Translate this page into:
A randomized, double-blind, placebo controlled, and half-head study to evaluate the effects of platelet-rich plasma on androgenetic alopecia

*Corresponding author: Shikha Verma, Department of Dermatology and STD, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India. shikha.b.thakur@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Verma S, Thakur BK, Nepram L. A randomized, double-blind, placebo controlled, and half-head study to evaluate the effects of platelet-rich plasma on androgenetic alopecia. CosmoDerma. 2024;4:53. doi: 10.25259/CSDM_36_2024
Abstract
Objectives:
The main objective of the study was to evaluate the clinical efficacy of autologous platelet-rich plasma (PRP) injections in the scalp of patients with male androgenetic alopecia (AGA) and to determine whether PRP could be used as adjuvant treatment of AGA with topical minoxidil.
Materials and Methods:
We enrolled 27 male patients with AGA in the study. The patients were randomized to receive a half-head treatment with PRP and the other half head with normal saline (placebo). Minoxidil 5% solution was applied twice daily throughout the study period. Hair counts were done at the first visit and one month after the third injection on 1 cm2 areas (tattooed) on both the right and left sides of the parietal scalp in all the patients. Results were statistically analyzed using the Statistical Package for Social Sciences version 10.
Results:
In PRP-treated areas, hair density increased from 106.48 ± 29.93 (baseline) to 119.29 ± 24.61 (four months) (P = 0.001). In normal saline-treated areas, hair density increased from 104.85 ± 27.29 (baseline) to 120.56 ± 26.86 (four months), (P < 0.001). However, the p-value for normal saline versus PRP was not significant (P = 0.964).
Conclusion:
Our study evaluates the efficacy of PRP as an adjuvant therapy with topical minoxidil in AGA in northeastern population of India.
Keywords
Androgenetic alopecia
Platelet-rich plasma
Minoxidil
Hair
Baldness
INTRODUCTION
Androgenetic alopecia (AGA) is a chronic, non-scarring hair loss disorder affecting 80% of men and 50% of women throughout their lifetime. It is postulated that the patterned clinical presentation of AGA may be attributed to the differential distribution of androgen receptors and steroid-converting enzymes of the outer root sheaths of the scalp hair follicles.[1]
In genetically predisposed individuals, the terminal hair follicles switch over to vellus-like hair. There is gradual non-scarring miniaturization of hair follicles and anagen to telogen ratio is decreased.[2]
There are various treatments suggested for this condition. However, topical minoxidil and oral finasteride are the only U.S. Food and Drug Administration approved drugs for AGA. Platelet-rich plasma (PRP), a treatment modality shown to promote wound healing, has also been explored as a potential treatment for AGA.[3,4]
Various growth factors (GFs) in activated alpha granules of platelets including platelet-derived growth factor, epidermal growth factor (EGF), interleukin-1, transforming growth factor, vascular endothelial growth factor, and insulin-like growth factor appear to act on stem cells in the bulge area of the hair follicles, where they promote hair growth.[5-7] However, the exact mechanism is not known. There are many studies that show the efficacy of PRP in AGA.[3,4] However, the randomized controlled trials, where PRP is used as adjuvant treatment of AGA with topical minoxidil are limited. We have performed a prospective, randomized, double blind, placebo controlled, and half-head study on 27 men with AGA. The main objective of the study was to evaluate the clinical efficacy of autologous PRP injections in the scalp of patients with male AGA and to determine whether PRP could be used as adjuvant treatment of AGA with topical minoxidil.
MATERIALS AND METHODS
Study design
The study was a randomized, double-blind, placebo-controlled, and half-head study conducted on patients with AGA. Institutional IEC approval was obtained. Clinical trials registry of India (CTRI) registration number was CTRI/2017/11/010542. The study has been performed in accordance with Indian Good Clinical Practices and the Indian Council of Medical Research guidelines. The study was conducted for one year in a tertiary care hospital. All the participants signed a written informed consent form.
Inclusion criteria
Males in good general health, aged 20–50 years with mild-to-moderate AGA Hamilton-Norwood grade III, III vertex, and IV were enrolled in the study.
