Translate this page into:
Nail changes in diabetes mellitus along with dermoscopic correlation: A cross-sectional observational study from a tertiary care institute in North India

*Corresponding author: Dr. Mohita Mahajan, Department of Dermatology, Venerology, and Leprosy, Government Medical College, Amritsar, Punjab, India. mohitamahajan96@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mahajan M, Mahajan BB. Nail changes in diabetes mellitus along with dermoscopic correlation: A cross-sectional observational study from a tertiary care institute in North India. CosmoDerma. 2024;4:138. doi: 10.25259/CSDM_155_2024
Abstract
Objectives:
The pathological process of diabetes that occurs systemically is reflected in the nail, thereby helping in the early diagnosis and optimal management of diabetes. Hence, the aim of our study was to study the nail changes in patients with diabetes mellitus (DM) and compare these nail changes with those occurring in non-DM patients.
Materials and Methods:
This cross-sectional observational study enrolled 200 patients which included 100 cases of DM and 100 non-DM age- and sex-matched controls and was conducted in a tertiary care institute over a period of 1.5 years. Onychoscopy was performed on all the patients.
Results:
Onychomycosis was present in 29% of diabetics as compared to 11% of controls (statistically significant – P = 0.001). Ragged cuticle was present in 63% of diabetics as compared to 22% of controls (P = 0.001). Longitudinal ridging was present in 46% of diabetics and 28% of controls (P = 0.008). Pitting was present in 8% (statistically not significant; P = 0.2), onychodystrophy in 4% (P = 0.04), paronychia in 16% (P = 0.002), and distal onycholysis (P = 0.05) of diabetics. Other nail changes included pterygium, leukonychia, Beau’s lines, thickening of the nail plate, melanonychia, and subungual hyperkeratosis.
Conclusion:
The nail changes are an important indicator of the underlying metabolic alterations. The nail changes that can be used as screening for DM include onychomycosis, paronychia, ragged cuticle, distal onycholysis, and onychodystrophy. Dermoscopy plays an important role in diagnosing these nail changes.
Keywords
Nail changes
Diabetes mellitus
Onychoscopy
INTRODUCTION
Skin changes occur in about a third of those with diabetes mellitus (DM), sometimes manifesting before the diagnosis is made.[1] Previous literature has detailed the cutaneous manifestations of diabetes, but reviews on the nail changes in this condition are limited.[1,2] The varied systemic pathologic alterations that occur in patients with DM may result in profound changes that affect the nail unit. These findings vary from simple onycholysis to a spectrum of infectious processes, as well as extensive, irreversible destruction. There may be a role of vascular and neurologic systems in producing these clinical findings. In this study, we describe the nail manifestations of DM. Knowledge of these alterations would ensure earlier diagnosis and more optimal management of diabetes. The objective of our study was to evaluate the different nail changes in patients with DM and compare these nail changes with those occurring in normal healthy controls along with onychoscopic changes. The relevance and rationale of the study is to find out the specific nail changes in diabetes along with dermoscopy which may be used for DM screening.
MATERIALS AND METHODS
This cross-sectional observational study enrolled 200 patients which included 100 cases of DM and 100 non-DM age- and sex-matched controls and was conducted in a tertiary care institute over a period of 1.5 years. Sample size calculation was done using convenience sampling. The Institutional Ethical Committee approved the study protocol. Adults (>18 years) diagnosed with Type 2 DM, based on the American Diabetic Association criteria[3] and consent to the study protocol, were included in the study. Patients with type 1 diabetes, trauma, patients with known connective tissue disease or on drugs affecting peripheral circulation, patients with esthetic treatments to the nail unit, in the past two weeks before the study, pregnant, and lactating females were excluded from the study. An informed written consent was taken from all the patients, before their inclusion in the study. Onychoscopy was performed in all the patients (both polarized and non-polarized modes were used, without interface) using a dermatoscope (AM 7515MZT DinoLite Edge Dermatoscope with ×20–×220 magnification) [Figure 1]. Potassium hydroxide (KOH) examination was done in patients clinically diagnosed as onychomycosis and those coming out as positive were considered.
