Translate this page into:
More ingredients may mean more allergens, in sunscreens: A screening analysis of commonly accessed sunscreens in India

*Corresponding author: C. Divyalakshmi, Department of Aesthetic Dermatology, Render Skin and Hair, Chennai, Tamil Nadu, India. drdivya@renderclinic.com
-
Received: ,
Accepted: ,
How to cite this article: Divyalakshmi C, Selvadurairaj S, Sukumaran P, Balasubramanian R, Thirunavukkarasu V, Anuba PA, et al. More ingredients may mean more allergens, in sunscreens: A screening analysis of commonly accessed sunscreens in India. CosmoDerma. 2025;5:6. doi: 10.25259/CSDM_171_2024
Abstract
Objective:
The objective of the study is to evaluate the presence of known allergens in topical sunscreens available in India.
Materials and Methods:
Sunscreens across commonly used online marketplaces were included. A total of 135 allergens were screened, with 48 being high prevalence and 87 being low prevalence.
Results:
The number of allergens in a sunscreen formulation increased with an increasing number of ingredients. Mineral sunscreens contained significantly fewer ingredients and allergens compared to chemical and hybrid sunscreens (P < 0.001).
Conclusion:
Patch and photopatch testing are recommended to determine the relevance of these high- and low-prevalence allergens for contemporary sunscreen formulations for Indian skin types. This study initiates the framework for better product selection by consumers and dermatologists.
Keywords
Sunscreens in India
Allergens
Octocrylene
SPF
Skin of color
INTRODUCTION
Sunscreens are important tools for dermatologists treating skin of color (SOC) individuals. Post-inflammatory hyperpigmentation (PIH), a dreaded complication of procedural treatments and naturally occurring conditions such as acne and trivial trauma, is reported in up to 58.2% of individuals.[1] The use of sunscreens post-laser treatments has been known to reduce PIH.[2,3] As a product that is left on, at times even on broken skin for prolonged periods of time, the potential for contact allergy with sunscreen use remains a concern. Keyes et al. noted the presence of allergens in all of the sunscreens evaluated by them.[4] Acknowledging the rapid increase in sunscreen use, we aimed to evaluate the presence of known allergens in topical sunscreens available in India.
MATERIALS AND METHODS
Sunscreens were selected using the search terms “sunscreen,” “sun screen,” “sunscreens,” and “sunscreen in India” across commonly used online marketplaces, including Amazon, Flipkart, SkinStore, Tata 1 mg, Nykaa, Purplle, and SkinStore.in and on Google Search and listings within the first 10 pages from each source included. Sunscreens without approved ultraviolet (UV) filters were excluded. Allergens concluded to be more prevalent (i.e., more than 1%) by the North American Contact Dermatitis Group (NACDG) core data (2019–2020) were considered high-prevalence allergens while allergens linked to allergic contact dermatitis in the literature but not listed by the NACDG were classified as low prevalence allergens.[4-7] Totally, 135 allergens were taken up for screening including 48 high prevalence and 87 low prevalence allergens.
RESULTS
We screened a total of 1048 sunscreens of which 918 sunscreens were eligible for analysis, comprising 159 mineral, 330 chemical, and 429 hybrid sunscreens, the latter containing both chemical and mineral UV filters. Allergens were noted in 899 (98%) sunscreens of the total sunscreens tested, 330 (100%) among chemical sunscreens, 143 (89.9%) among mineral sunscreens, and 426 (99.3%) among hybrid sunscreens, [Table 1]. The most common high-level allergens and the most common low-level allergens in our study were fragrances and octinoxate, respectively.
