Translate this page into:
An open-label, single-arm, clinical study to evaluate the efficacy and safety of Nyumi happy hair gummies in healthy adult subjects with hair fall

*Corresponding author: Ananya Agarwal, Ikaria Wellness Pvt. Ltd., Mumbai, Maharashtra, India. ananya@nyumi.com
-
Received: ,
Accepted: ,
How to cite this article: Karmase A, Agarwal A, Patel B, Sethi S, Joshi P, Shrivastava A. An open-label, single-arm, clinical study to evaluate the efficacy and safety of Nyumi happy hair gummies in healthy adult subjects with hair fall. CosmoDerma. 2024;4:142. doi: 10.25259/CSDM_165_2024
Abstract
Objectives:
Nutrition is pivotal for healthy hair growth. The clinical study was conducted to assess the safety and efficacy of multivitamins, minerals, and nutraceutical ingredients-based test gummies in Healthy Adult Subjects with hair fall.
Materials and Methods:
An open-label, single-arm, clinical study was conducted to evaluate the efficacy and safety of test Gummies in healthy adult participants with hair fall. Dermatological, instrumental, and subjective assessments were performed. Hair pluck test, hair thickness, hair density, and hair growth by CASlite Nova, 60-s comb test, hair pull test, hair gloss/shine by Glossymeter, dermatologist evaluation for the general appearance of scalp hair, scalp skin and nail brittleness, nail growth, and nail health satisfaction, along with subject satisfaction questionnaire were the study parameters. Safety assessment was also carried out based on local intolerance.
Results:
Thirty-two subjects completed all study visits. At the end of the study, the anagen and telogen ratio (A:T ratio) was improved by 28.27%. Hair density, hair growth, and hair thickness showed significant improvement by 15.0%, 98.7%, and 15.0%, respectively. Hair fall reduction in hairs with bulb and hairs without bulb (i.e., broken hairs) by 74.7% and 75% was recorded. A reduction of hair strands by 65.6% was also observed by the hair pull test. Improvement in hair gloss by 1.39 folds was observed. All subjects reported dense hair, good shininess, and a reduction in hair dullness and 93.75% of subjects reported full hair volume at the end of the study. There were no signs of scalp skin redness, roughness, dryness, or skin itchiness at the end of the study. Improvement in nail brittleness by 51.0%, nail growth by 1.17 folds, and nail health by 1.12 folds was also established. Moreover, no adverse event was recorded during the study conduction.
Conclusion:
Nyumi Happy Hair Gummies were found to be safe based on no apparent or experienced discomfort, reactions or any kind of intolerance or adverse skin reactions or events evidenced in the trial. The Gummies were found to be efficacious in improving A: T ratio, hair density, hair growth, hair thickness, hair strength, and hair gloss/shine. The test formulation also improved hair density, hair volume, and hair shininess in all participants. It also reduced hair fall, scalp skin redness, roughness and dryness, and scalp skin itchiness. Furthermore, improvement in nail health, nail growth, and nail brittleness was also observed. The test product was endorsed by all the subjects for palatable, chewy, easy to swallow, appealing taste, and appealing smell parameters.