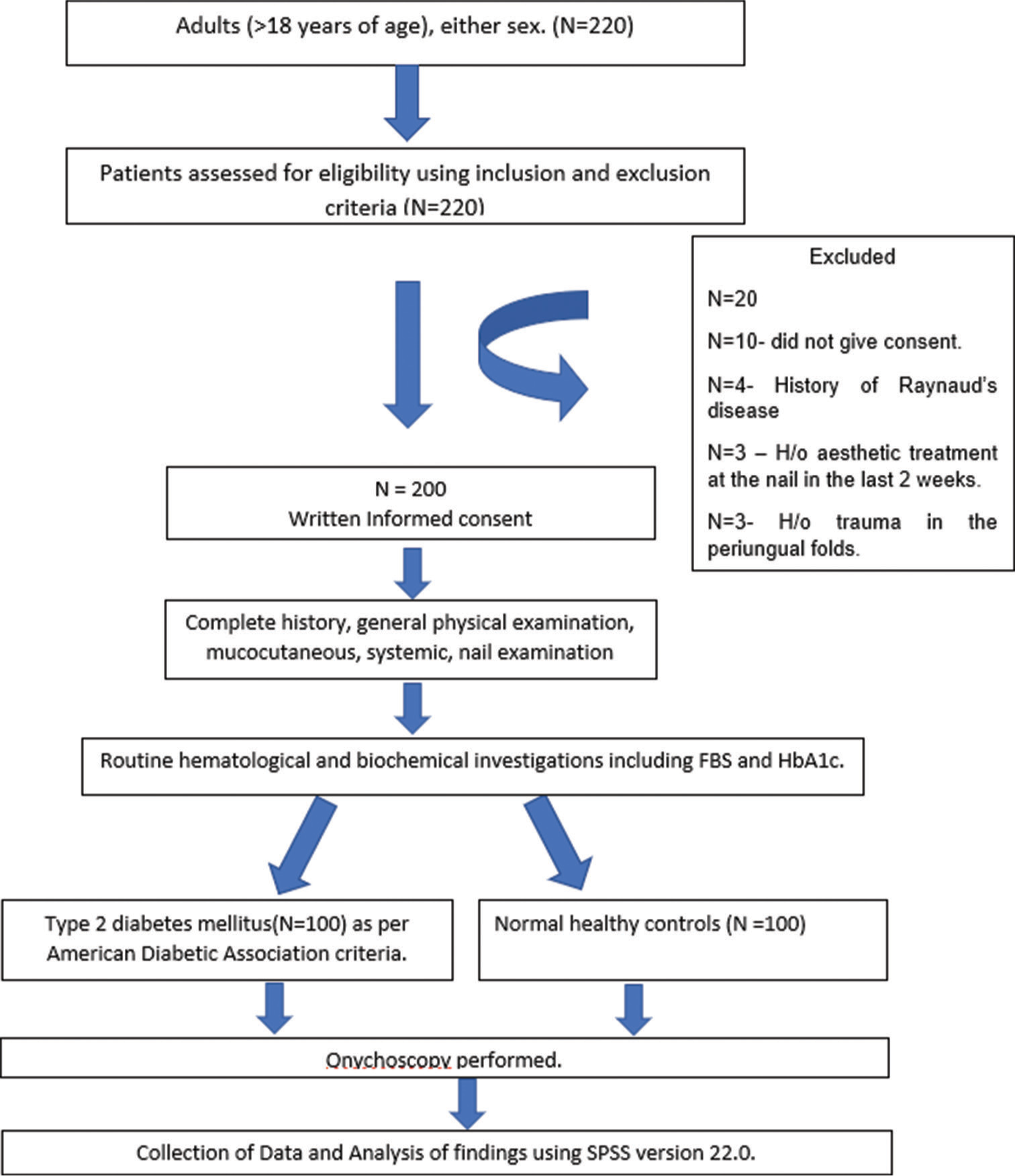
- The protocol of the study. H/o: History of, FBS: Fasting blood sugar, HbA1c: Hemoglobin A1c.
Results were tabulated and analyzed objectively at the end of the study statistically. Categorical variables were presented in number and percentage and continuous variables were presented as mean ± standard deviation (SD). Quantitative variables were compared using an unpaired t-test between two groups. Qualitative variables were correlated using the Chi-square test. “P” < 0.05 was considered statistically significant. The data were analyzed using the Statistical Package for the Social Sciences version 22.0.