| Variable | Physical (%) | Chemical (%) | Hybrid (%) | Total (%) |
|---|---|---|---|---|
| Number of sunscreens assessed | 159 (17.32) | 330 (35.95) | 429 (46.73) | 918 (100) |
| Prevalence of high-level allergens | 67 (42.14) | 226 (68.48) | 290 (67.60) | 583 (63.51) |
| Most common high-level allergens | Helianthus annuus(n=29, 18.24) | Propylene glycol (n=102, 30.90) | Fragrances (n=142, 33.10) | Fragrances (n=250, 27.23) |
| Prevalence of low-level allergens | 137 (86.16) | 329 (99.7) | 425 (99.07) | 891 (97.06) |
| Most common low-level allergens | Botanical extracts of Aloe vera(n=48, 30.19) | Phenoxyethanol (n=195, 59) | Octinoxate (n=320, 74.6) | Octinoxate (n=484, 52.7) |
| Prevalence of any allergen | 143 (89.94) | 330 (100) | 426 (99.3) | 899 (97.93) |
| Median SPF | 50 | 50 | 50 | 50 |
| Average number of ingredients (Mean±SD) | 22.97±11.26 | 26.75±10.32 | 28.38±12.76 | 26.86±11.82 |
| Average number of allergens (Mean±SD) | 2.68±2.08 | 7.55±3.06 | 7.26±3.16 | 6.57±3.46 |
| Number of sunscreens with SPF 30 | 50 (31.44) | 63 (19) | 69 (16) | 182 (19.83) |
| Average number of ingredients in sunscreens with SPF 30 (Mean±SD) | 23.94±11.30 | 25.44±9.74 | 26.25±12.283 | 25.34±11.16 |
| Average number of allergens in sunscreens with SPF 30 (Mean±SD) | 3.18±2.32 | 7.14±2.78 | 7.41±3.29 | 6.15±3.4 |
| Number of sunscreens with SPF 50 | 79 (49.69) | 195 (59) | 283 (65.97) | 557 (60.7) |
| Average number of ingredients in sunscreens with SPF 50 (Mean±SD) | 22.44±11.03 | 27.18±10.644 | 28.95±13.48 | 27.41±12.39 |
| Average number of allergens in sunscreens with SPF 50 (Mean±SD) | 2.16±1.77 | 7.71±2.843 | 7.13±3.19 | 6.63±3.44 |
SD: Standard deviation, SPF: Sun protection factor.
The number of allergens in a sunscreen formulation increased with an increasing number of ingredients, [Figure 1]. Mineral sunscreens contained significantly fewer ingredients and allergens compared to chemical and hybrid sunscreens (P < 0.001). In turn, chemical sunscreens had fewer ingredients than hybrid sunscreens (P = 0.03), but the number of allergens did not significantly differ between them. Sun protection factor (SPF) values did not correlate with the number of allergens. A higher number of ingredients correlated with higher SPF for hybrid sunscreens, but not for mineral or chemical sunscreens.
