Translate this page into:
A comparative study of assessment of clinical response of methotrexate versus apremilast in chronic plaque psoriasis

*Corresponding author: Pradeep S. Nair, Department of Dermatology and Venereology, Government T D Medical College, Alappuzha, Kerala, India dvmchtvm@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Iqbal HM, Samad KA, Nair PS. A comparative study of assessment of clinical response of methotrexate versus apremilast in chronic plaque psoriasis. CosmoDerma. 2024;4:104. doi: 10.25259/CSDM_123_2024
Abstract
Objectives:
Methotrexate (MTX) is one of the oldest conventional agents used for management of chronic plaque psoriasis, and apremilast (APM) is a recent small molecule for the treatment of chronic plaque psoriasis. The objective of this study was to compare the clinical response of patients with chronic plaque psoriasis treated with MTX versus APM.
Materials and Methods:
This is a 1½ year descriptive observational study of all new chronic plaque psoriasis patients. The sample size was 64 (31 in MTX group and 33 in APM group). Sixty-four patients diagnosed with chronic plaque psoriasis were treated with MTX or APM. The patients were evaluated for therapeutic outcome by psoriasis area and severity index (PASI) score at 4 weeks, 12 weeks, and 24 weeks. The efficacy of drugs was compared using PASI 75. The data obtained from the patient was entered in Microsoft Excel and analyzed using the Statistical Package for the Social Sciences software.
Results:
Out of the 64 patients, 31 were treated with MTX and 33 patients were treated with APM. At 12 weeks, 35.5% of patients receiving MTX group achieved PASI 75 whereas only 9% of patients achieved PASI 75 in APM group which was found to be statistically significant (P = 0.01). The PASI 75 was achieved by 93.5% of patients in the MTX group and 87.9% of patients in the APM group with no statistical significance at 24 weeks.
Conclusion:
The MTX and APM demonstrated equal efficacy in the treatment of chronic plaque psoriasis at the end of 24 weeks.
Keywords
Apremilast
Methotrexate
Psoriasis
INTRODUCTION
Chronic plaque psoriasis is the most common subtype of psoriasis and 10–20% of these cases require systemic disease modifying drugs. Methotrexate (MTX), a folic acid antagonist, is one of the first systemic agents used in the treatment of moderate-to-severe psoriasis. MTX is the drug of choice in managing severe psoriasis due to its high efficacy, low cost, oral administration, and benefit when there is also psoriatic arthritis.[1] The most common side effects include nausea, leukopenia, and liver enzyme elevation. Despite the potential side effects, it remains a frequently used cost-effective first-line drug. Apremilast (APM) is the first orally active phosphodiesterase-4 inhibitor approved drug for plaque psoriasis.[2] No routine monitoring of hematologic parameters is required for APM, which is a major advantage compared to other small molecules and conventional drugs.[2]
Very few studies compare APM with MTX, and hence, little knowledge about the relative comparative efficacy. Since such studies are lacking in India, this study was undertaken. The primary objective of this study was to compare the clinical response of chronic plaque psoriasis in patients treated with low-dose MTX versus APM using the psoriasis area severity index (PASI).
MATERIALS AND METHODS
This is a 1½ year descriptive observational non-randomized comparative study done in a tertiary care center. Convenience sampling was employed and the sample size was calculated to be 31 in each group of MTX and APM based on the previous study by Rathipriyadharshini et al.[3] The inclusion criteria were all clinically diagnosed, chronic plaque psoriasis patients, newly treated with APM or MTX having PASI >10, and above the age of 18 years. Patients already on other specific systemic treatment for the past 1 month, patients who were contraindicated for MTX and APM, and patients with arthropathy were excluded from the study. The independent variables were age, gender, and severity of psoriasis, while the dependent variable was PASI score. A structured pro forma was used to collect data after informed consent. Detailed clinical history, systemic, and dermatological details were recorded on preformatted pro forma. The data collected included demographic profile, duration of disease, site of involvement, size of plaque, extent, and severity of plaque measured by PASI score before starting the treatment. Routine investigations done were blood routine, urine routine examination, liver function test, blood sugar, renal function test, serum calcium levels, viral markers, Chest X-ray, and ultrasound abdomen. The dose of MTX was 7.5 mg weekly (low dose) and APM 30 mg twice daily. The clinical endpoints for response to treatment were assessed as mean % of improvement of PASI at 4, 12, and 24 weeks from the baseline PASI and PASI 75, which was defined as a 75% reduction in the PASI score from the original baseline at the end of 4, 12, and 24 weeks after starting treatment. Qualitative data was expressed in proportion or percentage. The quantitative variable was expressed as the mean. Frequency distribution analysis and descriptive statistics were calculated for nominal and ratio data, respectively. The Chi-square test/Fisher’s exact test was used to find out the difference between the two groups’ proportionate values. Mann–Whitney U-Test was used to compare the mean PASI score among the patients who were treated with MTX and the patients who were treated with APM. Statistical significance was considered at a 5% level.