RESULTS
A total of 100 patients with DM and 100 non-DM patients were evaluated. The baseline characteristics are summarized in Table 1. The severity of nail involvement among the diabetics and healthy controls is shown in Table 2. In diabetics, nail involvement was more common than in healthy controls. Mild nail involvement (1–3 nails) was seen in 35%, moderate (4–6 nails) in 21%, and severe (7–10 nails) in 13% as compared to healthy controls in which 20% had mild, 13% had moderate, and 9% had severe nail involvement [Table 2].
| Baseline characteristics | Cases (Diabetes mellitus) (n=100), n(%) | Controls (Non-Diabetes mellitus) (n=100), n(%) | P-value |
|---|---|---|---|
| Mean age±SD (years) | 58.32±9.00 | 56.43±7.62 | 0.926 |
| Number of males | 48 | 59 | |
| Number of females | 52 | 41 | |
| Mean disease duration±SD (years) | 6.20±4.658 | – | |
| BMI | 25.86±3.77 | 23.97±3.50 | |
| Mean fasting blood sugar±SD (mg/dL) | 177.01±48.40 | 94.91±3.75 | |
| Mean HbA1c±SD (%) | 8.80±1.60 | 5.43±0.21 |
BMI: Body mass index, HbA1c: Hemoglobin A1c, SD: Standard deviation. P<0.05 was taken as significant
| No. of nails involved | Cases | Controls | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Mild involvement (1–3) | 35 | 35.00 | 20 | 20.00 |
| Moderate involvement (4–6) | 21 | 21.00 | 13 | 13.00 |
| Severe involvement (7–10) | 13 | 13.00 | 9 | 9.00 |
| No nail involvement | 31 | 31.00 | 58 | 58.00 |
| Total | 100 | 100 | ||
The nail changes among the diabetics and healthy group patients are summarized in Table 3. Onychomycosis was present in 29% of diabetics as compared to 11% in controls (statistically significant – P = 0.001). Ragged cuticle was present in 63% of diabetics as compared to 22% in