Keywords
Hair fall
Hair growth
Hair strength
Hair shine
Nail health
INTRODUCTION
Hair is certainly a vital component of the body and the one of few physical characteristics throughout the ages that reflects in personality building and the general appearance of an individual. Healthy hairs are characterized by smooth texture, dense volume, natural color, shine, and more hairs in the growing phase. However, hair loss is a universal distressing condition involving various genetic, nutritional, medical, and environmental factors.[1] Deficiency of various nutrients can disrupt the normal cycling of a follicle and causes visible changes to the hair shaft.[2] Hair loss has significant social, psychological, and emotional impacts on an individual.[3]
Human beings are born with approximately 100,000 terminal hair follicles on the scalp, with a hair growth rate of 0.05 inches/month.[3,4] Anagen, catagen, telogen, and exogen are the four primary phases of the hair cycle. Among these, anagen is the most important phase, which is characterized by the production of the hair shaft from the hair follicle that comprises approximately 85–90% of hairs. Telogen (10–15% hairs) is a quiescence phase, characterized by the reduction of proliferation and biological activity of hair follicles. Whereas, catagen (1–2% hairs) describes regression and the resting phase of the follicle, respectively, resulting in hair shedding.[5] Anagen phase lasts 3–5 years, catagen 2–3 weeks, and telogen approximately 3–4 months, followed by shedding of hair.[1]
Anything that disrupts the normal hair growth cycle causes more hairs to enter the telogen phase, resulting greater amount of hair fall. Shedding of 50–100 hairs from the head a day is considered normal for any individual. However, a fall of more than 100 hairs in a day becomes a point of concern. Age, inflammation, hormones, stress, poor sleep quality, genetic, and environmental factors, along with nutritional deficiencies, are some of the key factors that promote anagen to telogen transition and may influence hair fall to a great extent.[5] The changes in the hair cycle dynamics, that is, a gradual decrease in the anagen phase and an increase in the telogen phase lead to miniaturization of hairs and finally thinning and balding.[6]
Hair care has gained a lot of importance in recent years. A balanced diet and proper nutrition are essential for anagen and telogen balance. Caloric or nutritional deficiency may negatively impact hair structure, growth, and pigmentation.[7,8] In addition to fatty acids and protein, various other nutritional components have been evaluated for their effects on hair structure and growth, including vitamins and minerals. The test product is a gummy vehicle by oral route and a unique combination of nutraceutical ingredients, namely, multivitamins (Vitamin A, B3, B5, B6, Folic Acid, B12, C, D2, and E), biotin, iodine, selenium, zinc, choline, inositol, and amla (Indian Gooseberry) fruit extract. The ingredients are well established in the literature for their hair nutrition and hair protectant action. In the present study, the cumulative effect of test formulation is intended to clinically establish its safety and efficacy for hair care properties and protection from hair fall in healthy adult human subjects with hair fall symptoms.
MATERIALS AND METHODS
Study design
An open-label, single-arm, and clinical study was conducted to evaluate the efficacy and safety of test Gummies in healthy adult participants with hair fall. The potential subjects were screened and enrolled in the study as per the inclusion and exclusion criteria after obtaining written informed consent. All eligible subjects were instructed to simply chew and swallow two gummies together after dinner for 90 days. Efficacy assessments by dermatologists, instruments, and subjective evaluation were performed on specified study visits. Safety was assessed throughout the study based on local intolerance.
The study duration was 90 days from the enrolment visit and consisted of a total of eight visits, including one screening visit, three shaving visits, and four evaluation visits.
Study participants
A total of 70 subjects were screened and signed the informed consent document (ICD) for the study. 56 subjects passed the screening, and 14 subjects failed during the screening visit as per inclusion-exclusion criteria. Thirty-six subjects who met all the study criteria were enrolled in the study. Of these, 32 subjects completed all the phases of the study. Four subjects were withdrawn from the study due to lost to follow up.
Among 36 enrolled subjects, there were 24 female and 12 male participants. Age of the subjects ranged between 21 and 44 years with an average being 35.6 years [Table 1].
| Variables | (n=36) (%) |
|---|---|
| Gender n (%) | |
| Female | 24 (66.67) |
| Male | 12 (33.33) |
| Race n (%) | |
| Asian | 36 (100) |
| Hair type n (%) | |
| Colored | 2 (5.56) |
| Dry | 5 (13.89) |
| Normal | 10 (27.78) |
| Oily | 19 (52.78) |
| Age (Years) | |
| N | 36 |
| Mean (SD) | 35.6 (5.88) |
| Median | 37.0 |
| Min, Max | 21, 44 |
SD: Standard deviation
Ethics
This study was carried out in accordance with “The Code of Ethics of the World Medical Association” (Declaration of Helsinki), the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH)-GCP (E6 R2), and applicable ethical guidelines for experiments involving humans. Prior approval on Study Protocol (C3B02404) and ICD was taken from the independent ethics committee before initiating the study. The trial was registered with the Clinical Trial Registry of India (CTRI) (CTRI/2022/08/044809) before the commencement of the study.