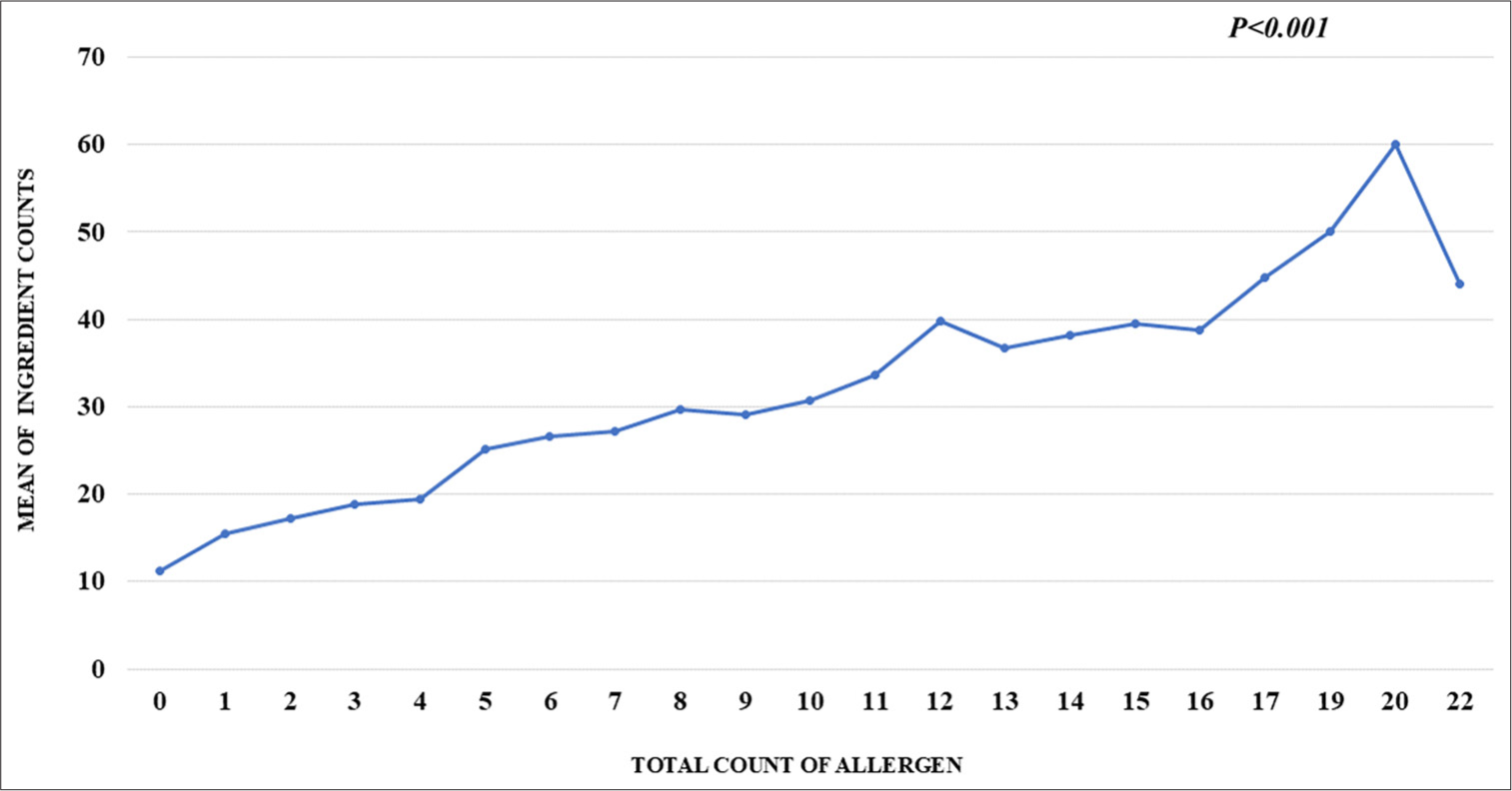
- Graph representing an increase in number of allergens with increasing number of ingredients.
DISCUSSION
Selecting a sunscreen that balances high effectiveness with low allergenicity is essential for the general population. To accomplish this, sunscreens must be assessed not only for their SPF and protective attributes but also for their ingredient composition, focusing on potential allergens.
Allergies to sunscreens have been increasingly reported in recent times, potentially reflecting a rise in global sunscreen use, with up to 95%[8-10] of positive photopatch tests being attributed to sunscreen filters, notably oxybenzone. The list of potential allergens noted in sunscreens based on current literature are listed below [Table 2]. In alignment with previous studies, fragrances emerged as the most common high-prevalence allergens in our dataset, identified in 98% of sunscreens. Literature from New Zealand[11] and other international studies[4] similarly reported fragrances as the predominant allergens in sunscreens. Our study identified octinoxate, a chemical UV filter, as the most common low-prevalence allergen. In contrast, previous literature highlights other chemical UV filters, such as octocrylene, oxybenzone, and avobenzone, as predominant low-prevalence allergens.[4] Our study notes a high prevalence of commonly acknowledged allergens in sunscreen products accessed by the Indian public, higher for chemical and hybrid sunscreens compared to mineral sunscreens. The increase in allergen number with increasing ingredient count is highly interesting, considering chemical sunscreens employed more ingredients for similar SPF as mineral and hybrid sunscreens. For the same SPF, choosing a sunscreen with a lesser number of total ingredients may translate into choosing a sunscreen with a lesser number of allergens.