controls (P = 0.001). Longitudinal ridging was present in 46% of diabetics and 28% of controls (P = 0.008). Pitting was present in 8% (statistically not significant; P = 0.2), onychodystrophy in 4% (P = 0.04), paronychia in 16% (P = 0.002), and distal onycholysis (P = 0.05) of diabetics. Other nail changes included pterygium, leukonychia, Beau’s lines, thickening of the nail plate, melanonychia, and subungual hyperkeratosis (statistically not significant) [Figure 2]. Onychoscopic findings are shown in Figure 3.
| Nail plate | Cases | Controls | P-value | ||
|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | ||
| Ragged cuticle | 63 | 63.00 | 22 | 22.00 | P=0.001* |
| Longitudinal ridging | 46 | 46.00 | 28 | 28.00 | P=0.008* |
| Onychomycosis | 29 | 29.00 | 11 | 11.00 | P=0.001* |
| Acute or chronic paronychia | 16 | 16.00 | 3 | 3.00 | P=0.002* |
| Melanonychia | 10 | 10.00 | 6 | 6.00 | P=0.297 |
| Pitting | 8 | 8.00 | 4 | 4.00 | P=0.234 |
| Distal onycholysis | 8 | 8.00 | 2 | 2.00 | P=0.052* |
| Thickening of the nail plate | 7 | 7.00 | 2 | 2.00 | P=0.088 |
| Onychodystrophy | 4 | 4.00 | 0 | 0.00 | P=0.043* |
| Pterygium | 3 | 3.00 | 1 | 1.00 | P=0.312 |
| Leukonychia | 3 | 3.00 | 8 | 8.00 | P=0.121 |
| Subungual hyperkeratosis | 2 | 2.00 | 0 | 0.00 | P=0.155 |
| Beau’s lines | 1 | 1.00 | 2 | 2.00 | P=0.561 |
| Median canaliform dystrophy | 0 | 0.00 | 1 | 1.00 | P=0.316 |
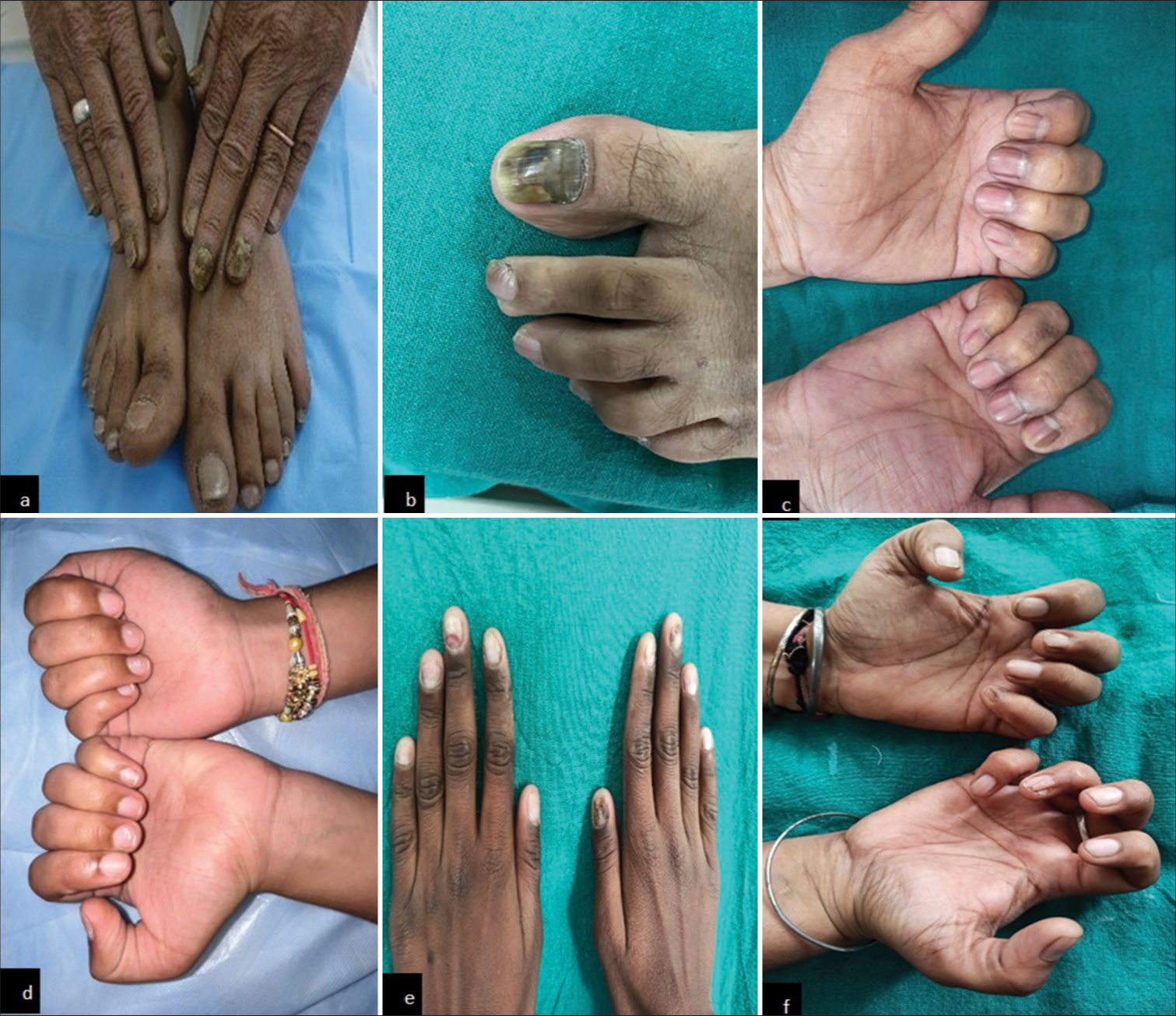
- Clinical findings in diabetes mellitus patients. (a) Showing onychomycosis (fingernails), (b) Showing onychomycosis (toenails), (c) Showing melanonychia (fingernails), (d) Showing leukonychia (fingernails), (e) Showing onychodystrophy (fingernails), and (f) Showing pterygium (fingernails).