Inclusion criteria
Healthy males and non-pregnant/non-lactating females of 20– 45 years, in the ratio of 1:2, were included in the present study. Females of childbearing potential must have a negative urine pregnancy test performed during screening. Subjects with thin hair, having 50–100 hair fall per day were included in the study. In addition, females with at least 50 counts of hair fall and males with 30–50 counts of hair fall from the 60s hair combing method were the key inclusion criteria. Subjects who did not apply any hair color/dye before inclusion into the study; male subjects with a minimum hair length of 2 inches; subjects committed to not use medicated/prescription shampoos/hair care products (containing Minoxidil/Anti-hair fall agents) or any other hair fall treatment/hair products other than the test product for the entire duration of the study; subjects willing to refrain from any other treatment for the main indications for which the study test products are being given during the course of the study; subjects willing to consume test product throughout the study period as instructed; subjects in good general health as determined by the investigator based on medical history and vital signs; subject must be able to understand and provide written informed consent to participate in the study; and subjects willing and able to follow the study protocol and study directions, to participate in the study, were some other inclusion criteria.
Exclusion criteria
Subjects with a history of any dermatological condition of the scalp other than hair loss and/or dandruff were excluded from the study. The other key exclusion criteria were subjects having applied topical treatment for hair loss for at least 4 weeks and any systemic treatment for at least three months, before their participation in the study; subjects having undergone hair growth treatment within three months before screening; subjects who were on chronic oral steroids three months before initial application and during the study period; subjects with hair loss due to autoimmune disorder/chemotherapy/immunosuppressive drugs/any major illness/polycystic ovary syndrome (PCOS) etc. by history; subjects with conditions known to cause premature greying such as progeria, Rothmund–Thomson syndrome, Werner’s syndrome, and so on; subjects with a history of cigarette smoking or medications; subjects having plans of shaving of scalp hair during the course of the study; subjects having any active scalp disease which may interfere in the study; subject with history of allergies to oral care/personal care consumer products or their ingredients; subjects on any prescription medicines that might interfere with the study outcome; pregnant or breastfeeding or planning pregnancy during the study period; subjects participating in other similar cosmetic or therapeutic trial within past four weeks; or any other condition which could warrant exclusion from the study, as per the Dermatologist’s/investigator’s discretion.
Test product
The test product (Nyumi Happy Hair Gummies of Ikaria Wellness Pvt. Ltd.), a blend of Indian and Western ingredients having a unique combination of Multivitamin (Vitamin A, B3, B5, B6, Folic Acid, B12, C, D2, and E), biotin, iodine, selenium, zinc, choline, inositol, and amla (Indian Gooseberry) fruit extract, etc., was provided to all study participants for 90 days. The subjects were asked to consume two gummies together once daily after dinner. Test product compliance was checked during all study visits.
Efficacy endpoints
Primary endpoint(s)
The primary efficacy endpoint was to assess the effect of the test product on anagen and telogen ratio (A: T ratio) by hair pluck test and microscopic evaluation at baseline on day 01 and end of treatment (EOT) that is, day 90.
Secondary endpoint(s)
The other study endpoints were hair thickness, hair density, and hair growth by CASlite Nova (Catseye System and Solution, India), hair fall reduction by 60 s combing method, hair strength by hair pull test, hair gloss/shine by Glossymeter, change in the general appearance of scalp hair, that is, hair volume, hair density, and hair shininess by a dermatologist, general appearance of scalp skin, that is, itchiness, redness, roughness, and scaliness of scalp by dermatologist, nail brittleness on the basis of global improvement rating performed by the dermatologist, nail growth, and nail health satisfaction by 0–10 visual analog scale (VAS) scale, along with Subject Satisfaction and Product perception Questionnaires. Digital photographs of the scalp and hairs were taken in addition for representation purposes.
Subjects were monitored throughout the study for undesirable/adverse events (AEs) either by self-reporting or at the time of every study visit.
Statistical analysis
All statistical tests of the hypothesis were employed with a level of significance of 0.05. If the P-value was observed ≤0.05, the test was considered statistically significant otherwise non-significant. The statistical analysis was done using Statistical Analysis System (SAS)® statistical software (Version: 9.4 or higher; SAS Institute Inc., USA). Continuous variables were summarized using tables of descriptive statistics (i.e., Mean, standard deviation, median, minimum, and maximum). Categorical variables were summarized using counts and percentages. For continuous variables, the within-treatment analyses were conducted to compare baseline to post-treatment data using paired t-tests. For categorical variables, the within-treatment analysis was done to compare baseline to post-treatment analysis using the Wilcoxon signed-rank test.
