators, providing immediate relief, and preventing further aggravation.[12] Hence, there is a requirement for alternative treatments that provide efficient relief of symptoms, and is patient compliant, while presenting a more favorable side effect profile. Given that hives are often itchy and swollen, the idea is that a topical formulation with anti-inflammatory, soothing, and hydrating properties could help provide rapid relief, calm the skin, reduce redness and swelling, and more effectively relieve the symptoms of urticaria.
Xyzal UC cream, a topical formulation containing the active ingredient Tazman pepper (Tasmannia lanceolata, commonly known as Tasmanian pepperberry), has shown promise as a novel treatment for urticaria. Research has identified key compounds in Tasmanian pepper berries, such as polygodial, warburganal, and 1β-acetoxy-9-deoxy-isomuzigadial. Polygodial’s presence in different organisms is linked to a wide array of biological activities beyond its pungent taste. These activities include antimicrobial, antifungal, antibiotic, antifeedant, antinociceptive, and anti-inflammatory properties.[13] Siligel, a silica gel-based material, and another major component of Xyzal UC cream, has been widely researched for its use in protective films and medical dressings.[14] Its capability to create a protective layer on the skin helps enhance hydration and shield against external irritants, proving useful for conditions such as eczema and chapped skin.[15]
Despite these promising indications, there is a lack of comprehensive clinical trials evaluating the effectiveness of Tazman pepper for urticaria. It was hypothesized that Xyzal UC cream, with its anti-inflammatory, soothing, and hydrating properties, can significantly reduce the severity and frequency of urticarial episodes by providing an instant cooling effect on irritated, sensitive skin, thereby alleviating symptoms such as redness and swelling. This prospective, single-group, and monocentric study was conducted to rigorously assess the efficacy and acceptability of Xyzal UC cream containing Tazman pepper in participants with urticaria.
MATERIALS AND METHODS
Study design
This was a single-center and prospective trial conducted to evaluate the effectiveness and patient satisfaction of Xyzal UC cream in individuals suffering from urticaria.
Ethical approval
The study was approved by the Royal Pune Independent Ethics Committee (IEC) (Approval No.: RPIEC181023; approved on October 27, 2023). The study was conducted in accordance with the protocol (Protocol No.: CL/097/0923/STU; Version No.: 1; dated October 09, 2023), principles of the Declaration of Helsinki, and its amendments in conformity with good clinical practices, Indian Council of Medical Research guidelines, and the New Drug and Clinical Trial rules 2019. This trial was registered with the Clinical Trial Registry of India (CTRI Registration No: CTRI/2023/11/059781).
Patient consent
Before the screening, the principal investigator explained the study procedures to all participants, with comprehensive information about the study, including its objectives, procedures, and potential risks. Participants were provided with the Informed Consent Document to review and understand. Any questions or concerns raised by the participants were addressed by the principal investigator. Those who agreed to participate provided their consent to participate in the trial.
Study participants
Thirty voluntary participants with mild-to-moderate urticaria were recruited based on specific inclusion and exclusion criteria or photographic evidence presented. Participants who did not experience an event during the study period were required to provide photographic evidence of a past event that had occurred within the previous week.
Inclusion criteria
The following criteria were included in the study:
Voluntary adult male and female patients between 18 and 55 years old with mild-to-moderate urticarial
Willing to cooperate, informed about the necessity and duration of the examinations, and ready to comply with protocol procedures
Having signed a consent form and informed both orally and in writing about all study procedures and objectives.
Exclusion criteria
The following criteria were excluded from the study:
Pregnant women (confirmed by urine pregnancy test) and lactating mothers
Patients with severe urticarial
Individuals with chronic illnesses that may affect study results
Those with any clinically significant systemic or cutaneous disease that might interfere with study treatments or procedures
Participants with hypersensitivity to any cosmetic product or raw material
Individuals on any systemic or topical medical treatment that may interfere with the study treatment, either currently or within the past month.
Endpoint(s)
Primary endpoints
To investigate the effectiveness of Xyzal UC cream in patients with urticaria:
Likert scale questionnaire to capture the effect of Xyzal UC cream on urticarial symptoms (redness, swelling, and itching) at 15 min, 30 min, 60 min, and 90 min post-application
Likert scale questionnaire for instant cooling/soothing effect at 1 min, 2 min, 3 min, 4 min, 5 min, 10 min, and 15 min post-application.