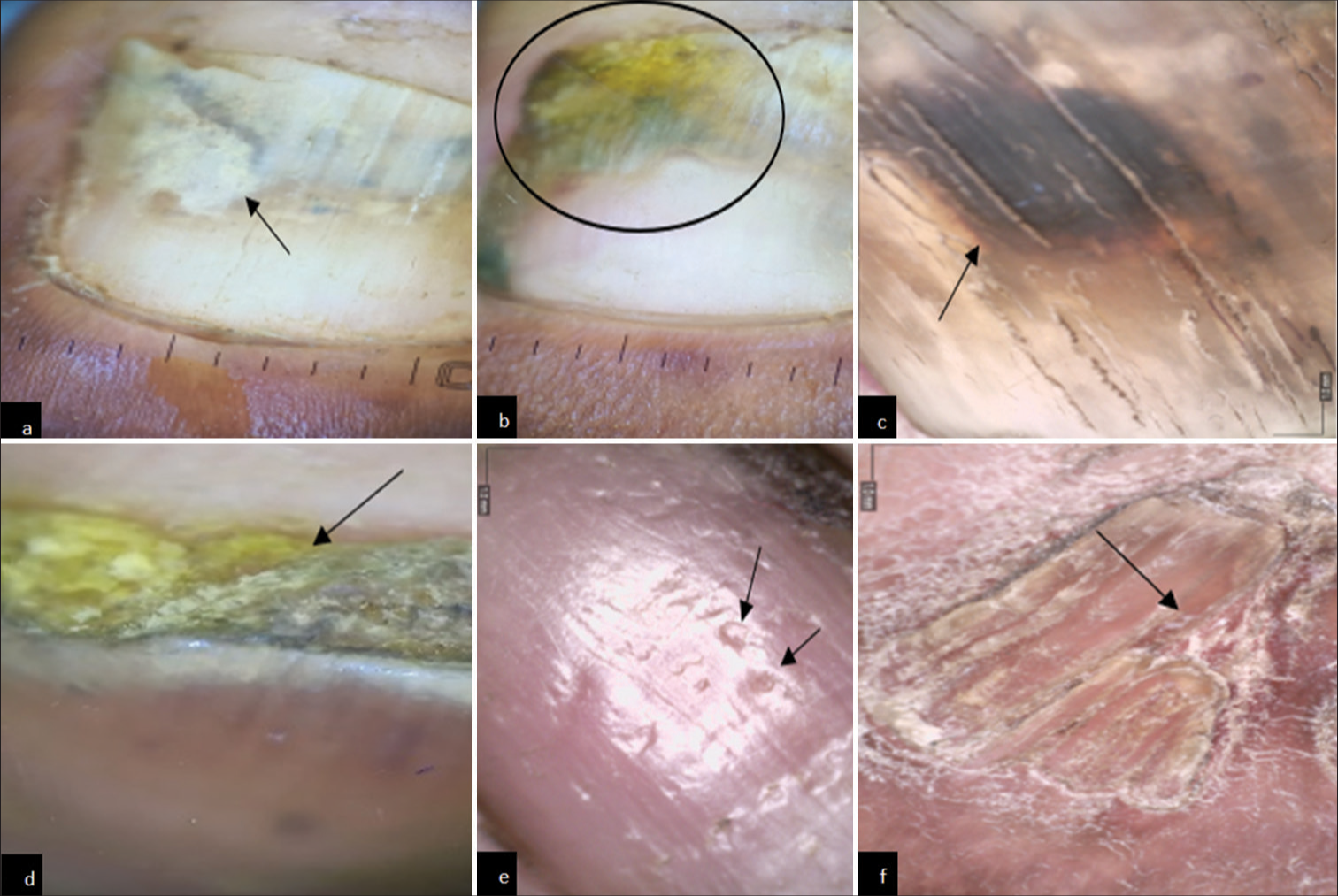
- Onychoscopic findings in diabetes mellitus patients. (a) Onychoscopy showing onychomycosis. (Onycholysis with jagged edge with spikes) (black arrow). (b) Onychoscopy showing onychomycosis (distal lateral subungual). (Yellow discoloration and spikes and onycholysis) (black circle). (c) Onychoscopy showing onychomycosis. (black discoloration) (black arrow). (d) Onychoscopy showing subungual hyperkeratosis (black arrow). (e) Onychoscopy showing pitting (black arrow). (f) Onychoscopy showing pterygium (black arrow).