RESULTS
Assessment of A:T ratio by hair pluck test and microscopic evaluation
After consuming the test product for 90 days, the mean score of the A: T ratio was increased to 3.66 from 2.86 at baseline, showing a significant improvement by 28.27% (P < 0.0001) [Figure 1]. This indicates that the test product effectively improved the growth phase of the hairs and decreased hair loss.
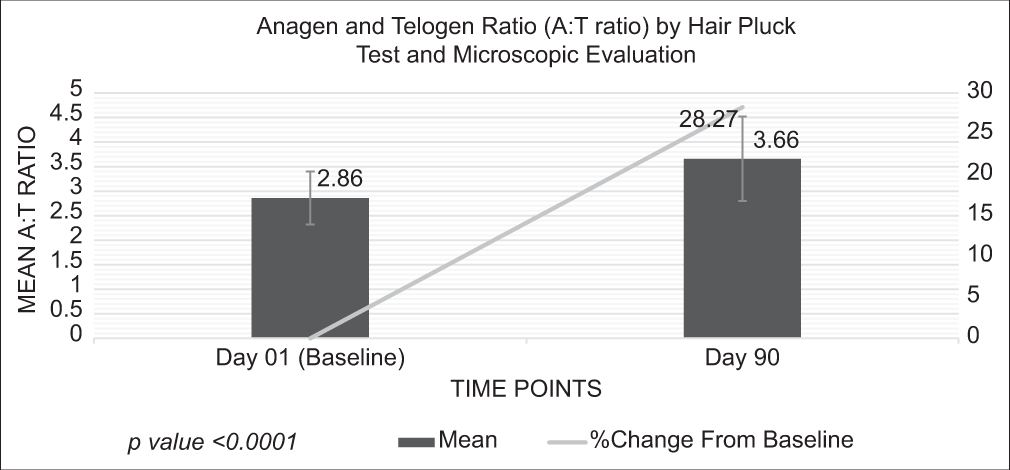
- Assessment of anagen and telogen ratio by hair pluck test.
Hair thickness, hair density, and hair growth by CASlite nova
Post-test product consumption, hair density exhibited significant improvement (all P < 0.0001) by 8.4% on day 50 and 15.0% on day 90, as compared to baseline. Significant improvement (all P < 0.0001) by 59.4% on day 50 and 98.7% on day 90 for hair growth and 8.1% on day 50 and 15.0% on day 90 for hair thickness was observed [Table 2 and Figure 2].
| Parameters | Visit 03 day 01 | Visit 06 day 50 | Visit 08 day 90 | ||||
|---|---|---|---|---|---|---|---|
| Baseline (SD) | Post baseline (SD) | CFB | %CFB | Post baseline (SD) | CFB | %CFB | |
| Mean Value | |||||||