Secondary endpoint
Tolerability of the test product: Participants were asked to report any intolerances post-Xyzal UC cream application.
Study treatment and procedures
The investigational product, Xyzal UC cream, was provided by the sponsor for the use in the study. It served as the sole test product involved in the trial. Participants were instructed to apply a sufficient quantity of Xyzal UC cream on affected areas during urticaria episodes. The application zones included any part of the body where hives appeared, such as legs, arms, trunk, or face. Participants graded urticarial symptoms (redness, swelling, and itching), along with cooling and soothing effects on a 5-point Likert scale (1 = strongly disagree, 2 = disagree, 3 = neither agree nor disagree, 4 = agree, and 5 = strongly agree). Participants were trained to document their symptoms using a Likert scale questionnaire and to capture photographs of the affected site at specific time points: Baseline (before application), immediately after application, and 90 min post-application or earlier if symptoms resolved. Relevant medical history was also recorded. Post 1 episode of urticaria, participants were asked to return to the study center wherein the questionnaires, photographs, and test products were retrieved. The brief chronology of events is depicted in Figure 1.
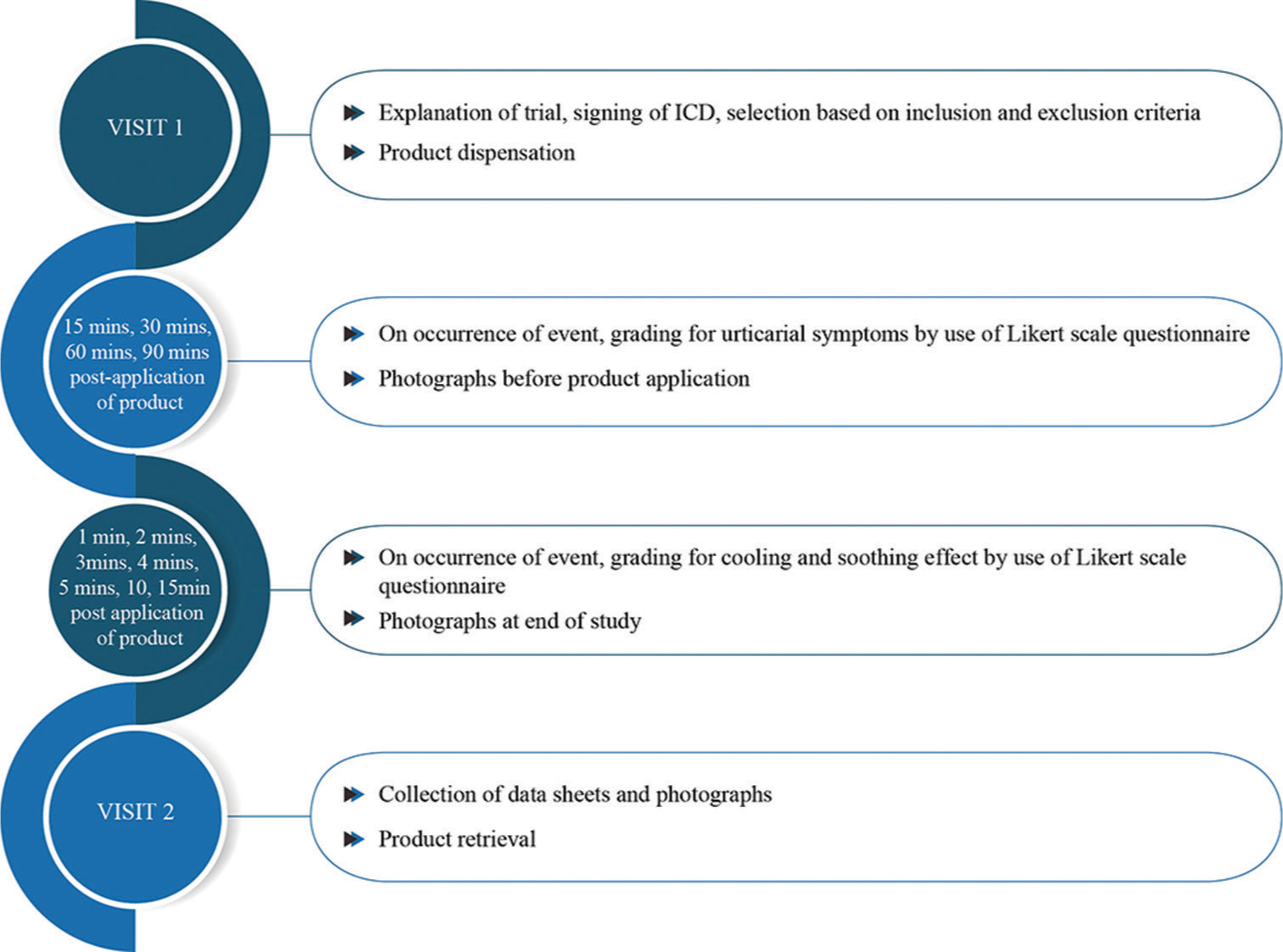
- Flowchart of the study, explaining events in chronological order. ICD: Informed consent document.