Exclusion criteria
Patients who received treatments for AGA within the past three months or with a history of smoking, active malignancies or platelets disorders, anemia, and/or bleeding disorders; on anticoagulants, non-steroidal anti-inflammatory drugs, chemotherapy, and un-cooperative patients or patients who are unable to understand the protocol or give informed consent and patients who have a keloidal tendency, known to be HIV, hepatitis B or C positive or otherwise immunocompromised and the subjects who have active skin disease or skin infection at the intended treatment area were excluded from the study.
Randomization
We enrolled 27 male patients with AGA in the study. The eligible patients were randomized in a ratio of 1:1 to receive a half-head treatment with PRP and the other half head with normal saline (placebo). The allocation was done randomly. The participants and assessor were blinded to treatment allocation till the analysis of data.
Preparation of PRP
PRP was separated from whole blood by light spin centrifugation, and subsequently, the platelets were concentrated by heavy spin centrifugation with the removal of the supernatant plasma. Initially, whole blood was collected from the antecubital vein of the patient (15 mL). The blood was then introduced into two tubes and centrifugated for 5 min at 1500 g using the laboratory centrifuge. Each tube contained a thixotropic gel and sodium citrate solution. After centrifugation, the blood was fractionated with the red blood cells being trapped under the gel and cellular elements settling on the surface of the gel. After the removal of 2 mL of upper supernatant platelet-poor plasma from each tube, 3 mL of PRP was obtained. The whole procedure was done in a strict aseptic manner in the blood bank.
Baseline hair counts were done on 1 cm2 areas (tattooed) on both the right and left sides of the parietal scalp in each patient on the first visit [Figure 1]. Intradermal injection of 3 mL PRP was given on the selected (tattooed) area of one half of the scalp (treated areas) while 3 mL normal saline (placebo) was given on the selected area (tattooed) of another half of the scalp (control areas) under aseptic precautions. No local anesthetic was used. An equal number of injections was given on each site in each sitting with 30-gauge needle. Injections were given monthly for three months in each patient. All patients were given topical 5% Minoxidil-1 mL to apply twice daily on the affected scalp. Final hair counts were done one month after the third injection on 1 cm2 areas (tattooed) on both the right and left sides of the parietal scalp in all the patients. The primary outcome was to observe the changes in hair count at baseline and at three months on each side of the scalp. The data collected was analyzed using statistics using the Statistical Package for the Social Sciences version 10.
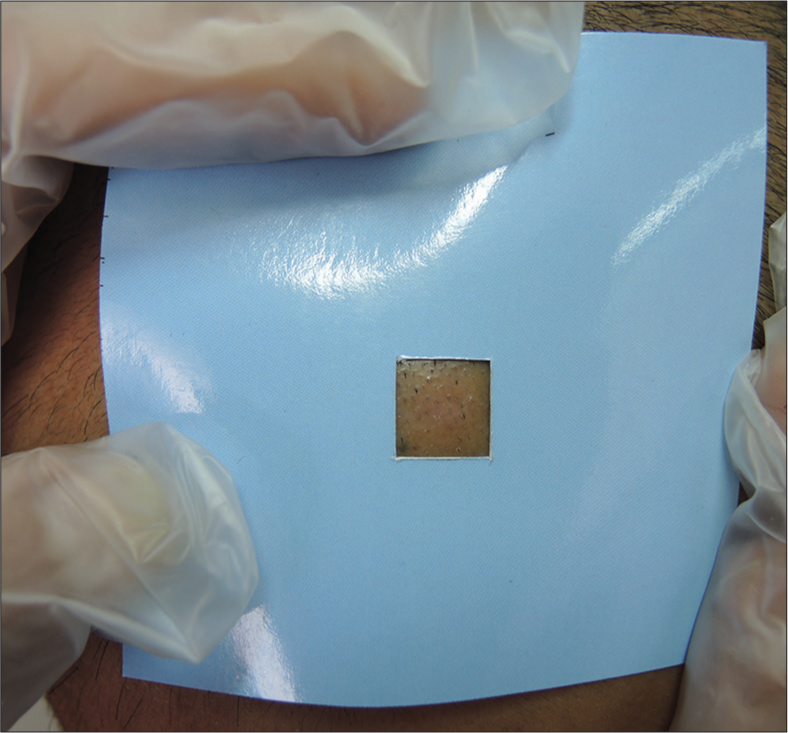
- Tattooed area on parietal scalp (1cm2).