DISCUSSION
DM is a metabolic disease characterized by many skin and nail changes. Due to hyperglycemia, there may be various systemic pathologic changes and metabolic events that affect nail unit structure and composition. There is the formation of advanced glycation end-products due to the nonenzymatic reaction of glucose with proteins and lipids which promote the production of proinflammatory cytokines.[4] Glycation products also accumulate in structural proteins of the microvasculature. Different pathologic processes are then activated, such as an increase in endothelial permeability and stimulation of growth factor synthesis by macrophages. As a result, the vessel wall thickens and loses its elasticity. Later, the glycation of hemoglobin leads to hypoxia, which is one of the prerequisites for microangiopathy.[5] They reduce collagen flexibility, leading to skin aging, and immunosuppression which affects wound healing and predisposes the individual to various infections.[6] Data suggest that glycation of nail proteins may be used as a diagnostic marker of diabetes and as an indicator of end-organ diabetic disease.[7] Chronic diabetes can cause atherosclerosis of small blood vessels in fingers and toes, which results in insufficiency of blood and oxygen supply to the nail matrix resulting in a change of shape, contour, and color of the nail. Nail changes are relatively common in diabetic patients, especially in chronic and insufficiently controlled diabetic patients. DM is a state of relative immune deficiency; hence, infections (fungal and bacterial) are more common. In our study, we found a high prevalence of nail changes due to patient-related negligence and lack of care of nails, as most of the patients were from rural areas (53%). Other causes for prevalence included agriculture work that brings patients in contact with soil, organic waste, and dirty water.
In our study, onychomycosis was present in 29% of diabetics as compared to 11% of healthy patients, which was consistent with the study done by Vidyasagar and Kumar,[5] in which onychomycosis was present in 43% of diabetic patients and 34.9% in a study done by Akkus et al.[8] The prevalence of onychomycosis is a significant predictor of diabetic foot ulcers.[9] Enlarged, dystrophic toenails cause increased pressure on the underlying toe, compromising the vascular supply and hence resulting in pressure ulcers.
Acute paronychia is often caused by a bacterial infection or trauma to the nail fold of one digit. Repetitive trauma, and excessive hand immersion in water result in the separation of the cuticle from the nail plate, facilitating the entrance of environmental particles and micro-organisms beneath the proximal nail fold. The presence of swollen and tender lateral or posterior nail folds with purulent fluid collections is diagnostic of acute paronychia. Chronic paronychia usually arises as a multifactorial inflammatory reaction of the proximal nail fold to irritants and allergens lasting longer than six weeks. Diabetes, prolonged exposure to water, irritating substances, manicures, nail trauma, and finger sucking all are implicated as predisposing factors.[10] In our study, paronychia was present in 16% of patients as compared to the study done by Vidyasagar and Kumar.[5] in which paronychia was present in 8.3% of patients. The temporary arrest of nail growth with infections can cause transverse depressions on the nail plate resulting in Beau’s lines. In severe infections, the nail can separate from the nailbed (distal and/or lateral separation) causing onycholysis. It is more commonly seen affecting the fingernails than toenails. In our study, distal onycholysis was present in 8% of patients as compared to the study done by Vidyasagar and Kumar.[5] in which it was present in 40% of patients.
In our study, pterygium was present in 3% of our patients as compared to the study done by Vidyasagar and Kumar.[5] in which it was present in 1.6% of patients. This may be attributed to the vascular insufficiency in diabetics. Other changes present included pitting, longitudinal ridging, melanonychia, and onychodystrophy. Diabetic neuropathy predisposes to nail dystrophy and dry skin. Lack of protective sensation (pain), temperature, and proprioception lead to trauma to hands and feet resulting in damage and distortion of nails.