| Allergen category | Low prevalence | High prevalence |
|---|---|---|
| UV filters | Octinoxate Avobenzone Octocrylene Octisalate Benzophenone-3 Homosalate |
|
| Preservatives | Phenoxyethanol Ethylhexylglycerin Sodium benzoate Benzyl alcohol Sorbic acid Benzyl benzoate Triclosan |
Methylisothiazolinone DMDM hydantoin Iodopropynyl Butylcarbamate Diazolidinyl urea Methylchloroisothiazolinone Imidazolidinyl urea |
| Botanicals | Botanical extracts Aloe vera Jojoba A-bisabolol A-tocopherol acetate M. alternifolia/melaleuca alternifolia |
H. annuus/sunflower Matricaria chamomilla Calendula officinalis Linne C. officinalis Carthamus tinctorius Linne Anthemis nobilis Linne L. Chrysanthemum Centaurea cyanus Linne Achillea millefolium Linne |
| Emulsifier | Triethanolamine Cetyl alcohol Sorbitan Stearate Sorbitan Oleate Sorbitan sesquioleate Sorbitan Laurate Sorbitan Palmitate |
Decyl glucoside |
| Fragrance | Butylated hydroxytoluene D-limonene Citronellol Stearyl alcohol Benzoic acid Benzyl salicylate Benzyl 2-hydroxybenzoate Vanillin Lilial Geranium oil Butylated hydroxyanisole Amyl cinnamal Coumarin Rosa Damascena flower oil Propyl gallate Lyral |
Fragrances Propylene glycol Linalool Hydroperoxide Geraniol Hydroxy citronellal Eugenol Cinnamyl alcohol |
| Others | Tocopheryl acetate Parabens Coco-glucoside A. mellifera/Honey L. angustifolia/lavender Panthenol Isopropyl myristate |
Lauryl glucoside Gold |
UV: Ultraviolet
An impaired epidermal barrier post-fractional laser resurfacing was shown to be associated with increased contact sensitization to octocrylene, compared to those with intact skin.[12] However, early use of sunscreens is important to reduce PIH after fractional laser treatments.[3] This raises a dilemma in deciding between sun protection and allergen avoidance for the dermatologist treating SOC patients. To choose sunscreens with lesser allergenicity, physicians may simply consider sunscreens with a lesser number of ingredients when advising patients on these special care circumstances, such as post-fractional laser ablation. Further, mineral sunscreens may be advisable for sensitive skin, in keeping with earlier studies.[8]
CONCLUSION
With an exponential rise in sunscreen awareness and use, it is important to remain vigilant for contact dermatitis to sunscreens. While it would be very useful to know which of these high- or low-level allergens are relevant in modern-day formulations in Indian skin types in prevalent weather through patch testing and photopatch testing, our study provides a good starting point to guide better product selection for consumers and dermatologists alike.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required, as there are no patients in this study.
Conflicts of interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Frequency and characteristics of acne-related post-inflammatory hyperpigmentation. J Dermatol. 2016;43:826-8.
- [CrossRef] [PubMed] [Google Scholar]
- Expert recommendations on supportive skin care for non-surgical and surgical procedures. J Eur Acad Dermatol Venereol. 2023;37(Suppl 3):16-33.
- [CrossRef] [PubMed] [Google Scholar]
- The use of sunscreen starting on the first day after ablative fractional skin resurfacing. J Eur Acad Dermatol Venereol. 2014;28:1522-8.
- [CrossRef] [PubMed] [Google Scholar]
- Potential allergenicity of commonly sold high SPF broad spectrum sunscreens in the United States; from the perspective of patients with autoimmune skin disease. Int J Womens Dermatol. 2019;5:227-32.
- [CrossRef] [PubMed] [Google Scholar]
- Allergens in cosmetics. U.S. Food and Drug Administration. Available from: https://www.fda.gov/cosmetics/cosmetic-ingredients/allergenscosmetics#common [Last accessed on 2024 Sep 17]
- [Google Scholar]
- North American Contact dermatitis group patch test results: 2019-2020. Dermatitis. 2023;34:90-104.
- [CrossRef] [PubMed] [Google Scholar]
- Allergic or not: Final interpretation of doubtful patch test reactions from the North American contact dermatitis group, 2019-2020. Dermatitis. 2024;35:138-43.
- [CrossRef] [PubMed] [Google Scholar]
- Photopatch testing in Bogota (Colombia): 2011-2013. Contact Dermatitis. 2016;74:11-7.
- [CrossRef] [PubMed] [Google Scholar]
- Sunscreens: A review of UV filters and their allergic potential. Dermatitis. 2023;34:176-90.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25:289-326.
- [CrossRef] [PubMed] [Google Scholar]
- Ultraviolet filter, fragrance and preservative allergens in New Zealand sunscreens. Aust J Dermatol. 2022;63:e21-5.
- [CrossRef] [PubMed] [Google Scholar]
- The sensitization potential of sunscreen after ablative fractional skin resurfacing using modified human repeated insult patch test. J Dermatolog Treat. 2015;26:485-8.
- [CrossRef] [PubMed] [Google Scholar]