RESULTS
A total of 27 men with AGA were enrolled in the study. The mean age of enrolled subjects (n = 27) was 29 years (Age range: 23–39 years). Nineteen patients (70.4%) had a family history of AGA. Twelve patients (44.4%) were smokers and 14 patients (51.8%) gave a history of alcohol intake. Maximum patients (12, 55.6%) showed grade 3 MAGA (Hamilton–Norwood scale) [Table 1].
| Case no | Age, years | Grading of Androgenetic Alopecia (Hamilton-Norwood) |
|---|---|---|
| 1 | 25 | Grade III |
| 2 | 39 | Grade IV |
| 3 | 27 | Grade II |
| 4 | 28 | Grade III |
| 5 | 26 | Grade III |
| 6 | 25 | Grade III |
| 7 | 35 | Grade III |
| 8 | 32 | Grade II |
| 9 | 32 | Grade V |
| 10 | 34 | Grade III |
| 11 | 25 | Grade III |
| 12 | 30 | Grade III |
| 13 | 35 | Grade III |
| 14 | 27 | Grade III |
| 15 | 25 | Grade II |
| 16 | 27 | Grade V |
| 17 | 28 | Grade IV |
| 18 | 32 | Grade VII |
| 19 | 37 | Grade VI |
| 20 | 26 | Grade IV |
| 21 | 33 | Grade III |
| 22 | 25 | Grade V |
| 23 | 25 | Grade IV |
| 24 | 23 | Grade III |
| 25 | 36 | Grade VI |
| 26 | 38 | Grade V |
| 27 | 23 | Grade IV |
The PRP was injected intradermally on the left side of the scalp in 14 patients and on the right side of the scalp in 13 patients (treated). Normal saline (Placebo) was injected intradermally on the other half of the scalp (control). Hair counts/cm2 were done at baseline (first visit) and one month after the third injection for PRP-treated and normal saline-treated half-head areas [Tables 2 and 3].
| Hair counts/cm2 (Platelet-rich plasma) | Hair counts/cm2 (Normal saline) | ||||
|---|---|---|---|---|---|
| Left side of scalp: 14 patients | Left side of scalp: 13 patients | ||||
| Right side of scalp: 13 patients | Right side of scalp: 14 patients | ||||
| Increased | No change | Decreased | Increased | No change | Decreased |
| 12 cases | 11 cases | 4 cases | 17 cases | 7 cases | 3 cases |
PRP: Platelet-rich plasma
| Mean hair count/cm2±SD | Platelet-Rich Plasma | P-value | Normal saline | P-value | Normal saline vs. PRP P-value | ||
|---|---|---|---|---|---|---|---|
| Baseline | 4th visit | Baseline | 4th visit | ||||
| 106.48±29.93 | 119.29±24.61 | 0.001 | 104.85±27.29 | 120.55±26.86 | <.001 | 0.964 | |
PRP: Platelet-rich plasma, SD: Standard deviation
The mean hair count/cm2 at four months increased in both PRP-treated and normal saline-treated areas. In PRP-treated areas, hair density increased from 106.48 ± 29.93 (baseline) to 119.29 ± 24.61 (four months) (P = 0.001). In normal saline-treated areas, hair density increased from 104.85 ± 27.29 (baseline) to 120.56 ± 26.86 (four months), (P<0.001). However, the p-value for normal saline versus PRP was not significant (P = 0.964).
Global photography showed an increase in scalp coverage in all the cases [Figure 2].