| Hair Thickness (µm) | 21.7* (±2.16) | 23.2* (±2.23) | 1.7 | 8.1 | 24.9* (±2.15) | 3.2 | 15.0 |
| Hair Density (cm2) | 141.7* (±26.53) | 155.2* (±23.40) | 11.0 | 8.4 | 161.5* (±23.39) | 19.8 | 15.0 |
| Hair Growth (µm) | 1015.8* (±243.24) | 1553.6* (±337.47) | 519.2 | 59.4 | 1916.0* (±296.83) | 900.2 | 98.7 |
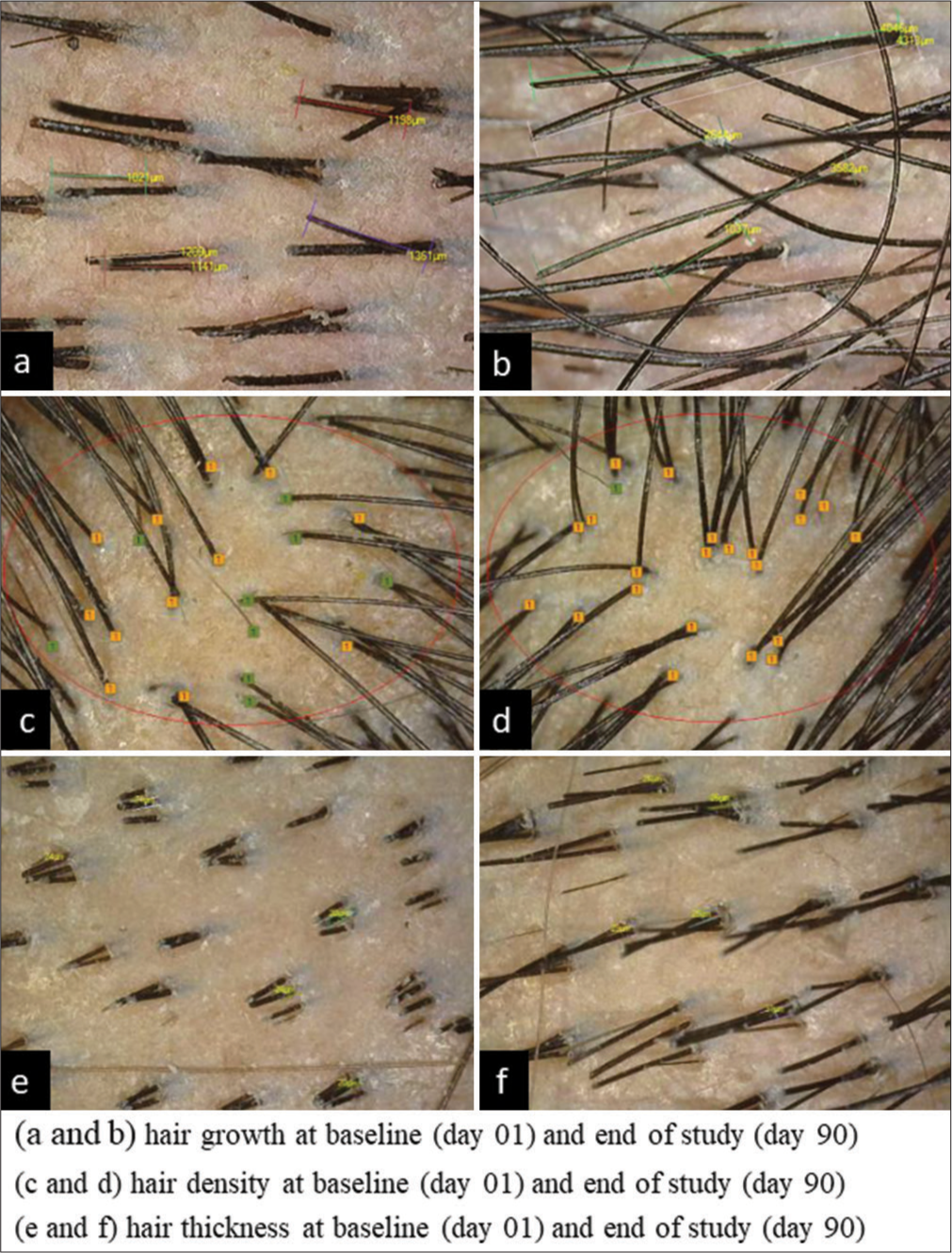
- Hair thickness, hair density and hair growth by CASlite nova. (a) CASlite nova images for hair growth. (b) CASlite nova images for hair density. (c) CASlite nova images for hair thickness.
Assessment of hair fall reduction by counting the number of hair breaks after the 60s combing method
The results of the 60-s comb test established a significant reduction (P < 0.0001) in hairs with bulbs by 17.3%, 52.5%, and 74.7% on day 25, day 50, and day 90, respectively, as compared to baseline. Significant reduction (P < 0.0001) of 18.9%, 55.5%, and 75.0% on day 25, day 50, and day 90, respectively, was also recorded for the hairs without bulbs (i.e., broken hairs). This clinically indicates that the test product significantly reduces hair fall [Table 3].