Statistical analysis
Data were recorded in tabular form and represented graphically using bar and line graphs. The percentage of participants agreeing or disagreeing with various parameters on the Likert scale was analyzed. No formal sample size determination was conducted. An interim analysis was carried out post-completion of the study for 15 participants which was notified to the Ethics Committee.
RESULTS
Between November 23, 2023, and April 02, 2024, 30 patients were recruited, wherein one subject dropped out. Data was analyzed for 30 participants. There were no protocol deviations.
Demography
The age range of participants was between 18.00 and 54.00 years, with an average age of 33.33 years. The distribution of gender among the participants revealed that a significant majority of the participants were female, comprising 83.3% (25 patients), while the remaining 16.7% (05 patients) were male.
Efficacy
Proportion of participants agreeing/disagreeing that redness of area due to urticaria has reduced over study duration
At the initial assessment point of 15 min post-application, 60.0% of participants expressed agreement or strong agreement that the product had effectively reduced redness. This proportion increased to 80.0% at the 30-min mark, and subsequent observations at 60 min revealed that 84.6% of the remaining 26 participants acknowledged a reduction in redness, with a further increase to 90.5% among the 21 participants assessed at the 90-min interval. When considering the entire cohort after 90 min, a significant 93.3% of participants reported agreement or strong agreement regarding the product’s efficacy in reducing redness associated with urticaria [Table 1]. A photographic representation of two participants before application, immediately after application, and 90 min post-application is provided in Figure 2.
| Assessment | Parameter – redness (n=30) | |||||||
|---|---|---|---|---|---|---|---|---|
| 15 min (n=30) | 30 min (n=30) | 60 min (n=26) | 90 min (n=21) | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Strongly disagree | - | - | - | - | - | - | - | - |
| Disagree | 07 | 23.3 | 03 | 10.0 | 02 | 07.7 | 02 | 09.5 |
| Neither agree nor disagree | 05 | 16.7 | 03 | 10.0 | 02 | 07.7 | - | - |
| Agree | 15 | 50.0 | 18 | 60.0 | 11 | 42.3 | 09 | 42.9 |
| Strongly agree | 03 | 10.0 | 06 | 20.0 | 11 | 42.3 | 10 | 47.6 |
| Top 2 scores (agree+strongly agree) | 18 | 60 | 24 | 80 | 22 | 84.6 | 19 | 90.5 |

- Representative photographs of participants before, during, and 90 min after application.