Permission to conduct the study was obtained from the Institutional Research and Ethical Committee.
RESULTS
This descriptive observational study comparing the efficacy of MTX versus APM had a sample size of 64 (n = 64), 31 cases in the MTX group (n = 31), and 33 patients in the APM group (n = 33). The salient demographic, clinical features, and response to treatment are given in Table 1. The age ranged from 21 to 68 years. There was no statistical significance between the age distribution in both MTX and APM groups (P = 0.69). Males outnumbered females in both groups [Table 1], but this was not statistically significant (P = 0.60). There were 54.5% (n = 8) cases in the MTX group and 48.4% (n = 16) cases in the APM group had a duration of psoriasis of more than 10 years, but this was not statistically significant (P = 0.80). In the MTX group, 38.7% (n = 12) cases and 24.2% (n = 8) cases in the APM had metabolic syndrome, but this was also not statistically significant (P = 0.21). The mean percentage improvement in PASI at 4, 12, and 24 weeks is given in Figure 1. At the end of 12 weeks, 35.4% (n = 11) cases in the MTX group and 9.1% (n = 3) cases in the APM achieved PASI 75 which was statistically significant (P = 0.011). At the end of 24 weeks, 93.5% (n = 29) cases in the MTX group and 87.8% (n = 29) cases in the APM group achieved PASI 75, which was not statistically significant (P = 0.673, Figure 2). In the MTX group, 6.4% (n = 2) cases and 12.1% (n = 4) cases in the APM group did not achieve PASI 75 at the end of 24 weeks which was not significant (P = 0.63).
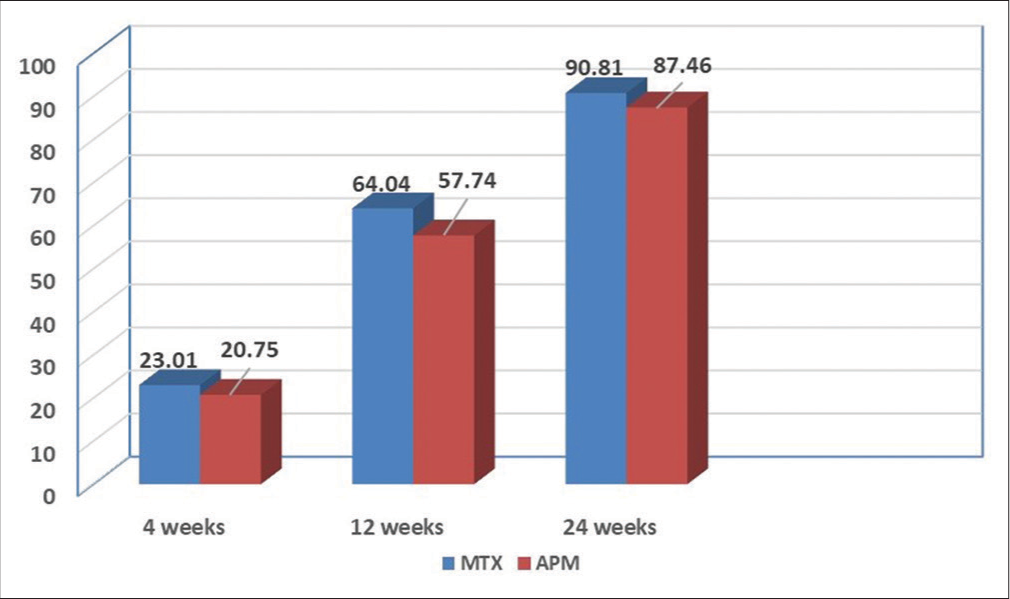
- Mean percentage improvement of psoriasis area and severity index at 4, 12, and 24 weeks. MTX: Methotrexate, APM: Apremilast.