| Visits | Hair fall reduction by 60 s combing method |
Hair strength by hair pull test | Hair gloss/shine by Glossymeter | Nail brittleness by dermatologist using Global improvement rating | Nail growth and Nail health satisfaction by 0–10 VAS scale | ||
|---|---|---|---|---|---|---|---|
| Hair fall with bulb | Hair fall without bulb | Nail Growth | Nail Health | ||||
| Visit 03 day 01 (Baseline) | |||||||
| Value (SD) | 28.3 (±2.27) | 27.1 (±2.33) | 3.5 (±0.62) | 0.768 (±0.2911) | 2.6 (±0.80) | 3.8 (±0.92) | 4.0 (±1.14) |
| Visit 04 day 25 | |||||||
| Value (SD) | 23.4 (±2.09) | 21.9 (±1.78) | 2.4 (±0.49) | 1.118 (±0.3154) | 2.7 (±0.47) | 5.4 (±0.71) | 5.3 (±0.74) |
| %CFB | −17.3 | −18.9 | −32.7 | 50.90 | 25.5 | 46.3 | 40.9 |
| Visit 06 day 50 | |||||||
| Value (SD) | 13.4 (±1.76) | 12.1 (±2.58) | 2.0 (±0.00) | 1.509 (±0.3178) | 3.0 (±0.00) | 6.7 (±0.53) | 6.7 (±0.53) |
| %CFB | −52.5 | −55.5 | −42.6 | 101.65 | 44.8 | 88.5 | 81.3 |
| Value (SD) | 7.1 (±2.12) | 6.7 (±2.28) | 1.2 (±0.40) | 1.712 (±0.3362) | 3.3 (±0.44) | 7.8 (±0.75) | 7.8 (±0.75) |
| %CFB | −74.7 | −75.0 | −65.6 | 139.18 | 51.0 | 117.7 | 112.0 |
CFB: Change from baseline, SD: Standard deviation, VAS: Visual analogue scale
Assessment of hair strength by the hair pull test
Statistically significant reduction (P < 0.0001) was recorded from baseline in number of hair strands by 32.7%, 42.6%, and 65.6% on day 25, day 50, and day 90, respectively, endorsing improvement in hair strength [Table 3].
Assessment of hair gloss/shine by Glossymeter GL 200
Instrumental assessment by Glossymeter showed significant improvement (P < 0.0001) by 50.9%, 101.65%, and 139.18% on day 25, day 50, and day 90 as compared to baseline, indicating the effectiveness of the test product in improving hair gloss/shine and reducing hair dullness [Table 3].
General appearance of scalp hair and scalp skin
At baseline, all subjects (100%) had thin hairs (i.e., less hair density) and none had dense hair. On day 25, 65.63% of subjects reported dense hair. The ratio was further improved to 100% on day 50 and day 90, whereas none of the subjects had thin hairs. About 100% of subjects had medium hair volume and none had full hair volume at baseline. However, on day 25, day 50, and day 90, 15.63%, 27.59%, and 93.75% of subjects had full hair volume, respectively. In a similar way, 100% of subjects had good shininess and a reduction in hair dullness at the end of the study. There was a complete absence of scalp skin redness, roughness, and dryness throughout the study. For scalp skin itchiness, 40.63% of subjects reported at baseline that they felt itchiness. There was a complete absence in scalp skin itchiness in 100% of subjects at the end of the study.
Assessment of nail brittleness by a dermatologist using global improvement rating
On consuming the test product for 25 days, there was an improvement in nail brittleness by 25.5%, though it was not significant. After consuming the test product for 50 days and 90 days, there was significant improvement by 44.8% (P = 0.0078) and 51.0% (P < 0.0001), respectively [Table 3].
Assessment of nail growth and nail health satisfaction by 0-10 VAS scale
Significant improvement (all P < 0.0001) in nail growth by 46.3%, 88.5%, and 117.7% and in nail health by 40.9%, 81.3%, and 112.0% was observed on day 25, day 50, and day 90, respectively, as compared to baseline [Table 3].
Subjective assessment
On specified visits, that is, day 25, day 50, and day 90, 100% of subjects agreed to strongly agreed that the test Gummies reduced hair fall and nail brittleness; and improved hair density, thickness, softness, smoothness, luster, and shine, and nail growth. All study subjects (100%) also endorsed that it is palatable, chewy, easy to swallow, has an appealing taste, and smell, and overall likeability.
Safety assessment
No apparent AEs were observed during the study conduction.