Proportion of participants agreeing/disagreeing that swelling of the area due to urticaria has reduced over the study duration
The study also assessed participants’ perceptions regarding the reduction of swelling associated with urticaria following the application of the product at various time intervals. At the initial assessment point of 15 min after application, 66.6% of total participants (20/30) strongly agreed or agreed that the product had effectively reduced swelling. This proportion remained stable at 80.0% at the 30-min mark and increased slightly to 84.7% (22/26) at the 60-min interval among the 26 participants assessed. Subsequent observations at the 90-min interval revealed that 90.4% of the 21 participants reported agreement or strong agreement to the reduction of swelling. After 90 min of applying the product, 93.3% of all participants agreed or strongly agreed that the swelling from urticaria had decreased [Table 2].
| Assessment | Parameter – swelling (n=30) | |||||||
|---|---|---|---|---|---|---|---|---|
| 15 min (n=30) | 30 min (n=30) | 60 min (n=26) | 90 min (n=21) | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Strongly disagree | - | - | - | - | - | - | - | - |
| Disagree | 05 | 16.7 | 02 | 06.7 | 01 | 03.8 | 01 | 04.8 |
| Neither agree nor disagree | 05 | 16.7 | 04 | 13.3 | 03 | 11.5 | 01 | 04.8 |
| Agree | 16 | 53.3 | 16 | 53.3 | 13 | 50.0 | 08 | 38.0 |
| Strongly agree | 04 | 13.3 | 08 | 26.7 | 09 | 34.7 | 11 | 52.4 |
| Top 2 scores (agree+strongly agree) | 20 | 66.6 | 24 | 80.0 | 22 | 84.7 | 19 | 90.4 |
Proportion of participants agreeing/disagreeing that itching of the area due to urticaria has reduced over the study duration
Initially, at 15 min post-application, a significant 83.4% of participants agreed or strongly agreed that the product effectively alleviated itching. This proportion increased further to 86.6% at the 30-min mark, indicating continued improvement. Subsequent assessments at 60 min revealed that 88.5% of the 26 participants acknowledged a reduction in itching and a 95.3% agreement among the 21 participants evaluated at the 90-min interval. At 90-min interval, an overwhelming 96.7% agreed or strongly agreed regarding the product’s efficacy in diminishing itching associated with urticaria [Table 3].
| Assessment | Parameter – itching (n=30) | |||||||
|---|---|---|---|---|---|---|---|---|
| 15 min (n=30) | 30 min (n=30) | 60 min (n=26) | 90 min (n=21) | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Strongly disagree | - | - | - | - | - | - | - | - |
| Disagree | 04 | 13.3 | 01 | 03.3 | 01 | 03.8 | 01 | 04.7 |
| Neither agree nor disagree | 01 | 03.3 | 03 | 10.1 | 02 | 07.7 | - | - |
| Agree | 17 | 56.7 | 13 | 43.3 | 11 | 42.3 | 06 | 28.6 |
| Strongly agree | 08 | 26.7 | 13 | 43.3 | 12 | 46.2 | 14 | 66.7 |
| Top 2 scores (agree+strongly agree) | 25 | 83.4 | 26 | 86.6 | 23 | 88.5 | 20 | 95.3 |
Proportion of participants agreeing/disagreeing on instant cooling effect after product application
The study also evaluated participants’ perceptions regarding the presence of an instant cooling effect following the application of the product at various time intervals. Remarkably, 100% of participants agreed or strongly agreed to the presence of an instant cooling effect after just 1 and 2 min of product application. As time progressed, a high percentage of participants continued to perceive this cooling effect, with 96.6%, 93.4%, and 86.6% agreeing or strongly agreeing after 3, 4, and 5 min, respectively [Table 4]. However, there was a notable decline in reported cooling effects over longer durations, with 60% and 40% of participants agreeing or strongly agreeing after 10 and 15 min, respectively [Figure 3, Table 4].