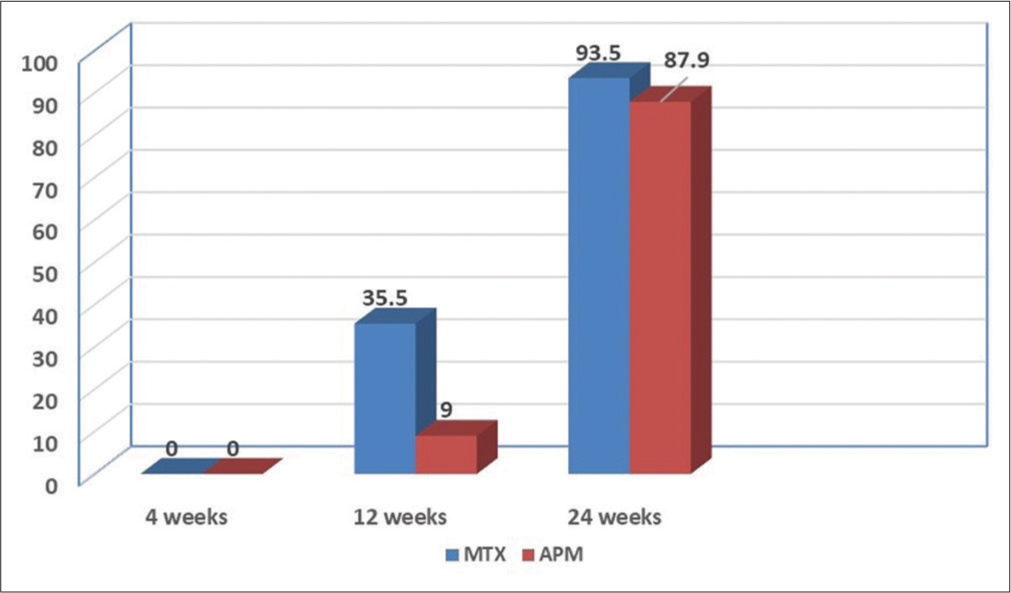
- Psoriasis area and severity index 75 at the end of 24 weeks.
| Drug | Mean Age (Years) | Commonest age group | Male/Female | Duration of psoriasis >10 years | Metabolic syndrome present | Baseline PASI | PASI 75 at 12 weeks | P-value | PASI 75 at 24 weeks | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| MTX | 56.3 | 50–59 14 cases (41.5%) |
26/5 (83.9%/16.1%) |
18 (58.07%) |
12 (38.71%) | 18.3 | 11 (35.5%) | 0.011 | 29 (93.5%) | 0.673 |
| APM | 53.7 | 50–59 9 cases (27.3%) |
26/7 (78.8%/21.1%) |
16 (48.49%) |
8 (24.24%) | 16.5 | 3 (9%) | 29 (87.9%) |
MTX: Methotrexate, APM: Apremilast, PASI: Psoriasis area severity index.
DISCUSSION
The MTX is an old, cheap, efficacious, and conventional drug used in psoriasis for a very long time. The MTX is potentially a bone marrow suppressor and long-term cumulative hepatotoxic drug; however, a low dose (7.5 mg) as used in this study is considered safe. The APM is a new generation small molecule devoid of organ-specific toxicity, but expensive compared to MTX. The treatment of psoriasis is now being revolutionized by the advent of biologicals, especially the interleukin-17 inhibitors. However, in a resource-poor country like India, they may not be the first option for most patients. This descriptive observation study compared the efficacy of an old drug, MTX versus a new, but relatively expensive drug, APM. The demographic parameters in this study were in concordance with the previous studies.[4-6] The baseline PASI in this study was comparable to the study by Rathipriyadharshini et al., but lower than other studies by Rathipriyadharshini et al. and Reddy and Pratheepa.[3,4,7] At 24 weeks, 90.6% of the total patients in the study achieved PASI 75 while only 9% did not, similar to other studies by Reddy and Pratheepa.[7] The mean baseline PASI score in both groups was almost similar [Table 1]. The baseline PASI score significantly determines the treatment outcome in psoriasis, a very high baseline score indicates a delayed response to treatment.[8] At the end of 4 weeks, there was not much improvement in the mean percentage of PASI [Figure 1]. This indicates that drugs such as MTX and APM may take time for their response to show clinical improvement and they may not be the first-line option in cases of severe psoriasis including erythroderma and arthropathy where cyclosporin or biologics can be considered.