DISCUSSION
Nutrition plays an important role in the overall health of an individual, hair health in particular. Different nutritional components have been evaluated for their effect on hair structure and growth. Numerous studies have been conducted to establish the correlation between nutritional deficiency and hair loss. Some micronutrients directly impact the normal hair follicle cycle and foster cellular turnover of matrix cells in hair follicle bulbs.[8] The role of nutrition and diet in treating hair loss represents a dynamic and growing area of inquiry.[8] An analysis of hair shows that it is composed of iron, oxygen, hydrogen, nitrogen, and sulfur that need to be transported to the scalp for proper nourishment and hair growth.[9]
Some minerals, namely, calcium, copper, chromium, iodine, zinc, and magnesium are necessary to retain healthy hairs. Deficiency of any of them may result in a reduction in blood circulation, which is inevitable for healthy hair growth. Blood circulation is also important for balancing thyroid hormones that prevent dry hair, hair loss, and defects in hair pigmentation.[10]
The Vitamin B complex plays an important role in cell metabolism. Researches conducted on the role of riboflavin, biotin, folate, and Vitamin B12 deficiencies suggest that hair loss is closely associated with their insufficiency. Moreover, iron, Vitamin D, folate, Vitamin B12, and selenium are involved in hair greying/whitening during childhood or early adulthood. Genetic biotin deficiency is associated with severe dermatitis and alopecia and acquired biotin deficiency is characterized by alopecia and brittle nails.[8] Supplementing these deficient micronutrients can improve premature greying.[8] Folate and Vitamin B12 play significant role in nucleic acid production, which suggests their role in the highly proliferative hair follicle.[11] Vitamin C plays an essential role in the absorption of iron in the intestine due to its chelating and reducing effect.[12] Vitamin C intake therefore plays a significant role in individuals with hair loss associated with iron deficiency. Being a rich source of Vitamin C, the Indian gooseberry provides nutrition and helps in hair loss protection. It also stimulates the proliferation of dermal papillae and enhances the hair growth.
Vitamin D is an essential nutrient that supports the absorption of calcium in the gut. Theory suggests that the expression of the Vitamin D receptor is required for a normal hair cycle,[13] including anagen initiation.[14] Comparatively low Vitamin D levels have been observed in trial participants with hair loss, both in men and women in many studies. Although Vitamin D monotherapy did not result in significant improvement in a clinical study, oral Vitamin D supplementation with minoxidil was found to be significantly more effective than minoxidil alone in female pattern baldness.[15] Vitamin E has good antioxidant properties that help to protect against free-radical damage, hence considered effective in hair count increase. It also aids real circulation in the scalp due to increased oxygen uptake in blood, which, therefore, plays an important role in promoting hair growth and preventing hair loss.
Iron, zinc, and selenium are minerals that have been implicated in hair cycle regulation. Zinc is a key component of various metalloenzymes that regulate protein synthesis and cell division. Earlier studies have established the benefit of its supplementation in androgenic alopecia and telogen effluvium. Selenium is another micronutrient that protects from oxidative damage and hair follicle morphogenesis. In previous clinical studies, its supplementation led to hair re-pigmentation[16] and improvement of alopecia.[17]
It is therefore evident that the active ingredients of test products are well established in the literature for their proven efficacy in hair growth, hair pigmentation, and hair fall protection activity. The test Gummies are a unique combination of nutraceutical ingredients, that is, multivitamins (Vitamin A, B3, B5, B6, Folic Acid, B12, C, D2, and E), biotin, iodine, selenium, zinc, choline, inositol, and amla (Indian Gooseberry) fruit extract. The results of the present clinical study indicate that the gummies effectively promote hair growth and prevent hair loss. At the end of the study, the mean score of the A: T ratio was improved by 28.27% indicating improvement in the growth phase of the hair and decrease in the hair loss. Hair density, growth, and thickness showed significant improvement by 15.0%, 98.7%, and 15.0%, respectively. Hair fall reduction in hairs with bulb and hairs without bulb (i.e., broken hairs) by 74.7% and 75% was recorded. Reduction of hair strands by 65.6% was also observed in the hair pull test. This clinically indicates that the test product significantly reduces hair fall. Improvement in hair gloss by 1.39 folds by Glossymeter indicates the effectiveness of the test product in improving hair gloss/shine and reducing hair dullness. The outcome of subjective assessments established the fact that the gummies improve hair shine, volume, and density. During the evaluation, all subjects reported dense hair, good shininess, and a reduction in hair dullness and 93.75% of subjects reported full hair volume at the end of the study. Moreover, there were no signs of scalp skin redness, roughness, dryness, or skin itchiness at the end of the study. The test Gummies were also found effective for nail health, which was evident from the improvement in nail brittleness by 51.0%, nail growth by 1.17 folds, and nail health by 1.12 folds. About 100% of subjects strongly agreed that the test product reduces hair fall and nail brittleness, improves hair density, thickness, softness, smoothness, luster, and shine and nail growth. All participants also endorsed the product for its palatability, chewy, easy to swallow, appealing taste, and smell and overall likeability parameters.