| Assessment parameter | Time points | ||||||
|---|---|---|---|---|---|---|---|
| Instant cooling (n=30) | 1 min n(%) | 2 min n(%) | 3 min n(%) | 4 min n(%) | 5 min n(%) | 10 min n(%) | 15 min n(%) |
| Strongly disagree | - | - | - | - | - | 1 (3.3) | 2 (6.7) |
| Disagree | - | - | - | 1 (3.3) | 2 (6.7) | 2 (6.7) | 3 (10.0) |
| Neither agree nor disagree | - | - | 1 (3.3) | 1 (3.3) | 2 (6.7) | 9 (30.3) | 13 (43.3) |
| Agree | 13 (43.3) | 14 (46.7) | 16 (53.3) | 18 (60.1) | 20 (66.7) | 14 (46.7) | 11 (36.7) |
| Strongly agree | 17 (56.7) | 16 (53.3) | 13 (43.3) | 10 (33.3) | 6 (19.9) | 4 (13.3) | 1 (3.3) |
| Top 2 scores (agree+strongly agree) |
30 (100.0) | 30 (100.0) | 29 (96.6) | 28 (93.4) | 26 (86.6) | 18 (60.0) | 12 (40.0) |
| Instant soothing (n=30) | 1 min n(%) | 2 min n(%) | 3 min n(%) | 4 min n(%) | 5 min n(%) | 10 min n(%) | 15 min n(%) |
| Strongly disagree | - | - | - | 1 (3.3) | 1 (3.3) | 1 (3.3) | 1 (3.3) |
| Disagree | - | - | - | - | - | - | - |
| Neither agree nor disagree | - | - | 1 (3.3) | 1 (3.3) | 1 (3.3) | 3 (10.0) | 4 (13.3) |
| Agree | 15 (50.0) | 16 (53.3) | 17 (56.7) | 17 (56.7) | 18 (60.1) | 15 (50.0) | 15 (50.0) |
| Strongly agree | 15 (50.0) | 14 (46.7) | 12 (40.0) | 11 (36.7) | 10 (33.3) | 11 (36.7) | 10 (33.3) |
| Top 2 scores (agree+strongly agree) | 30 (100.0) | 30 (100.0) | 29 (96.7) | 28 (93.4) | 28 (93.4) | 26 (86.7) | 25 (83.3) |
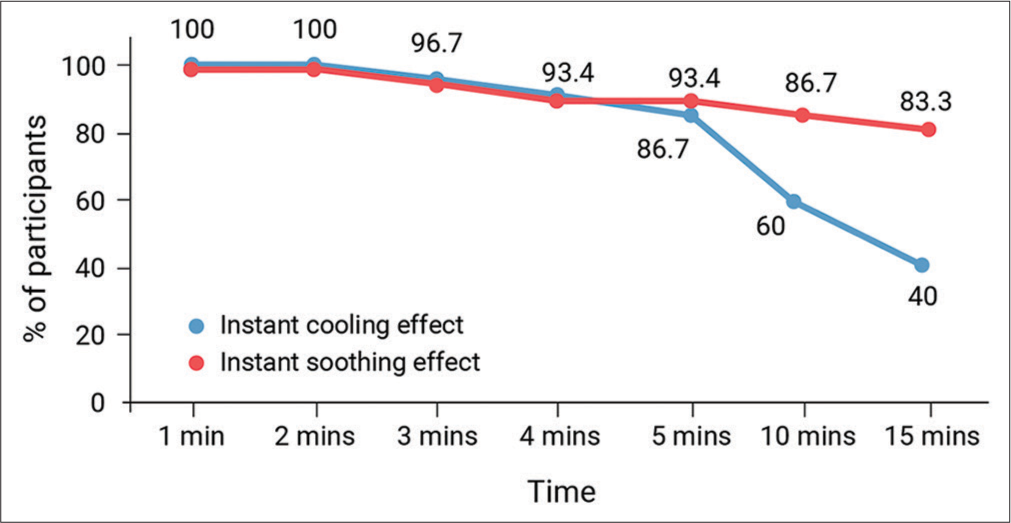
- Percentage of participants agreeing on instant cooling/instant soothing effect after product application.
Proportion of participants agreeing/disagreeing on instant soothing effect after product application
The study also explored how participants perceived an instant soothing effect following the application of Xyzal UC cream at different time intervals. Initial responses were unanimously positive, with all participants (100%) feeling a strong soothing effect within 1–2 min of application (agree or strongly agree). As time elapsed, the majority maintained this perception, with 96.7%, 93.4%, and 93.4% still agreeing or strongly agreeing after 3, 4, and 5 min, respectively [Table 4]. Even at the 10- and 15-min mark, a significant number of participants, 86.7% and 83.3%, respectively, reported a continued strong soothing sensation [Figure 3, Table 4].
Exceptionally, all participants (100%) agreed or strongly agreed that the cream provided an instant cooling and soothing effect within just 1–2 min of application.
Safety assessment
No adverse events, serious adverse events, or intolerances were reported by any participant throughout the study.