[9] At the end of 12 weeks, there was considerable improvement in the mean percentage of PASI improvement in both groups, but not significant [Figure 1]. At the end of 24 weeks, there was a 90% and 87% mean percentage PASI improvement, but not significant. PASI 75 was the ultimate clinical endpoint in this study. At the end of 4 weeks, no cases achieved PASI 75. This is similar to other studies as it is well known that MTX and APM take much longer to achieve 75 compared to the biologics.[10] At the end of 12 weeks, 35.5% in the MTX group achieved PASI compared to APM which was significant (P = 0.011). This is a unique finding in this study, as other studies have shown similar efficacy at the end of 12 weeks, keeping in mind that a low dose (7.5 mg) of MTX was used in this study, but showed faster clinical response than APM. We have observed in our department that this low dose is highly efficacious in most patients, devoid of toxicity, and affordable for the patient; hence, this low dose was used in the present study. This finding can be exploited in the context of poor patients in a country like India since MTX is a very cheap drug and may be a first-line option for these patients.[11,12] However, at the end of 24 weeks, the efficacy was similar in both groups with no significance [Figures 3 and 4]. As per Cochrane review (2017) on systemic pharmacological treatments for chronic plaque psoriasis, no difference in efficacy was shown between APM and MTX.[13] However, a recent study done in Cuttack, India, has shown slightly better efficacy for APM, but the sample size was small.[14] There are also isolated reports indicating better efficacy and tolerability of MTX over APM.[5] However, in the context of psoriatic arthropathy, the dose of MTX is higher to achieve control (15–25 mg), at which levels related toxicities may come into the picture and in this situation, APM may be the superior alternative.[15] However, the toxicity profile was not a part of the present study. Interestingly, a recent study done in India demonstrated that a combination of MTX and APM was superior to MTX alone in treating palmoplantar psoriasis.[16]
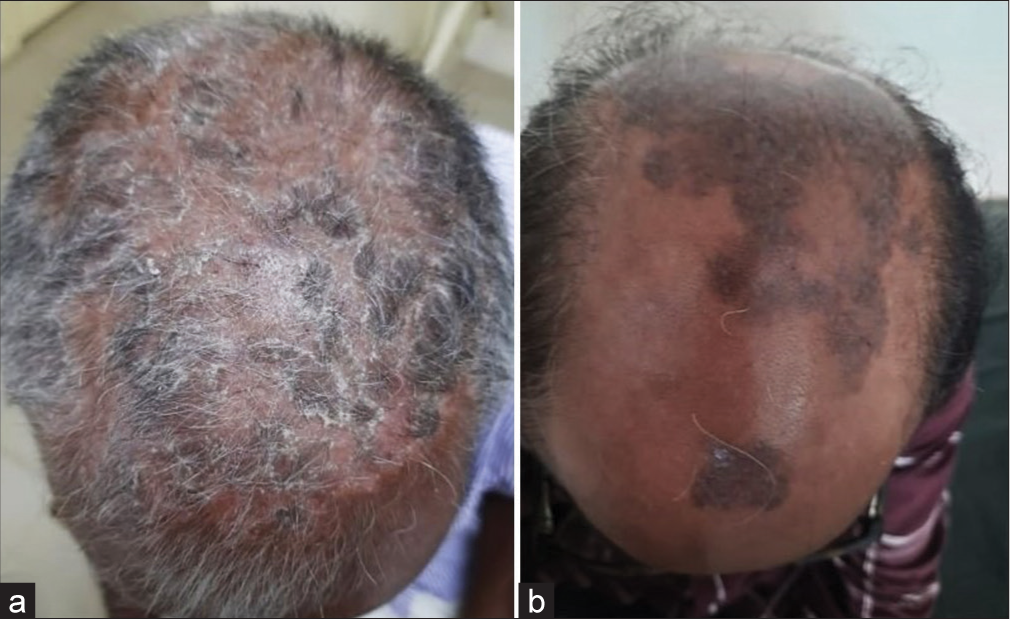
- Treatment with methotrexate: (a) Before and (b) After.