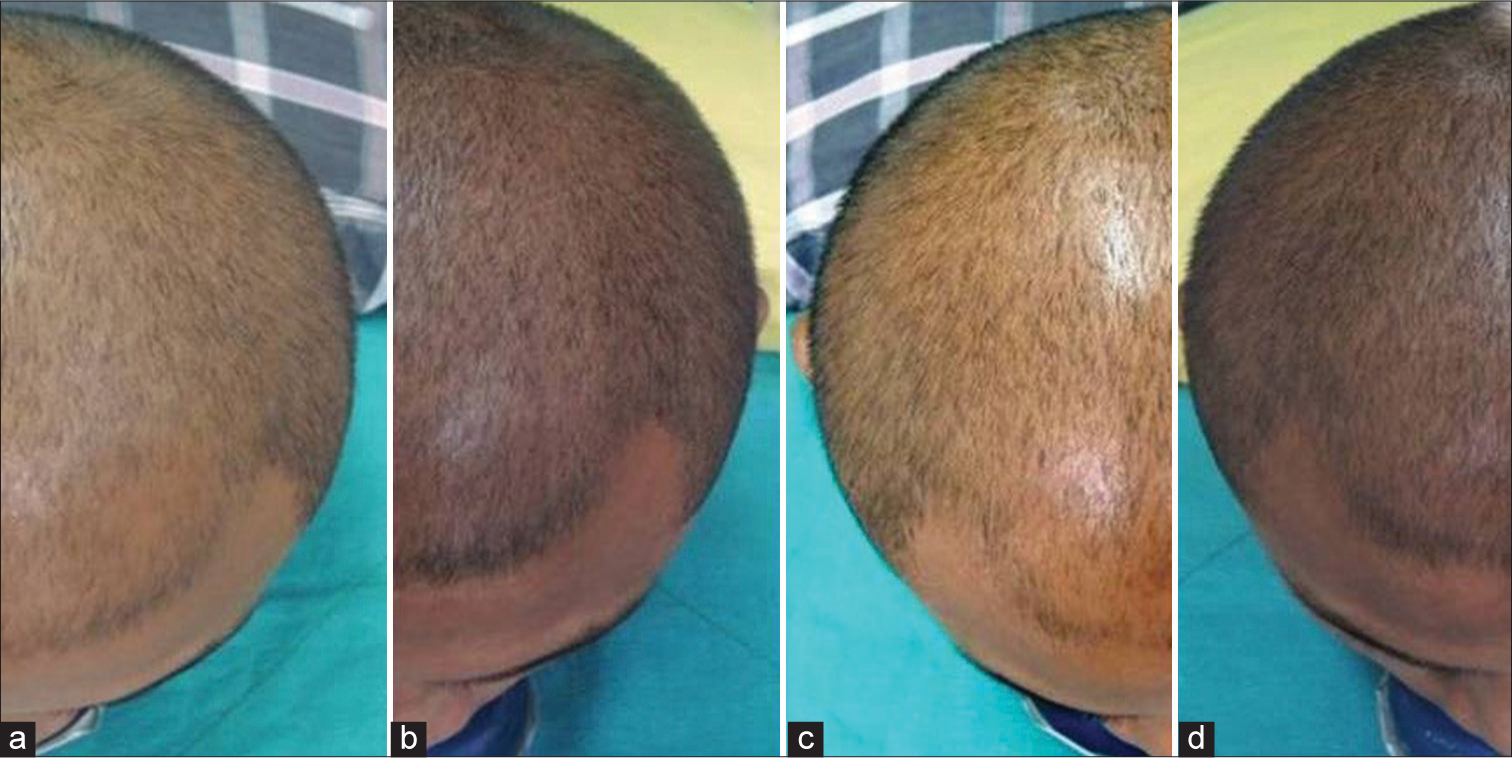
- Global photography comparing scalp hair at baseline (a and c) and at fourth month (b and d).