The authors acknowledge certain limitations of the present study. Open-label study design, without comparator agent, lower sample size, and short study duration are a few of them. Further study on a larger population is required to establish the safety and efficacy of the product in a larger group. Randomized control clinical trial for longer duration needs to be done to further confirm the efficacy of the test product.
CONCLUSION
At the end of the study, the Gummies were found to be efficacious in improving A: T ratio, hair density, hair growth, hair thickness, hair strength, and hair gloss/shine. The test formulation also improved hair density, hair volume, and hair shininess in all the study subjects. It also reduces hair fall, scalp skin redness, roughness, and dryness and also scalp skin itchiness. Furthermore, improvement in nail health, nail growth, and nail brittleness was also observed. Safety of the test Gummies was also established based on no apparent or experienced discomfort, reactions, or any kind of intolerance during the trial.
Ethical approval
The research/study approved by the Institutional Review Board at OM – Institutional Ethics Committee, number C3B02404, dated August 13, 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Aniket Karmase and Ananya Agarwal are employees of Ikaria Wellness Pvt. Ltd., which is the sponsor of this study and owns brand Nyumi. The other authors declare no conflict of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Appropriate funding for study conduction was received from Ikaria Wellness Pvt. Ltd., India (which owns the brand Nyumi) for clinical trial conduction.
References
- Clinical study to evaluate the efficacy and safety of a hair serum product in healthy adult male and female volunteers with hair fall. Clin Cosmet Investig Dermatol. 2020;13:691-700.
- [CrossRef] [PubMed] [Google Scholar]
- Role and mechanisms of phytochemicals in hair growth and health. Pharmaceuticals. 2023;16:206.
- [CrossRef] [PubMed] [Google Scholar]
- Integrative and mechanistic approach to the hair growth cycle and hair loss. J Clin Med. 2023;12:893.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective efficacy and safety study of an innovative Kerascalp hair growth serum in mild-to-moderate alopecia in India: Regrowth study. Cureus. 2023;15:e38742.
- [CrossRef] [Google Scholar]
- Diet and hair loss: Effects of nutrient deficiency and supplement use. Dermatol Pract Concept. 2017;7:1-10.
- [CrossRef] [PubMed] [Google Scholar]
- The role of vitamins and minerals in hair loss: A review. Dermatol Ther (Heidelb). 2019;9:51-70.
- [CrossRef] [PubMed] [Google Scholar]
- Hair growth and rejuvenation: An overview. J Dermatol Treat. 2011;22:123-32.
- [CrossRef] [PubMed] [Google Scholar]
- Three of the B vitamins: Folate, vitamin B6, and vitamin B12 Boston, MA: Harvard TH, Chan School of Public Health; 2018.
- [Google Scholar]
- The role of vitamin D in non-scarring alopecia. Int J Mol Sci. 2017;18:2653.
- [CrossRef] [PubMed] [Google Scholar]
- Does D matter? The role of vitamin D in hair disorders and hair follicle cycling. Dermatol Online J. 2010;16:3.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment with oral vitamin D alone, topical minoxidil, or combination of both in patients with female pattern hair loss: A comparative clinical and dermoscopic study. J Cosmet Dermatol. 2022;21:3917-24.
- [CrossRef] [PubMed] [Google Scholar]
- Macrocytosis and pseudoalbinism: Manifestations of selenium deficiency. J Pediatr. 1987;111:711-71.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features of selenium deficiency in infants receiving long-term nutritional support. Nutrition. 2007;23:782-7.
- [CrossRef] [PubMed] [Google Scholar]