In diabetics, nail involvement was more common than in healthy controls. Mild nail involvement (1–3 nails) was seen in 35%, moderate (4–6 nails) in 21%, and severe (7–10 nails) in 13% of patients. In a study done by Agrawal et al., the severity of nail involvement was compared with the hemoglobin A1c levels, and it was found to be statistically significant.[11] There are various techniques to detect and monitor diabetes in the nail, which include estimation of glycated nail protein (fructosamine), analysis of the molecular structure of human fingernail proteins, and laser-induced breakdown spectroscopy.[12] A study done on fingernails showed that patients with diabetes have higher levels of furosine-lysine (a marker of non-enzymatic glycation).[13] These investigations may prove to be valuable noninvasive methods of monitoring diabetes in the future.
In our study, an onychoscopy was done and the findings were correlated with the clinical findings. In a study done by Litaiem et al.,[14] the main dermoscopic signs of onychomycosis were ruin appearance, longitudinal striae, and spikes on the proximal margin of onycholytic areas, with a specificity of 99.38%, 83.78%, and 85.64%, respectively. A jagged edge with spikes was observed in 58% of patients, yellow discoloration in 57%, and black discoloration in 24% of patients similar to our study where we observed onycholysis with jagged edge with spikes and yellow and black discoloration. In the systematic review by Litaiem et al.,[14] it was shown that dermoscopic signs of onychomycosis have good specificity and are useful in distinguishing nail psoriasis, trauma, and onychomycosis. Although dermoscopy in onychomycosis has good specificity, KOH examination or fungal culture is required for confirmation of the disease. Hence, it is a non-invasive tool that can help in early diagnosis and management of disease.
CONCLUSION
The nail changes are an important indicator of the underlying metabolic alterations. The nail changes that can be used as screening for DM include onychomycosis, paronychia, ragged cuticle, distal onycholysis, and onychodystrophy. The pathological processes of diabetes that occur systemically are reflected in the nail, thereby helping in the early diagnosis and optimal management of diabetes. Dermoscopy plays an important role in diagnosing these nail changes. In this study, there may be chances of Berksonian bias since the patients coming to the hospital were recruited. Hence, community screening is advised in diabetic patients for the early detection of systemic involvement since nail changes are a mirror to the underlying metabolic alterations.
Ethical approval
The research/study approved by the Institutional Review Board at Government Medical College Amritsar, number 3385/D-26/2020, dated 2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Nail changes associated with diabetes mellitus. J Am Acad Dermatol. 1987;16:1015-21.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of diabetes: Synopsis of the 2016 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2016;164:542-52.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous manifestations of diabetes mellitus: A review. Am J Clin Dermatol. 2017;18:541-53.
- [CrossRef] [PubMed] [Google Scholar]
- Toe nail changes in diabetes mellitus. IP Indian J Clin Exp Dermatol. 2021;7:40-6.
- [CrossRef] [Google Scholar]
- Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatol. 2018;36:89-93.
- [CrossRef] [PubMed] [Google Scholar]
- Glycation of nail proteins: from basic biochemical findings to a representative marker for diabetic glycation-associated target organ damage. PLoS One. 2015;10:e0120112.
- [CrossRef] [PubMed] [Google Scholar]
- Tinea pedis and onychomycosis frequency in diabetes mellitus patients and diabetic foot ulcers. A cross sectional-observational study. Pakistan J Med Sci. 2016;32:891-5.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of diabetic foot ulcer occurrence using commonly available clinical information: The Seattle Diabetic Foot Study. Diabetes Care. 2006;29:1202-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of onychomycosis in patients with diabetes mellitus: A cross-sectional study from a tertiary care hospital in North India. Indian J Dermatol Venereol Leprol. 2023;89:710-7.
- [CrossRef] [PubMed] [Google Scholar]
- The nail as an investigative tool in medicine: What a dermatologist ought to know. Indian J Dermatol Venereol Leprol. 2017;83:635-43.
- [CrossRef] [PubMed] [Google Scholar]
- Glycosylation levels of nail proteins in diabetic patients with retinopathy and neuropathy. Kobe J Med Sci. 1985;31:183-8.
- [Google Scholar]
- Dermoscopy of onychomycosis: A systematic review. Dermatol Pract Concept. 2023;13:e2023072.
- [CrossRef] [PubMed] [Google Scholar]