DISCUSSION
The AGA is distinguished by short anagen and miniaturization of hair. Current therapies target cellular proliferation and hair cycle differentiation.[8] However, no treatment is absolutely satisfactory. Minoxidil stimulates potassium channels and prostaglandin endoperoxide synthase-1, which prolongs anagen and increases hair follicle size.[9] Prolongation of anagen is also induced by 5 alpha reductase inhibitor finasteride.[1]
The PRP is an autologous preparation of platelets in concentrated plasma. The platelet is a natural source of different GFs and cytokines. The regenerative potential of PRP depends on these GFs.[7] These GFs act on stem cells in the bulge area of the follicles, stimulate the development of new follicles, and promote neovascularization. Activated autologous PRP has been reported to be antiapoptotic. It also induces the proliferation of dermal papilla cells by up-regulating fibroblast growth factor 7 and b-catenin as well as an extracellular signal-related kinase and Akt signaling.[10]
Various studies have demonstrated an increase in hair density with PRP intradermal injections.[10-14] European guidelines for AGA treatment focus on PRP therapy but recognize its insufficient evidence base.[15] The clinical data demonstrating the efficacy of PRP therapy as adjuvant with minoxidil is lacking.[16,17] However, many studies have shown the benefits of PRP therapy as an adjuvant treatment for AGA.[16-18]
Our study methodology removed biases such as age and alopecia grade, as it was a half-head study and the same patient acted as a treated and control subject. In the present study, we have shown that intradermal injections of PRP as well as normal saline (placebo) show a statistically significant improvement in the total hair count at 3 months when given as an adjuvant therapy with topical 5% minoxidil. However, the difference between the increase in total hair count on intradermal PRP-treated area and normal saline-treated area was not statistically significant. Hair growth even after intradermal normal saline injections may be attributed to the release of platelet-derived growth factor and EGF from activated platelets during wound healing.
CONCLUSION
Intradermal PRP and normal saline injections have equivocal results in male AGA. Furthermore, our study is unique as it evaluates the efficacy of intradermal PRP vis-a-vis normal saline injections as adjuvant therapy with topical minoxidil in AGA in northeastern population of India.
Ethical approval
The research/study approved by the Institutional Review Board at North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, number NEIGR/IEC/2013/27, dated June 26, 2014.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Different levels of 5α-reductase type I and II, aromatase and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol. 1997;109:296-300.
- [CrossRef] [PubMed] [Google Scholar]
- S1 guideline for diagnostic alopecia in men, women and adolescents. Br J Dermatol. 2011;164:5-15.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-7.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of efficacy of platelet-rich plasma therapy for androgenetic alopecia. J. Dermatolog Treat. 2017;28:55-8.
- [CrossRef] [PubMed] [Google Scholar]
- Increased vibrissa growth in transgenic mice expressing insulin-like growth factor 1. J Invest Dermatol. 1999;112:245-8.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of growth hormone receptor, insulin-like growth factor 1 (IGF-1) and IGF-1 receptor mRNA and proteins in human skin. J Invest Dermatol. 1992;99:343-9.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg. 2004;62:489-96.
- [CrossRef] [PubMed] [Google Scholar]
- Study of platelet-rich-plasma injections in the treatment of androgenetic alopecia through a one-year period. J Cutan Aesthet Surg. 2014;7:213-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hair growth and rejuvenation: An overview. J Dermatolog Treat. 2011;22:123-32.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38:1040-6.
- [CrossRef] [PubMed] [Google Scholar]
- Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg. 2011;37:1721-9.
- [CrossRef] [PubMed] [Google Scholar]
- The role of platelet plasma growth factors in male pattern pattern baldness surgery. Plast Reconstr Surg. 2006;118:1458-66.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: Clinical and histomorphometric evaluation. Biomed Res Int. 2014;2014:760709.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous platelet-rich plasma as a potential therapeutic tool in androgenetic alopecia. J Am Acad Dermatol. 2013;68:SAB103.
- [CrossRef] [Google Scholar]
- Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men-Short version. J Eur Acad Dermatol Venereol. 2018;32:11-22.
- [CrossRef] [PubMed] [Google Scholar]
- Combination therapy with platelet-rich plasma and minoxidil leads to better clinical results than monotherapy with these methods in men with androgenetic alopecia. Int J Trichol. 2022;14:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma in combination with 5% minoxidil topical solution and 1 mg oral finasteride for the treatment of androgenetic alopecia: A randomized placebo-controlled, double-blind, half-head study. Dermatol Surg. 2018;44:126-30.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of microneedling with platelet-rich plasma plus topical minoxidil (5%) and topical minoxidil (5%) alone in androgenetic alopecia. Int J Trichology. 2017;9:14-8.
- [CrossRef] [PubMed] [Google Scholar]