DISCUSSION
Urticaria, a prevalent dermatological condition characterized by the appearance of wheals, angioedema, or both, often accompanied by pruritus or a sensation of burning, necessitates a multifaceted approach to itch management.[16] This approach includes both pharmacological and non-pharmacological interventions that aim to decrease or minimize exacerbating factors, as well as assist in enhancing the patient’s overall quality of life. Topical agents with cooling and anti-pruritic properties can effectively curb the urge to scratch and may complement systemic therapies. However, the use of topical antihistamines is cautioned against due to their tendency to cause photo-allergy. Furthermore, topical corticosteroids are contraindicated in urticaria treatment.[17] European guidelines advocate for the use of moisturizers to fortify the skin’s natural barrier and alleviate itchiness.[18] The escalating demand for natural alternatives in response to growing awareness of the potential health risks associated with synthetic substances has propelled the popularity of herbal products, now utilized by approximately 20% of the American population.[19] The principal objective of the present investigation was to evaluate the efficacy and tolerability of Xyzal UC cream, enriched with Tasmanian pepper (T. lanceolata), in the management of urticaria. This research aimed to address the necessity for alternative treatments boasting a superior side effect profile. The lack of toxicity of the T. lanceolata extracts and their potent broad-spectrum inhibitory bioactivity against bacteria and fungi further supports their efficacy in defending against cutaneous microbial infections.[20] A soothing and cooling cream can ameliorate dermal manifestations by reducing abnormal skin temperatures and assuaging the itching and inflammation associated with urticaria. The majority of itch-alleviating creams exert their effect by enhancing the hydration levels of the stratum corneum.[21] Subjective feedback from participants constituted the primary means of evaluating the cream’s efficacy in this study. The outcomes revealed that a single application of Xyzal UC cream resulted in a statistically significant reduction in urticarial symptoms, encompassing redness, swelling, and itchiness. Patient satisfaction with therapy is commonly measured using the Likert scale, a psychometric ordinal scale presented in the form of a questionnaire. This scale is typically used to assess a person’s level of agreement or the amount of contentment, which is ranked on a five-point scale: Strongly disagree, disagree, neither agree nor disagree, and agree or strongly agree. Participant responses, analyzed through Likert scale assessments at various time points, underscored substantial amelioration.[22] Evidently, at the 90-min time point, 93.4% of respondents indicated a decrease in redness, 93.4% witnessed a reduction in swelling, and 96.7% achieved alleviation from itchiness. These findings underscored a consistent and increasingly positive perception among participants regarding the product’s effectiveness in relieving itching throughout the study. Furthermore, the study underscored the immediate cooling and soothing effects engendered by the cream. All participants (100%) acknowledged experiencing a cooling sensation immediately following application, with a significant proportion continuing to perceive this effect up to 15 min post-application. Such effects are particularly invaluable in providing instantaneous relief from the discomfort associated with urticaria [Figure 2].
The active component, Tasmanian pepper, has previously been recognized for its anti-inflammatory attributes, primarily linked to substances such as polygodial. Siligel’s presence, a material based on silica gel, also aids in the effectiveness of the cream by forming a protective shield on the skin, improving moisture levels, and guarding against external irritants. These elements collectively enhance the efficiency of the cream in relieving symptoms of urticaria. Participants were requested to communicate any intolerances post-application, and the absence of negative reactions among all individuals suggests that the cream is well-received. This discovery highlights the potential of Xyzal UC cream as a good alternative to traditional therapies, which often come with the risk of side effects.
However, the study had several limitations including the relatively small sample size of 30 participants and the monocentric structure of the study which may have restricted the relevance of the outcomes. In addition, the absence of a placebo control group in the study limited the thorough comparison for assessing the cream’s efficacy.
CONCLUSION
This research offers preliminary proof backing the utilization of Xyzal UC cream in lessening urticaria symptoms. The Xyzal UC cream’s anti-inflammatory, calming, and moisturizing features add to its efficacy in relieving redness, swelling, and itching, with high levels of participant satisfaction and a positive tolerance profile. The instant cooling and soothing properties of the cream offer immediate relief, positioning it as a promising substitute for conventional therapies often associated with adverse effects. These findings highlight the potential of Xyzal UC cream as a viable and patient-compliant choice for managing mild-to-moderate urticaria. Although more extensive, placebo-controlled investigations are required to validate these results.