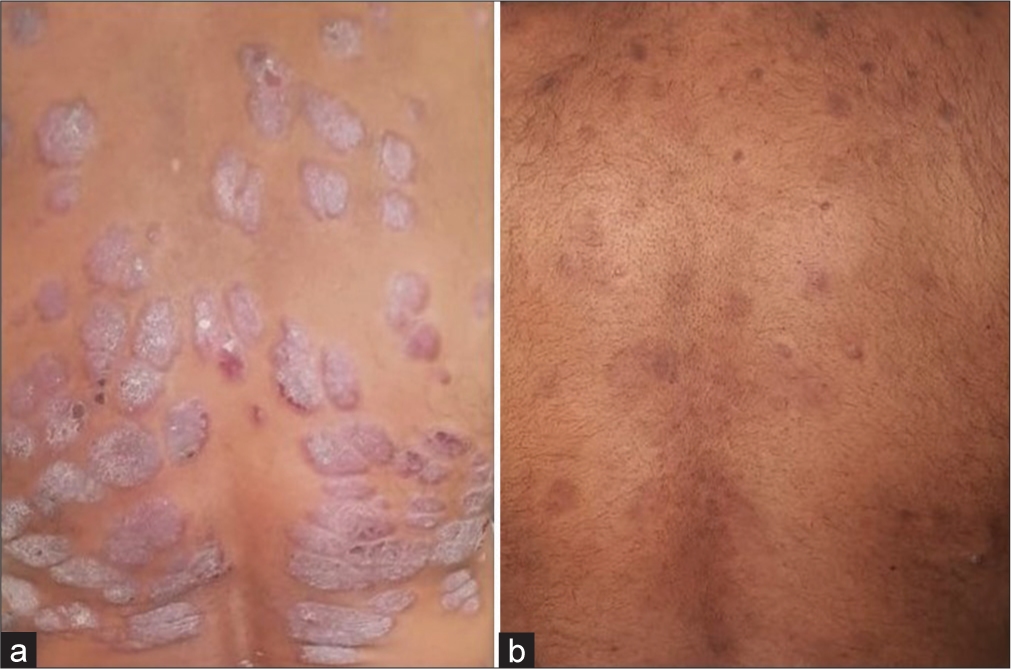
- Treatment with apremilast: (a) Before and (b) After.
Limitations of study
Small sample size, lack of randomization, and not including drug toxicity profile and no follow-up after 24 weeks.
CONCLUSION
This study showed similar efficacy of MTX and APM at the end of 24 weeks in achieving PASI 75. However, MTX showed a significantly faster response rate of improvement compared to APM at the end of 12 weeks. Low-dose MTX is still a cheap, effective, and safe first-line drug for psoriasis in resource-poor settings.
Ethical approval
The research/study approved by the Institutional Review Board at Institution Ethics Committee, Government T D Medical College Alappuzha, number EC 22/2021, dated March 29, 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Joint American Academy of Dermatology-National Psoriasis Foundation guidelines care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-86.
- [CrossRef] [Google Scholar]
- Apremilast in psoriasis and beyond: Big hopes on a small molecule. Indian Dermatol Online J. 2019;10:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- An open labelled randomised evaluation of therapeutic efficacy and safety of apremilast versus methotrexate in the treatment of patients with chronic plaque psoriasis. Ann Trop Med Pub Health. 2020;23:S517.
- [CrossRef] [Google Scholar]
- A comparative study of the efficacy and safety of oral apremilast versus oral methotrexate in patients with moderate to severe chronic plaque psoriasis. Int J Res Dermatol. 2018;4:563-9.
- [CrossRef] [Google Scholar]
- What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;4(Suppl 2):10-6.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative efficacy of methotrexate versus apremilast for methotrexate-naive psoriasis patients: An indirect comparison. J Am Acad Dermatol. 2015;72:563-72.
- [CrossRef] [Google Scholar]
- A correlative research of methotrexate and apremilast's efficacy in the management of moderate to severe plaque psoriasis. Eur J Mol Clin Med. 2023;10:5520-41.
- [Google Scholar]
- The use of apremilast in psoriasis: An Indian perspective on real-world scenarios. Psoriasis Targets Ther. 2021;11:109-22.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative effectiveness of less commonly used systemic monotherapies and common combination therapies for moderate to severe psoriasis in the clinical setting. J Am Acad Dermatol. 2014;71:1167-75.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: A phase III randomized control trial (ESTEEM 2) Br J Dermatol. 2015;173:1387-99.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: Results of an open-label, active-controlled, randomized trial (RESTORE1) Br J Dermatol. 2011;165:1109-17.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of methotrexate in psoriasis: A meta-analysis of published trials. PloS One. 2016;5:e0153740.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic pharmacological treatments for chronic plaque psoriasis: A network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD011535.
- [CrossRef] [PubMed] [Google Scholar]
- Apremilast or methotrexate: The arrows in the quiver for psoriasis. Cureus. 2023;15:e38802.
- [CrossRef] [Google Scholar]
- Comparison between methotrexate and apremilast in psoriatic arthritis-a single blind randomised control trial. Rheumatol Int. 2023;43:841-8.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate monotherapy versus methotrexate and apremilast combination therapy in the treatment of palmo-plantar psoriasis. A prospective, randomized, assessor blinded, comparative study. Indian J Dermatol Venereol Leprol. 2023;89:213-20.
- [CrossRef] [PubMed] [Google Scholar]