Acknowledgments
The authors thank the study participants, principal investigators, and site (Site name: C.L.A.I.M.S. Pvt. Ltd) staff. The authors also thank NeoCrest Life Sciences Consulting Private Limited for providing medical writing assistance for this manuscript.
Ethical approval
The research/study was approved by the Institutional Review Board at Royal Pune IEC, number RPIEC181023, dated October 27, 2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Akshay Kumar Jain has no conflicts of interest. The authors Devesh Kumar Joshi, Seema Vikas Bhagat, Arti Sanghavi, Monil Gala, Snehal S. Muchhala, Sagar Katare, and Bhavesh P. Kotak are employees of Dr. Reddy’s Laboratories Ltd., Hyderabad, Telangana, India.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
The study was funded by Dr. Reddy’s Laboratories Ltd., Hyderabad, Telangana, India.
References
- The International EAACI/GA2LEN/EuroGuiDerm/APAAACI Guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. 2022;77:734-66.
- [CrossRef] [PubMed] [Google Scholar]
- Global epidemiology of urticaria: Increasing burden among children, females and low-income regions. Acta Derm Venereol. 2021;101:adv00433.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of urticaria in primary care. North Clin Istanb. 2019;6:93-9.
- [Google Scholar]
- Evidence-based use of antihistamines for treatment of allergic conditions. Ann Allergy Asthma Immunol. 2023;131:412-20.
- [CrossRef] [PubMed] [Google Scholar]
- The role of antihistamines in the treatment of chronic urticaria. J Allergy Clin Immunol. 1990;86:662-5.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence-based diagnosis and treatment of chronic urticaria/angioedema. Allergy Asthma Proc. 2014;35:10-6.
- [CrossRef] [PubMed] [Google Scholar]
- Unmet medical needs in chronic, non-communicable inflammatory skin diseases. Front Med (Lausanne). 2022;9:875492.
- [CrossRef] [PubMed] [Google Scholar]
- Physiology and pathophysiology of itch. Physiol Rev. 2020;100:945-82.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci Lett. 1995;187:157-60.
- [CrossRef] [PubMed] [Google Scholar]
- Structure-pungency relationships and TRP channel activation of drimane sesquiterpenes in Tasmanian Pepper (Tasmannia lanceolata) J Agric Food Chem. 2017;65:5700-12.
- [CrossRef] [PubMed] [Google Scholar]
- Silica hydrogels as platform for delivery of hyaluronic acid. Pharmaceutics. 2022;15:77.
- [CrossRef] [PubMed] [Google Scholar]
- Silika gel termodifikasi dimetilamin sebagai penyerap anion fosfat Indonesia: Universitas Negeri Padang; 2022.
- [CrossRef] [Google Scholar]
- Urticaria and angioedema. Allergy Asthma Clin Immunol. 2018;14:59.
- [CrossRef] [PubMed] [Google Scholar]
- Itch in urticaria management. Curr Probl Dermatol. 2016;50:77-85.
- [CrossRef] [PubMed] [Google Scholar]
- European S2k guideline on chronic pruritus. Acta Derm Venereol. 2019;99:469-506.
- [CrossRef] [PubMed] [Google Scholar]
- Herbal medicine in the United States: Review of efficacy, safety, and regulation: Grand rounds at University of California, San Francisco Medical Center. J Gen Intern Med. 2008;23:854-9.
- [CrossRef] [PubMed] [Google Scholar]
- The potential of Tasmannia lanceolata as a natural preservative and medicinal agent: Antimicrobial activity and toxicity. Pharmacogn Commun. 2014;4:42-52.
- [CrossRef] [Google Scholar]
- Emollient and antipruritic effect of Itch cream in dermatological disorders: A randomized controlled trial. Indian J Pharmacol. 2005;37:253-4.
- [CrossRef] [Google Scholar]
- Medical therapies for melasma. J Cosmet Dermatol. 2022;21:3707-28.
- [CrossRef] [PubMed] [Google Scholar]






